Leaching Behavior of As and Pb in Lead–Zinc Mining Waste Rock under Mine Drainage and Rainwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
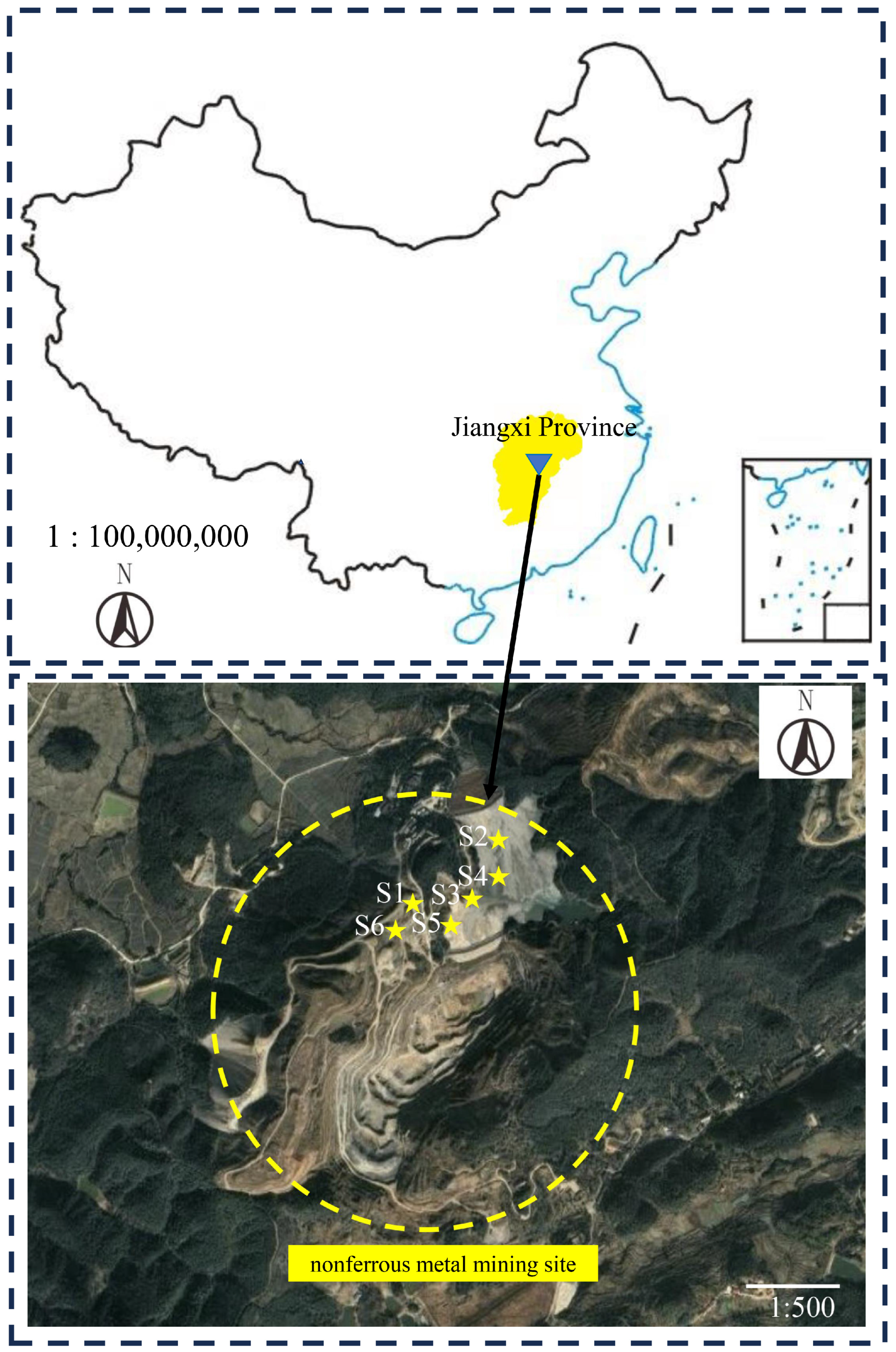
2.2. Waste Rock Sample Collection and Pretreatment
2.3. Characterizations
2.4. Batch Leaching Tests
2.5. Statistical Analysis
3. Results and Discussion
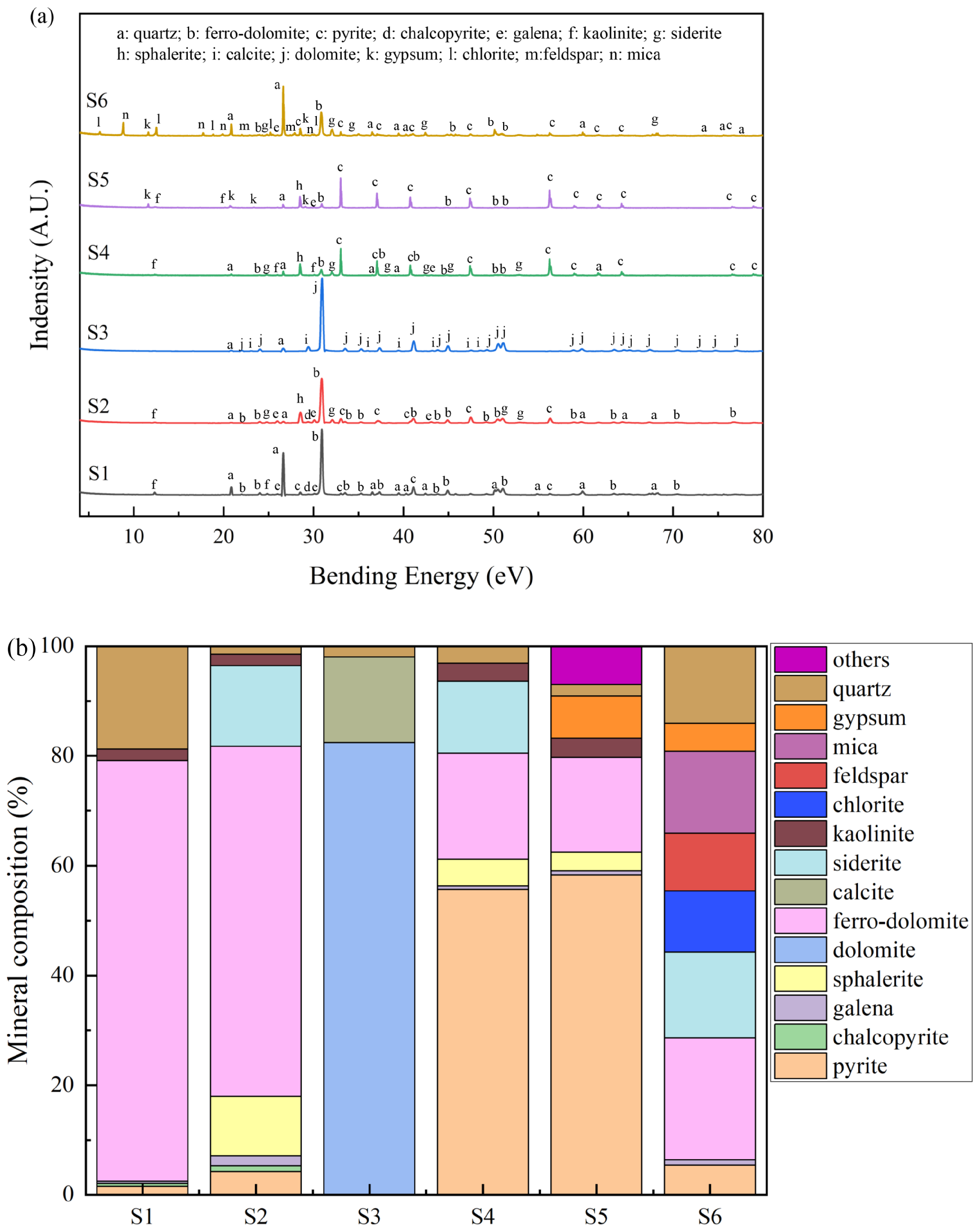
3.1. Mineral Component of Waste Rocks
3.2. Chemical Composition of Waste Rocks
3.3. Morphology and Element Distribution of Waste Rocks
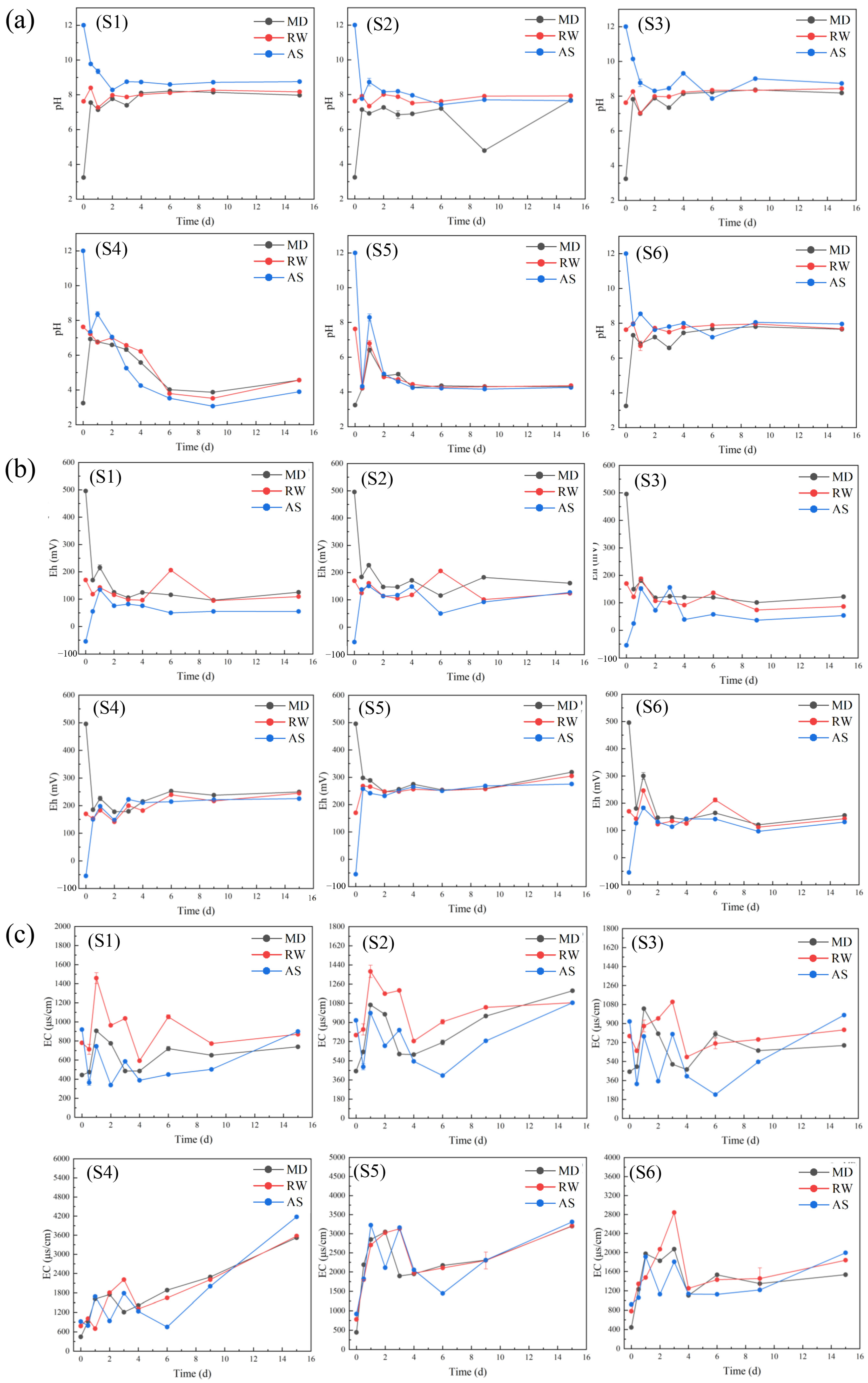
3.4. The Changes in pH, Eh, and EC Values of Leaching Solutions
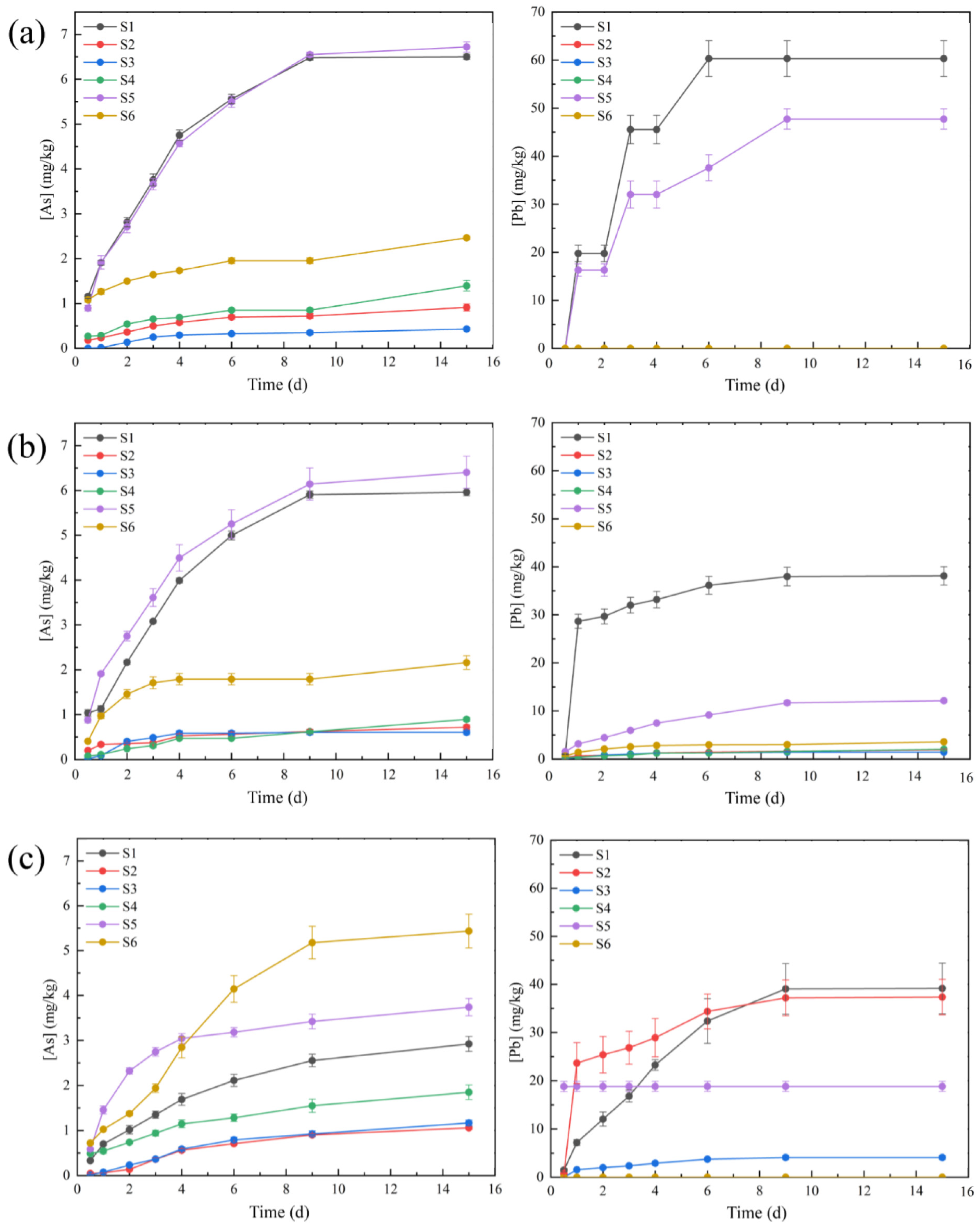
3.5. The Change in Total Concentrations of As and Pb in Leaching Solutions
3.6. Correlation between Release of As and Pb and Mineral Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, J.; Liu, L.; Zhang, Z.; Xia, X.; Yang, L.; Ning, Z.; Liu, C.; Qiu, G. Sulfate-accelerated photochemical oxidation of arsenopyrite in acidic systems under oxic conditions: Formation and function of schwertmannite. J. Hazard. Mater. 2022, 433, 128716. [Google Scholar] [CrossRef]
- Jain, M.K.; Das, A. Impact of Mine Waste Leachates on Aquatic Environment: A Review. Curr. Pollut. Rep. 2017, 3, 31–37. [Google Scholar] [CrossRef]
- Zhai, M.; Hu, R.; Wang, Y.; Jiang, S.; Wang, R.; Li, J.; Chen, H.; Yang, Z.; Lü, Q.; Qi, T. Mineral resource science in China: Review and perspective. Geogr. Sustain. 2021, 2, 107–114. [Google Scholar] [CrossRef]
- Khaboushan, A.S.; Osanloo, M.; Esfahanipour, A. Optimization of open pit to underground transition depth: An idea for reducing waste rock contamination while maximizing economic benefits. J. Clean. Prod. 2020, 277, 123530. [Google Scholar] [CrossRef]
- Peng, J.-Y.; Zhang, S.; Wang, Y.-J.; Zhao, R.-F.; Zhou, Y.-L.; Zhou, J.-W. Identification of priority pollutants and key factors affecting environmental risks of lead-zinc mine tailing sites. Sci. Total Environ. 2023, 889, 164039. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Wu, Y.; Zhang, S.; Li, G.; An, T. Pollution Profiles, Source Identification and Health Risk Assessment of Heavy Metals in Soil near a Non-Ferrous Metal Smelting Plant. Int. J. Environ. Res. Public Health 2023, 20, 1004. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.C.; Guo, Z.H.; Peng, C.; Liu, X.; Zhou, Z.R.; Xiao, X.Y. Heavy metals in soils around non-ferrous smelteries in China: Status, health risks and control measures. Environ. Pollut. 2021, 282, 117038. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, H.; Peng, T.; Ding, W.; Liu, B.; Liu, Q. Comprehensive evaluation of environmental availability, pollution level and leaching heavy metals behavior in non-ferrous metal tailings. J. Environ. Manag. 2021, 290, 112639. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Contamination of soils with heavy metals and metalloids and its ecological hazard (analytic review). Eurasian Soil Sci. 2013, 46, 793–801. [Google Scholar] [CrossRef]
