Genome-Wide Profiling of Transcriptome and DNA Methylome in Human Embryonic Stem Cells Exposed to Extractable Organic Matter from PM2.5
Abstract
1. Introduction
2. Materials and Methods
2.1. PM2.5 Collection and EOM Extraction
2.2. Human Embryonic Stem Cell Culture
2.3. Cell Viability Assay
2.4. Immunofluorescence Staining
2.5. RNA Preparations and Quantitative Real Time PCR
2.6. Genomic-Wide mRNA Expression Analysis
2.7. Genomic-Wide DNA Methylation Profiling
2.8. Statistical Analyses
3. Results
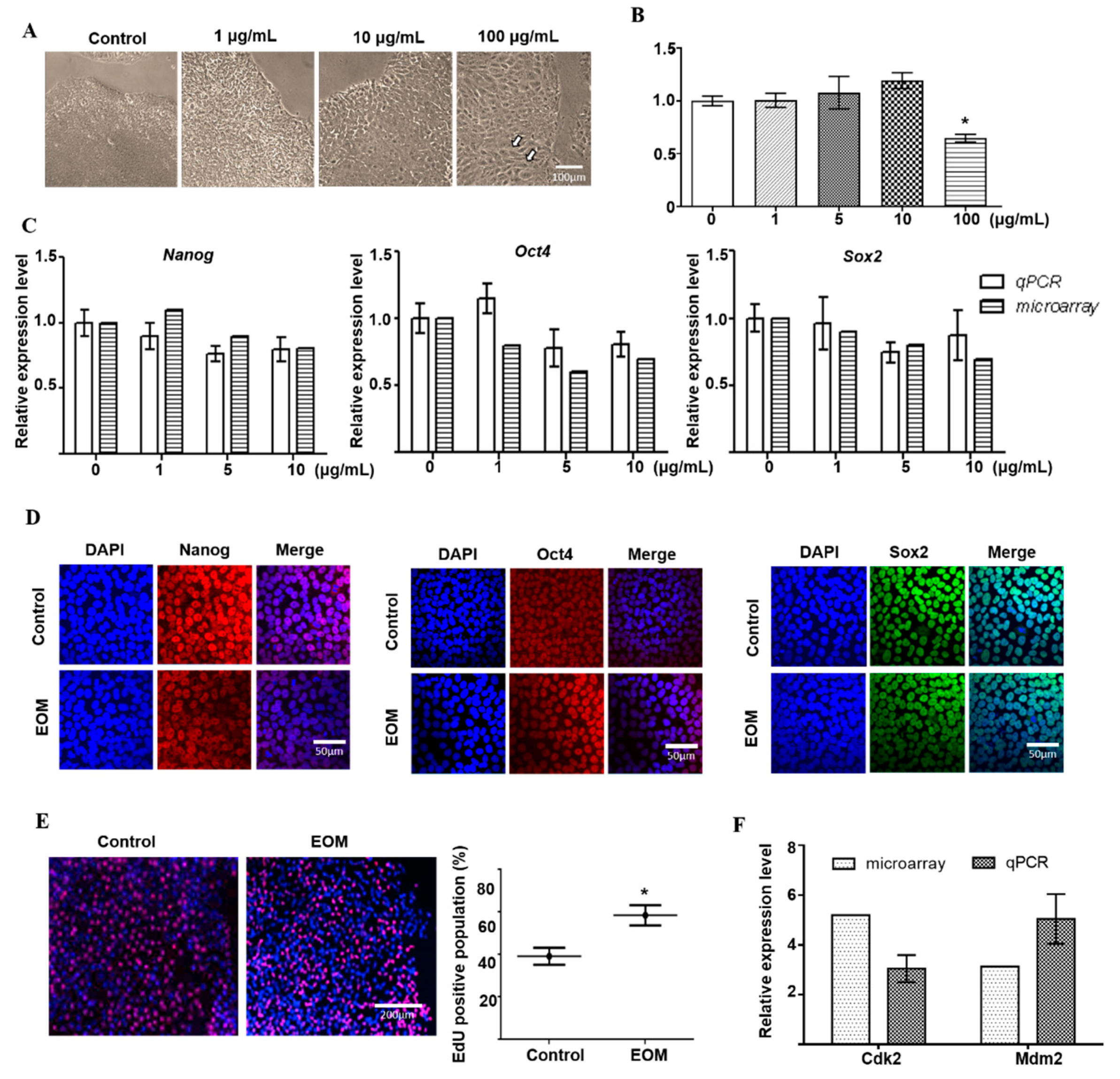
3.1. Effects of EOM on Viability and Characteristics of hESCs
3.2. EOM-Induced Transcriptomic Changes in hESCs
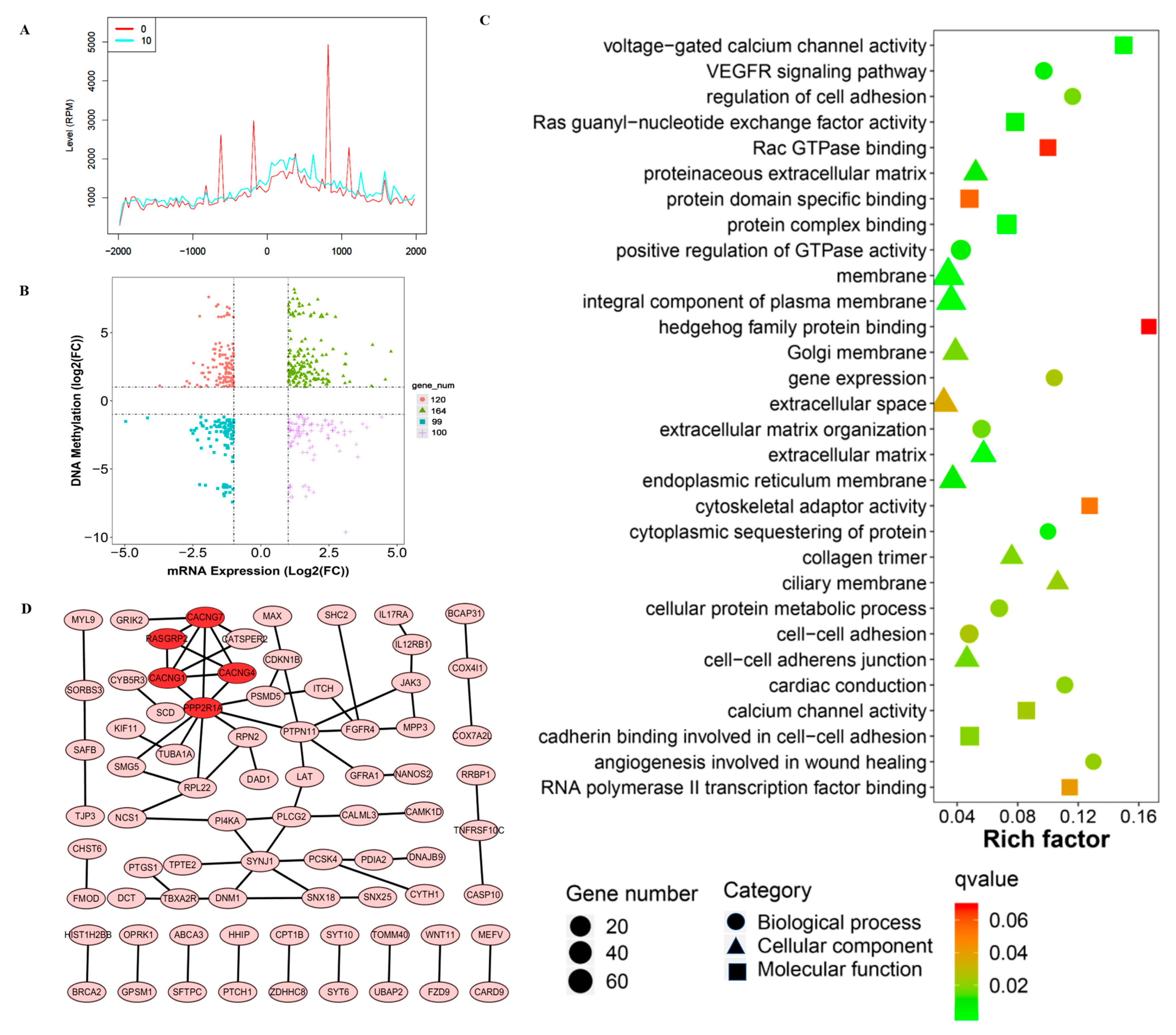
3.3. EOM Changed the DNA Methylation Patterns in hESCs
3.4. Integrated Analysis of Differentially Methylated Genes and Differentially Expressed Genes after EOM Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Carré, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R. Does air pollution play a role in infertility? A systematic review. Environ. Health 2017, 16, 82. [Google Scholar] [CrossRef]
- Dehbi, H.-M.; Blangiardo, M.; Gulliver, J.; Fecht, D.; de Hoogh, K.; Al-Kanaani, Z.; Tillin, T.; Hardy, R.; Chaturvedi, N.; Hansell, A.L. Air pollution and cardiovascular mortality with over 25years follow-up: A combined analysis of two British cohorts. Environ. Int. 2017, 99, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Coker, E.; Ghosh, J.; Jerrett, M.; Gomez-Rubio, V.; Beckerman, B.; Cockburn, M.; Liverani, S.; Su, J.; Li, A.; Kile, M.L.; et al. Modeling spatial effects of PM2.5 on term low birth weight in Los Angeles County. Environ. Res. 2015, 142, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Schembari, A.; de Hoogh, K.; Pedersen, M.; Dadvand, P.; Martinez, D.; Hoek, G.; Petherick, E.S.; Wright, J.; Nieuwenhuijsen, M.J. Ambient Air Pollution and Newborn Size and Adiposity at Birth: Differences by Maternal Ethnicity (the Born in Bradford Study Cohort). Environ. Health Perspect. 2015, 123, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.; Torres-Padilla, M.-E. Chromatin dynamics in the regulation of cell fate allocation during early embryogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 723–735. [Google Scholar] [CrossRef]