- Zeng, J.; Luo, X.; Cheng, Y.; Ke, W.; Hartley, W.; Li, C.; Jiang, J.; Zhu, F.; Xue, S. Spatial distribution of toxic metal(loid)s at an abandoned zinc smelting site, Southern China. J. Hazard. Mater. 2022, 425, 127970. [Google Scholar] [CrossRef]
- Huang, S.; Yuan, C.; Li, Q.; Yang, Y.; Tang, C.; Ouyang, K.; Wang, B. Distribution and risk assessment of heavy metals in soils from a typical Pb-Zn mining area. Pol. J. Environ. Stud. 2017, 26, 1105–1112. [Google Scholar] [CrossRef]
- Song, L.; Qian, J.Z.; Zhang, F.W.; Kong, X.K.; Li, H.; Luan, S.; Zhang, Q.J.; Kang, Z.Q.; Han, Z.T.; Zhang, Z.J. An ecological remediation model combining optimal substrate amelioration and native hyperaccumulator colonization in non-ferrous metal tailings pond. J. Environ. Manag. 2022, 322, 116141. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, C.; Li, K.; Zhu, X. Distribution characteristics and pollution assessment of soil heavy metals over a typical nonferrous metal mine area in Chifeng, Inner Mongolia, China. Environ. Earth Sci. 2018, 77, 638. [Google Scholar] [CrossRef]
- Obiora, S.C.; Chukwu, A.; Chibuike, G.; Nwegbu, A.N. Potentially harmful elements and their health implications in cultivable soils and food crops around lead-zinc mines in Ishiagu, Southeastern Nigeria. J. Geochem. Explor. 2019, 204, 289–296. [Google Scholar] [CrossRef]
- Vural, A. Assessment of metal pollution associated with an alteration area: Old Gumushane, NE Black Sea. Environ. Sci. Pollut. Res. 2015, 22, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.Q.; Dong, Y.Q.; Feng, L.; Zhou, M.; Hou, H.B. Contamination and health risk assessment of heavy metals in China’s lead-zinc mine tailings: A meta-analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef] [PubMed]
- Bigot, M.; Guterres, J.; Rossato, L.; Pudmenzky, A.; Doley, D.; Whittaker, M.; Pillai-McGarry, U.; Schmidt, S. Metal-binding hydrogel particles alleviate soil toxicity and facilitate healthy plant establishment of the native metallophyte grass Astrebla lappacea in mine waste rock and tailings. J. Hazard. Mater. 2013, 248, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.B.; Chen, D.N.; Lin, H. The behavior of heavy metal release from sulfide waste rock under microbial action and different environmental factors. Environ. Sci. Pollut. Res. 2022, 29, 75293–75306. [Google Scholar] [CrossRef] [PubMed]
- Demers, I.; Bouda, M.; Mbonimpa, M.; Benzaazoua, M.; Bois, D.; Gagnon, M. Valorization of acid mine drainage treatment sludge as remediation component to control acid generation from mine wastes, part 2: Field experimentation. Miner. Eng. 2015, 76, 117–125. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Z.H.; Hu, Y.N.; Cheng, H.F. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total Environ. 2019, 695, 133893. [Google Scholar] [CrossRef]
- Yin, T.T.; Lin, H.; Dong, Y.B.; Wei, Z.S.; Li, B.; Liu, C.J.; Chen, X. Inhibition of cadmium releasing from sulfide tailings into the environment by carbonate-mineralized bacteria. J. Hazard. Mater. 2021, 419, 126479. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.N.; Wu, P.X.; Huang, Z.J.; Li, Y.Y.; Yang, S.S.; Dang, Z.; Ruan, B.; Kang, C.X. Efficient inhibition of heavy metal release from mine tailings against acid rain exposure by triethylenetetramine intercalated montmorillonite (TETA-Mt). J. Hazard. Mater. 2016, 318, 396–406. [Google Scholar] [CrossRef]
- Ollson, C.J.; Smith, E.; Scheckel, K.G.; Betts, A.R.; Juhasz, A.L. Assessment of arsenic speciation and bioaccessibility in mine-impacted materials. J. Hazard. Mater. 2016, 313, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.X.; Chen, M.; Wang, X.T.; Chen, Y.D.; Dong, K. Ecological Risk Assessment and Source Analysis of Heavy Metals in the Soils of a Lead-Zinc Mining Watershed Area. Water 2023, 15, 15010113. [Google Scholar] [CrossRef]
- Aykol, A.; Budakoglu, M.; Kumral, M.; Gultekin, A.H.; Turhan, M.; Esenli, V.; Yavuz, F.; Orgun, Y. Heavy metal pollution and acid drainage from the abandoned Balya Pb-Zn sulfide Mine, NW Anatolia, Turkey. Environ. Geol. 2003, 45, 198–208. [Google Scholar] [CrossRef]
- Elghali, A.; Benzaazoua, M.; Taha, Y.; Amar, H.; Ait-khouia, Y.; Bouzahzah, H.; Hakkou, R. Prediction of acid mine drainage: Where we are. Earth-Sci. Rev. 2023, 241, 104421. [Google Scholar] [CrossRef]
- Grathwohl, P.; Susset, B. Comparison of percolation to batch and sequential leaching tests: Theory and data. Waste Manag. 2009, 29, 2681–2688. [Google Scholar] [CrossRef]
- Hama, J.R.; Jorgensen, D.B.G.; Diamantopoulos, E.; Bucheli, T.D.; Hansen, H.C.B.; Strobel, B.W. Indole and quinolizidine alkaloids from blue lupin leach to agricultural drainage water. Sci. Total Environ. 2022, 834, 155283. [Google Scholar] [CrossRef]
- Yin, K.; Chan, W.P.; Dou, X.M.; Ren, F.; Chang, V.W.C. Measurements, factor analysis and modeling of element leaching from incineration bottom ashes for quantitative component effects. J. Clean. Prod. 2017, 165, 477–490. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Lottermoser, B.; Bradshaw, D. Evaluating waste rock mineralogy and microtexture during kinetic testing for improved acid rock drainage prediction. Miner. Eng. 2013, 52, 111–124. [Google Scholar] [CrossRef]
- Chai, J.C.; Onitsuk, K.; Hayashi, S. Cr(VI) concentration from batch contact/tank leaching and column percolation test using fly ash with additives. J. Hazard. Mater. 2009, 166, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Grosselli, M.; Gonzalez, P.; Martinez, D.; Gil, R. Batch leaching tests of motherboards to assess environmental contamination by bromine, platinum group elements and other selected heavy metals. Chemosphere 2016, 144, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Ohtsubo, M.; Higashi, T.; Yamaoka, S.; Morishita, T. Leachability of municipal solid waste ashes in simulated landfill conditions. Waste Manag. 2007, 27, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Raygan, S.; Rezaei, A.; Jafari, A. Leaching of nickel from a secondary source by sulfuric acid. J. Environ. Chem. Eng. 2017, 5, 3922–3929. [Google Scholar] [CrossRef]