- Chen, T.; Jin, H.; Wang, H.; Yao, Y.; Aniagu, S.; Tong, J.; Jiang, Y. Aryl hydrocarbon receptor mediates the cardiac developmental toxicity of EOM from PM(2.5) in P19 embryonic carcinoma cells. Chemosphere 2019, 216, 372–378. [Google Scholar] [CrossRef]
- Ren, F.; Ji, C.; Huang, Y.; Aniagu, S.; Jiang, Y.; Chen, T. AHR-mediated ROS production contributes to the cardiac developmental toxicity of PM2.5 in zebrafish embryos. Sci. Total. Environ. 2020, 719, 135097. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, W. Epigenome in Early Mammalian Development: Inheritance, Reprogramming and Establishment. Trends Cell Biol. 2018, 28, 237–253. [Google Scholar] [CrossRef]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef]
- Greenberg, M.V.C.; Bourc’His, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Ren, F.; Ji, C.; Aniagu, S.; Chen, T. PM2.5-induced extensive DNA methylation changes in the heart of zebrafish embryos and the protective effect of folic acid. Environ. Pollut. 2019, 255 Pt 3, 113331. [Google Scholar] [CrossRef]
- Ren, F.; Huang, Y.; Tao, Y.; Ji, C.; Aniagu, S.; Jiang, Y.; Chen, T. Resveratrol protects against PM2.5-induced heart defects in zebrafish embryos as an antioxidant rather than as an AHR antagonist. Toxicol. Appl. Pharmacol. 2020, 398, 115029. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, H.; Cui, S.; Han, B.; Zhou, L.; Zhang, N.; Su, X.; Niu, Y.; Chen, W.; Chen, R.; et al. Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J. Hazard. Mater. 2019, 369, 180–190. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Liu, L.; Wang, Q.; Zeng, J.; Chen, C. PM2.5 exposure perturbs lung microbiome and its metabolic profile in mice. Sci. Total. Environ. 2020, 721, 137432. [Google Scholar] [CrossRef]