- Lv, J.F.; Zheng, Y.X.; Tong, X.; Li, X. Clean utilization of waste rocks as a novel adsorbent to treat the beneficiation wastewater containing arsenic and fluorine. J. Clean. Prod. 2021, 293, 126160. [Google Scholar] [CrossRef]
- Kappen, P.; Ferrando-Miguel, G.; Reichman, S.M.; Innes, L.; Welter, E.; Pigram, P.J. Antimony leaching and chemical species analyses in an industrial solid waste: Surface and bulk speciation using ToF-SIMS and XANES. J. Hazard. Mater. 2017, 329, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ahmad, F.; Yusoff, P.S.M.M. Effect of boric acid and melamine on the intumescent fire-retardant coating composition for the fire protection of structural steel substrates. J. Appl. Polym. Sci. 2013, 128, 2983–2993. [Google Scholar] [CrossRef]
- Soltan, A.M.M.; Pöhler, K.; Fuchs, F.; Abd El-Raoof, F.; El-Kaliouby, B.A.H.; Koenig, A.; Pöllmann, H. Clay-bricks from recycled rock tailings. Ceram. Int. 2016, 42, 16685–16696. [Google Scholar] [CrossRef]
- Wang, D.F.; Zhang, Y.S.; Li, Z.H.; Shi, J.S.; Liu, C.; Pang, B.; Chen, Y.D.; Liu, G.J.; Sun, G.W. Systemical investigation on the determination of sulfate in cement-based materials based on a promoted conductometric titrator. Measurement 2022, 203, 111909. [Google Scholar] [CrossRef]
- Sonnichsen, C.; Atamanchuk, D.; Hendricks, A.; Morgan, S.; Smith, J.; Grundke, I.; Luy, E.; Sieben, V.J. An Automated Microfluidic Analyzer for In Situ Monitoring of Total Alkalinity. ACS Sens. 2023, 8, 344–352. [Google Scholar] [CrossRef]
- Jia, G.; Wang, Y.; Yang, F.; Ma, Z. Preparation of CFB fly ash/sewage sludge ceramsite and the morphological transformation and release properties of sulfur. Construction and Building Materials 2023, 373, 130864. [Google Scholar] [CrossRef]
- Xu, F.; Chu, M.; Chang, Z.; Gu, Z.; Sun, X. Sulfur release and transformation during the pyrolysis of lignite with different particle sizes. J. Anal. Appl. Pyrolysis 2021, 156, 105162. [Google Scholar] [CrossRef]
- Yang, J.J.; Guo, Z.W.; Jiang, L.H.; Sarkodie, E.K.; Li, K.W.; Shi, J.X.; Deng, Y.; Zhang, Z.C.; Liu, H.W.; Liang, Y.L.; et al. Cadmium, lead and arsenic contamination in an abandoned nonferrous metal smelting site in southern China: Chemical speciation and mobility. Ecotoxicol. Environ. Saf. 2022, 239, 113617. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Tabelin, C.B.; Gao, W.; Tang, L.; Luo, X.; Ke, W.; Jiang, J.; Xue, S. Heterogeneous distributions of heavy metals in the soil-groundwater system empowers the knowledge of the pollution migration at a smelting site. Chem. Eng. J. 2023, 454, 140307. [Google Scholar] [CrossRef]
- Ma, T.; Luo, H.; Huang, K.; Pan, Y.; Tang, T.; Tao, X.; Lu, G. Integrated ecological risk assessment of heavy metals in an oil shale mining area after restoration. J. Environ. Manag. 2021, 300, 113797. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Akcil, A.; Mishra, S.; Erust, C. Synergistic effect of biogenic Fe3+ coupled to S° oxidation on simultaneous bioleaching of Cu, Co, Zn and as from hazardous Pyrite Ash Waste. J. Hazard. Mater. 2017, 325, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Le, Z.; Li, Z.; Liu, J.M.; Ren, A. Placental concentrations of mercury, lead, cadmium, and arsenic and the risk of neural tube defects in a Chinese population. Reprod. Toxicol. 2013, 35, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Elghali, A.; Benzaazoua, M.; Bouzahzah, H.; Abdelmoula, M.; Dynes, J.J.; Jamieson, H.E. Role of secondary minerals in the acid generating potential of weathered mine tailings: Crystal-chemistry characterization and closed mine site management involvement. Sci. Total Environ. 2021, 784, 147105. [Google Scholar] [CrossRef]
- Jennings, S.R.; Dollhopf, D.J.; Inskeep, W.P. Acid production from sulfide minerals using hydrogen peroxide weathering. Appl. Geochem. 2000, 15, 235–243. [Google Scholar] [CrossRef]
- Sakala, E.; Fourie, F.; Gomo, M.; Madzivire, G. Natural Attenuation of Acid Mine Drainage by Various Rocks in the Witbank, Ermelo and Highveld Coalfields, South Africa. Nat. Resour. Res. 2021, 30, 557–570. [Google Scholar] [CrossRef]
- Holmstrom, H.; Ljungberg, J.; Ohlander, B. Role of carbonates in mitigation of metal release from mining waste. Evidence from humidity cells tests. Environ. Geol. 1999, 37, 267–280. [Google Scholar] [CrossRef]
- Gu, S.Q.; Kang, X.N.; Wang, L.; Lichtfouse, E.; Wang, C.Y. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Du, H.H.; Chen, W.L.; Cai, P.; Rong, X.M.; Feng, X.H.; Huang, Q.Y. Competitive adsorption of Pb and Cd on bacteria-montmorillonite composite. Environ. Pollut. 2016, 218, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Fakhreddine, S.; Fendorf, S. The effect of porewater ionic composition on arsenate adsorption to clay minerals. Sci. Total Environ. 2021, 785, 147096. [Google Scholar] [CrossRef] [PubMed]
- Covelo, E.F.; Vega, F.A.; Andrade, M.L. Heavy metal sorption and desorption capacity of soils containing endogenous contaminants. J. Hazard. Mater. 2007, 143, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Kastyuchik, A.; Karam, A.; Aider, M. Effectiveness of alkaline amendments in acid mine drainage remediation. Environ. Technol. Innov. 2016, 6, 49–59. [Google Scholar] [CrossRef]
- Li, Y.; Yin, H.; Cai, Y.; Luo, H.; Yan, C.; Dang, Z. Regulating the exposed crystal facets of alpha-Fe2O3 to promote Fe2O3-modified biochar performance in heavy metals adsorption. Chemosphere 2023, 311, 136976. [Google Scholar] [CrossRef]