- Fritsche, E.; Haarmann-Stemmann, T.; Kapr, J.; Galanjuk, S.; Hartmann, J.; Mertens, P.R.; Kämpfer, A.A.M.; Schins, R.P.F.; Tigges, J.; Koch, K. Stem Cells for Next Level Toxicity Testing in the 21st Century. Small 2021, 17, e2006252. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jiang, B.; Teng, Z.; Liu, T.; Wang, J.; Aniagu, S.; Zhang, G.; Chen, T.; Jiang, Y. Cx43 overexpression is involved in the hyper-proliferation effect of trichloroethylene on human embryonic stem cells. Toxicology 2022, 465, 153065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yao, Y.; Chen, Y.; Yue, C.; Chen, J.; Tong, J.; Jiang, Y.; Chen, T. Crosstalk between AhR and wnt/beta-catenin signal pathways in the cardiac developmental toxicity of PM2.5 in zebrafish embryos. Toxicology 2016, 355–356, 31–38. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, S. A Global Perspective of Fine Particulate Matter Pollution and Its Health Effects. Rev. Environ. Contam. Toxicol. 2018, 244, 5–51. [Google Scholar] [CrossRef]
- Jin, L.; Ni, J.; Tao, Y.; Weng, X.; Zhu, Y.; Yan, J.; Hu, B. N-acetylcysteine attenuates PM2.5-induced apoptosis by ROS-mediated Nrf2 pathway in human embryonic stem cells. Sci. Total Environ. 2019, 666, 713–720. [Google Scholar] [CrossRef]
- Sarsour, E.H.; Kumar, M.G.; Chaudhuri, L.; Kalen, A.L.; Goswami, P.C.; Liu, G.-Y.; Sun, Y.-Z.; Zhou, N.; Du, X.-M.; Yang, J.; et al. Redox Control of the Cell Cycle in Health and Disease. Antioxidants Redox Signal. 2009, 11, 2985–3011. [Google Scholar] [CrossRef]
- Pyle, A.D.; Donovan, P.J.; Lock, L.F. Chipping away at ‘stemness’. Genome Biol. 2004, 5, 235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Talbot, P.; Lin, S. The effect of cigarette smoke on fertilization and pre-implantation development: Assessment using animal models, clinical data, and stem cells. Biol. Res. 2011, 44, 189–194. [Google Scholar] [CrossRef]
- Eischen, C.M.; Lozano, G. The Mdm Network and Its Regulation of p53 Activities: A Rheostat of Cancer Risk. Hum. Mutat. 2014, 35, 728–737. [Google Scholar] [CrossRef]
- Janssen, B.G.; Godderis, L.; Pieters, N.; Poels, K.; Kiciński, M.; Cuypers, A.; Fierens, F.; Penders, J.; Plusquin, M.; Gyselaers, W.; et al. Placental DNA hypomethylation in association with particulate air pollution in early life. Part. Fibre Toxicol. 2013, 10, 22. [Google Scholar] [CrossRef]