- Zhang, H.P.; Gu, L.Q.; Zhang, L.; Zheng, S.R.; Wan, H.Q.; Sun, J.Y.; Zhu, D.Q.; Xu, Z.Y. Removal of aqueous Pb(II) by adsorption on Al2O3-pillared layered MnO2. Appl. Surf. Sci. 2017, 406, 330–338. [Google Scholar] [CrossRef]
- Faheem; Yu, H.X.; Liu, J.; Shen, J.Y.; Sun, X.Y.; Li, J.S.; Wang, L.J. Preparation of MnOx-loaded biochar for Pb2+ removal: Adsorption performance and possible mechanism. J. Taiwan Inst. Chem. Eng. 2016, 66, 313–320. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Smith, D.L. Geology and mineralogy of the Ashram Zone carbonatite, Eldor Complex, Quebec. Ore Geol. Rev. 2017, 86, 784–806. [Google Scholar] [CrossRef]
- Mbamba, C.K.; Harrison, S.T.L.; Franzidis, J.P.; Broadhurst, J.L. Mitigating acid rock drainage risks while recovering low-sulfur coal from ultrafine colliery wastes using froth flotation. Miner. Eng. 2012, 29, 13–21. [Google Scholar] [CrossRef]
- Liu, Y.B.; Cui, J.; Peng, Y.; Lu, Y.F.; Yao, D.R.; Yang, J.; He, Y. Atmospheric deposition of hazardous elements and its accumulation in both soil and grain of winter wheat in a lead-zinc smelter contaminated area, Central China. Sci. Total Environ. 2020, 707, 135789. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.R.; Yin, W.Z.; Yang, B.; Han, F. Simultaneous separation of quartz and dolomite from magnesite using monosodium phosphate as a regulator via reverse flotation. Miner. Eng. 2021, 172, 107185. [Google Scholar] [CrossRef]
- Qiu, G.; Gao, T.; Hong, J.; Tan, W.; Liu, F.; Zheng, L. Mechanisms of arsenic-containing pyrite oxidation by aqueous arsenate under anoxic conditions. Geochim. Cosmochim. Acta 2017, 217, 306–319. [Google Scholar] [CrossRef]
- Ramírez-Aldaba, H.; Valles, O.P.; Vazquez-Arenas, J.; Rojas-Contreras, J.A.; Valdez-Pérez, D.; Ruiz-Baca, E.; Meraz-Rodríguez, M.; Sosa-Rodríguez, F.S.; Rodríguez, A.G.; Lara, R.H. Chemical and surface analysis during evolution of arsenopyrite oxidation by Acidithiobacillus thiooxidans in the presence and absence of supplementary arsenic. Sci. Total Environ. 2016, 566, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Pumure, I.; Renton, J.J.; Smart, R.B. The interstitial location of selenium and arsenic in rocks associated with coal mining using ultrasound extractions and principal component analysis (PCA). J. Hazard. Mater. 2011, 198, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Playter, T.; Konhauser, K.; Owttrim, G.; Hodgson, C.; Warchola, T.; Mloszewska, A.M.; Sutherland, B.; Bekker, A.; Zonneveld, J.P.; Pemberton, S.G.; et al. Microbe-clay interactions as a mechanism for the preservation of organic matter and trace metal biosignatures in black shales. Chem. Geol. 2017, 459, 75–90. [Google Scholar] [CrossRef]
- Deng, M.G.; Zhao, J.X.; Liu, F.X.; Yu, H.J.; Sun, B.D.; Liu, F.; Li, S.B. Discussion on sources of metallogenic fluids and materials of the Shuitoushan Pb-Zn deposit in Zhenkang, western Yunnan: Evidence from H, O, S and Pb isotopes. Acta Petrol. Sin. 2017, 33, 2001–2017. [Google Scholar]
- Su, M.; Han, F.Y.; Wang, M.X.; Ma, J.X.; Wang, X.W.; Wang, Z.J.; Hu, S.J.; Li, Z. Clay-assisted protection of Enterobacter sp. from Pb (II) stress. Ecotoxicol. Environ. Saf. 2021, 208, 111704. [Google Scholar] [CrossRef]
- Perez-Lopez, R.; Nieto, J.M.; de Almodovar, G.R. Utilization of fly ash to improve the quality of the acid mine drainage generated by oxidation of a sulphide-rich mining waste: Column experiments. Chemosphere 2007, 67, 1637–1646. [Google Scholar] [CrossRef]
- Minamikawa, K.; Sakai, N. The effect of water management based on soil redox potential on methane emission from two kinds of paddy soils in Japan. Agric. Ecosyst. Environ. 2005, 107, 397–407. [Google Scholar] [CrossRef]
- Yao, B.M.; Wang, S.Q.; Xie, S.T.; Li, G.; Sun, G.X. Optimal soil Eh, pH for simultaneous decrease of bioavailable Cd, as in co-contaminated paddy soil under water management strategies. Sci. Total Environ. 2022, 806, 151342. [Google Scholar] [CrossRef] [PubMed]
- Asael, D.; Matthews, A.; Oszczepalski, S.; Bar-Matthews, M.; Halicz, L. Fluid speciation controls of low temperature copper isotope fractionation applied to the Kupferschiefer and Timna ore deposits. Chem. Geol. 2009, 262, 147–158. [Google Scholar] [CrossRef]
- Kasemodel, M.C.; Sakamoto, I.K.; Varesche, M.B.A.; Rodrigues, V.G.S. Potentially toxic metal contamination and microbial community analysis in an abandoned Pb and Zn mining waste deposit. Sci. Total Environ. 2019, 675, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Opiso, E.M.; Sato, T.; Morimoto, K.; Asai, A.; Anraku, S.; Numako, C.; Yoneda, T. Incorporation of arsenic during the formation of Mg-bearing minerals at alkaline condition. Miner. Eng. 2010, 23, 230–237. [Google Scholar] [CrossRef]
- Kong, L.H.; Hu, X.Y.; Peng, X.J.; Wang, X.L. Specific H2S Release from Thiosulfate Promoted by UV Irradiation for Removal of Arsenic and Heavy Metals from Strongly Acidic Wastewater. Environ. Sci. Technol. 2020, 54, 14076–14084. [Google Scholar] [CrossRef] [PubMed]
- Mukwaturi, M.; Lin, C.X. Mobilization of heavy metals from urban contaminated soils under water inundation conditions. J. Hazard. Mater. 2015, 285, 445–452. [Google Scholar] [CrossRef]
- Zhang, J.F.; Xie, X.D.; Meng, X.G.; Li, Y.; Zhu, W.H. The critical role of oxidative debris in the adsorption and desorption of Pb(II) to graphene oxides under alkaline groundwater conditions. Sci. Total Environ. 2020, 704, 135254. [Google Scholar] [CrossRef]
- Lee, P.-K.; Kang, M.-J.; Jeong, Y.-J.; Kwon, Y.K.; Yu, S. Lead isotopes combined with geochemical and mineralogical analyses for source identification of arsenic in agricultural soils surrounding a zinc smelter. J. Hazard. Mater. 2020, 382, 121044. [Google Scholar] [CrossRef]