- Mostafavi, N.; Vermeulen, R.; Ghantous, A.; Hoek, G.; Probst-Hensch, N.; Herceg, Z.; Tarallo, S.; Naccarati, A.; Kleinjans, J.C.; Imboden, M.; et al. Acute changes in DNA methylation in relation to 24 h personal air pollution exposure measurements: A panel study in four European countries. Environ. Int. 2018, 120, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ren, X.; Sun, Z.; Duan, J. The critical role of epigenetic mechanism in PM2.5-induced cardiovascular diseases. Genes Environ. 2021, 43, 47. [Google Scholar] [CrossRef]
- Sempou, E.; Kostiuk, V.; Zhu, J.; Guerra, M.C.; Tyan, L.; Hwang, W.; Camacho-Aguilar, E.; Caplan, M.J.; Zenisek, D.; Warmflash, A.; et al. Membrane potential drives the exit from pluripotency and cell fate commitment via calcium and mTOR. Nat. Commun. 2022, 13, 6681. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.R.; Nelson, D.A.; DeSantis, K.A.; Morrissey, J.M.; Larsen, M. Endothelial cell regulation of salivary gland epithelial patterning. Development 2017, 144, 211–220. [Google Scholar] [CrossRef]
- Loganathan, R.; Rongish, B.J.; Smith, C.M.; Filla, M.B.; Czirok, A.; Bénazéraf, B.; Little, C.D. Extracellular matrix motion and early morphogenesis. Development 2016, 143, 2056–2065. [Google Scholar] [CrossRef]


| Genes | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| GAPDH | CAGGAGGCATTGCTGATGAT | GAAGGCTGGGGCTCATTT |
| OCT4 | GGGAGATTGATAACTGGTGTGTT | GTGTATATCCCAGGGTGATCCTC |
| Nanog | TTTGTGGGCCTGAAGAAAACT | AGGGCTGTCCTGAATAAGCAG |
| SOX2 | TACAGCATGTCCTACTCGCAG | GAGGAAGAGGTAACCACAGGG |
| KEGG Term | Count | % | p-Value | Genes |
|---|---|---|---|---|
| hsa05200:Pathways in cancer | 13 | 6.565657 | 0.010758 | CDKN1B, MAX, HHIP, PTCH1, FZD9, CALML3, BRCA2, RASGRP2, WNT11, PLCG2, IL12RB1, FGFR4, JAK3 |
| hsa04260:Cardiac muscle contraction | 5 | 2.525253 | 0.013998 | CACNG7, COX7A2L, COX4I1, CACNG1, CACNG4 |
| hsa04921:Oxytocin signaling pathway | 6 | 3.030303 | 0.024599 | CACNG7, CAMK1D, CALML3, CACNG1, MYL9, CACNG4 |
| hsa04625:C-type lectin receptor signaling pathway | 5 | 2.525253 | 0.025232 | CLEC4M, CARD9, PLCG2, PTPN11, CALML3 |
| hsa05217:Basal cell carcinoma | 4 | 2.020202 | 0.029833 | WNT11, PTCH1, HHIP, FZD9 |