- Shi, S.Y.; Fang, Z.H. Bioleaching of marmatite flotation concentrate by Acidithiobacillus ferrooxidans. Hydrometallurgy 2004, 75, 1–10. [Google Scholar] [CrossRef]
- Tum, S.; Matsumoto, S.; Nishikata, M.; Yasutaka, T. Assessment of seasonal changes in groundwater quality of waste rock dump in temperate continental climate, northern Japan. Chemosphere 2023, 327, 138482. [Google Scholar] [CrossRef]
- Kim, M.J.; Nriagu, J.; Haack, S. Carbonate ions and arsenic dissolution by groundwater. Environ. Sci. Technol. 2000, 34, 3094–3100. [Google Scholar] [CrossRef]




| Chemical Parameter | MD | RW | AS |
|---|---|---|---|
| pH | 3.24 | 7.63 | 12.00 |
| Eh (mV) | 495.67 | 170.00 | −54.67 |
| EC (μS/cm) | 443.23 | 780.90 | 919.63 |
| As | N.D. | N.D. | N.D. |
| Pb | N.D. | N.D. | N.D. |
| Chemical Composition | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Na2O | 0.11% | 0.78% | 1.31% | N.D. | 1.51% | N.D. |
| MgO | 2.45% | 1.53% | 2.88% | 17.66% | 23.97% | 29.44% |
| Al2O3 | 4.85% | 4.44% | 22.96% | 6.99% | 2.96% | 0.62% |
| SiO2 | 7.79% | 5.70% | 32.93% | 30.24% | 3.87% | 3.70% |
| P2O5 | 0.07% | 0.07% | 0.26% | 0.06% | 0.09% | N.D. |
| K2O | 0.06% | 0.02% | 2.84% | 0.17% | 0.06% | 0.04% |
| CaO | 4.24% | 4.05% | 7.36% | 30.22% | 41.41% | 56.84% |
| MnO | 0.43% | 0.23% | 0.79% | 0.70% | 1.69% | 0.57% |
| Fe2O3 | 73.50% | 77.42% | 23.82% | 8.82% | 1.13% | 4.83% |
| As | 0.02% | 0.03% | N.D. | 0.03% | 0.04% | 0.06% |
| Pb | 0.33% | 1.99% | 0.03% | 0.51% | 0.71% | 0.34% |
| Total carbon | 6.75% | 8.66% | 10.80% | 1.55% | 0.71% | 3.56% |
| Total sulfur | 1.42% | 42.54% | 0.17% | 48.60% | 49.92% | 3.78% |
| Sulfide–sulfur | 1.01% | 41.23% | 0.01% | 47.40% | 46.14% | 2.29% |
| Sulfate–sulfur | 0.41% | 1.31% | 0.16% | 1.20% | 3.78% | 1.49% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Yang, J.; Li, K.; Shi, J.; Peng, Y.; Sarkodie, E.K.; Miao, B.; Liu, H.; Liu, X.; Jiang, L. Leaching Behavior of As and Pb in Lead–Zinc Mining Waste Rock under Mine Drainage and Rainwater. Toxics 2023, 11, 943. https://doi.org/10.3390/toxics11110943
Guo Z, Yang J, Li K, Shi J, Peng Y, Sarkodie EK, Miao B, Liu H, Liu X, Jiang L. Leaching Behavior of As and Pb in Lead–Zinc Mining Waste Rock under Mine Drainage and Rainwater. Toxics. 2023; 11(11):943. https://doi.org/10.3390/toxics11110943
Chicago/Turabian StyleGuo, Ziwen, Jiejie Yang, Kewei Li, Jiaxin Shi, Yulong Peng, Emmanuel Konadu Sarkodie, Bo Miao, Hongwei Liu, Xueduan Liu, and Luhua Jiang. 2023. "Leaching Behavior of As and Pb in Lead–Zinc Mining Waste Rock under Mine Drainage and Rainwater" Toxics 11, no. 11: 943. https://doi.org/10.3390/toxics11110943
APA StyleGuo, Z., Yang, J., Li, K., Shi, J., Peng, Y., Sarkodie, E. K., Miao, B., Liu, H., Liu, X., & Jiang, L. (2023). Leaching Behavior of As and Pb in Lead–Zinc Mining Waste Rock under Mine Drainage and Rainwater. Toxics, 11(11), 943. https://doi.org/10.3390/toxics11110943