| hsa04014:Ras signaling pathway | 7 | 3.535354 | 0.04077 | SHC2, PLCG2, PTPN11, CALML3, FGFR4, RASGRP2, LAT |
| hsa00562:Inositol phosphate metabolism | 4 | 2.020202 | 0.043319 | MIOX, SYNJ1, PI4KA, PLCG2 |
| hsa05214:Glioma | 4 | 2.020202 | 0.046326 | SHC2, CAMK1D, PLCG2, CALML3 |
| hsa04072:Phospholipase D signaling pathway | 5 | 2.525253 | 0.074274 | SHC2, PLCG2, PTPN11, CYTH1, DNM1 |
| hsa04261:Adrenergic signaling in cardiomyocytes | 5 | 2.525253 | 0.07719 | CACNG7, PPP2R1A, CALML3, CACNG1, CACNG4 |
| hsa04934:Cushing syndrome | 5 | 2.525253 | 0.084729 | CDKN1B, WNT11, FZD9, PDE8A, CYP17A1 |
| hsa03015:mRNA surveillance pathway | 4 | 2.020202 | 0.085772 | PPP2R1A, PABPC3, TARDBP, SMG5 |
| hsa04070:Phosphatidylinositol signaling system | 4 | 2.020202 | 0.085772 | SYNJ1, PI4KA, PLCG2, CALML3 |
| hsa04916:Melanogenesis | 4 | 2.020202 | 0.094103 | WNT11, DCT, FZD9, CALML3 |
| hsa04010:MAPK signaling pathway | 7 | 3.535354 | 0.09527 | CACNG7, MAX, SRF, CACNG1, FGFR4, RASGRP2, CACNG4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, T.; Wang, J.; Chen, T.; Jiang, Y. Genome-Wide Profiling of Transcriptome and DNA Methylome in Human Embryonic Stem Cells Exposed to Extractable Organic Matter from PM2.5. Toxics 2023, 11, 840. https://doi.org/10.3390/toxics11100840
Wang J, Liu T, Wang J, Chen T, Jiang Y. Genome-Wide Profiling of Transcriptome and DNA Methylome in Human Embryonic Stem Cells Exposed to Extractable Organic Matter from PM2.5. Toxics. 2023; 11(10):840. https://doi.org/10.3390/toxics11100840
Chicago/Turabian StyleWang, Jianming, Tiantian Liu, Jin Wang, Tao Chen, and Yan Jiang. 2023. "Genome-Wide Profiling of Transcriptome and DNA Methylome in Human Embryonic Stem Cells Exposed to Extractable Organic Matter from PM2.5" Toxics 11, no. 10: 840. https://doi.org/10.3390/toxics11100840
APA StyleWang, J., Liu, T., Wang, J., Chen, T., & Jiang, Y. (2023). Genome-Wide Profiling of Transcriptome and DNA Methylome in Human Embryonic Stem Cells Exposed to Extractable Organic Matter from PM2.5. Toxics, 11(10), 840. https://doi.org/10.3390/toxics11100840







