Evaluation of the Toxic Effect of Bauhinia purpurea Mediated Synthesized Silver Nanoparticles against In-vitro and In-vivo Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Synthesis of Silver Nanoparticle using Bauhinia purpurea Gum
2.3. Bio-Physical Characterization of Silver Nanoparticles
2.4. Collection, Maintenance, and Pre-Treatment of Earthworms and Zebrafishes
2.5. Breeding, Spawning and Maintenance of Zebrafishes Embryos
2.6. Exposure to Silver Nanoparticles
2.7. Observation of Phenotypical Changes
2.8. Histopathological Studies and ICP–OES
3. Results
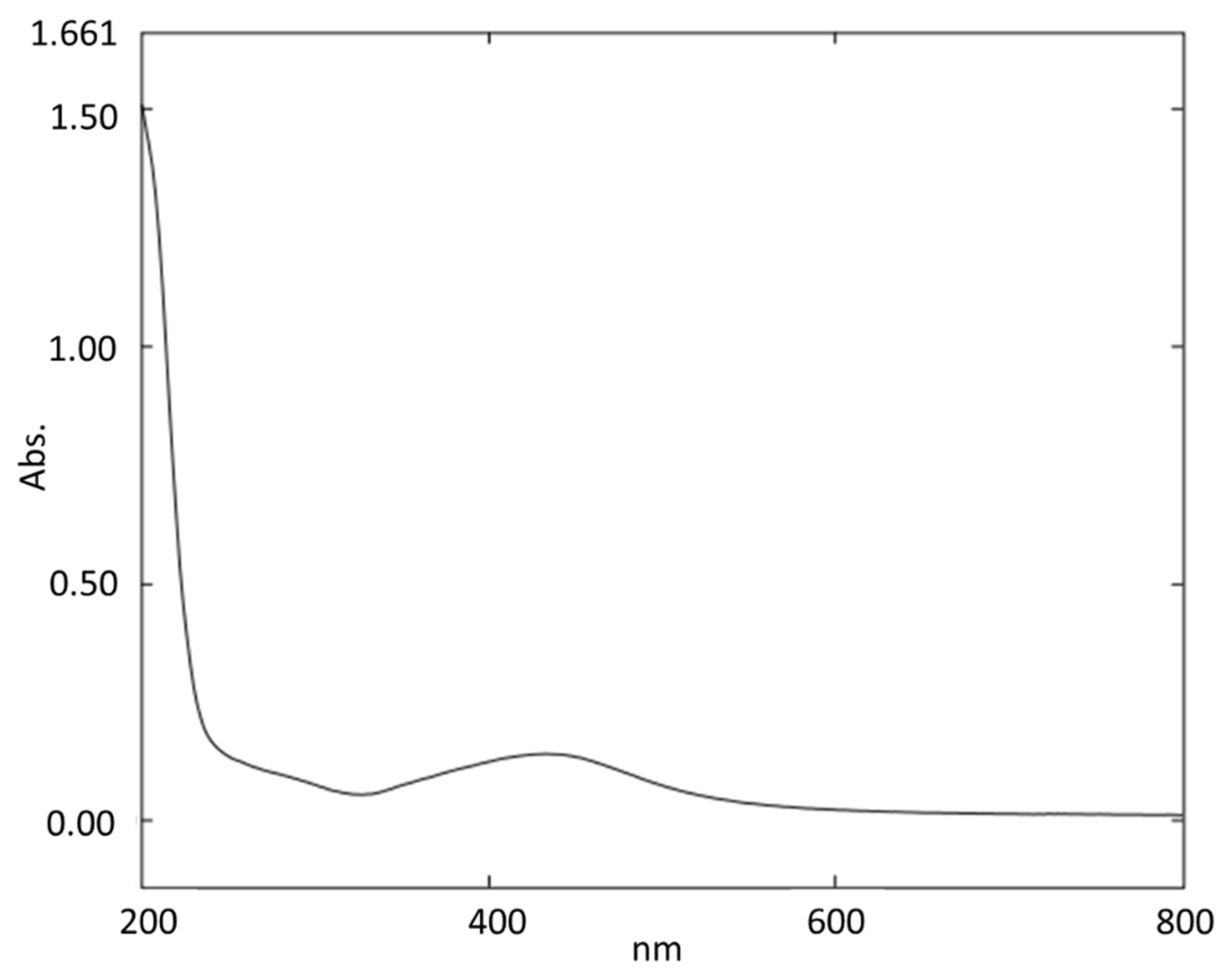
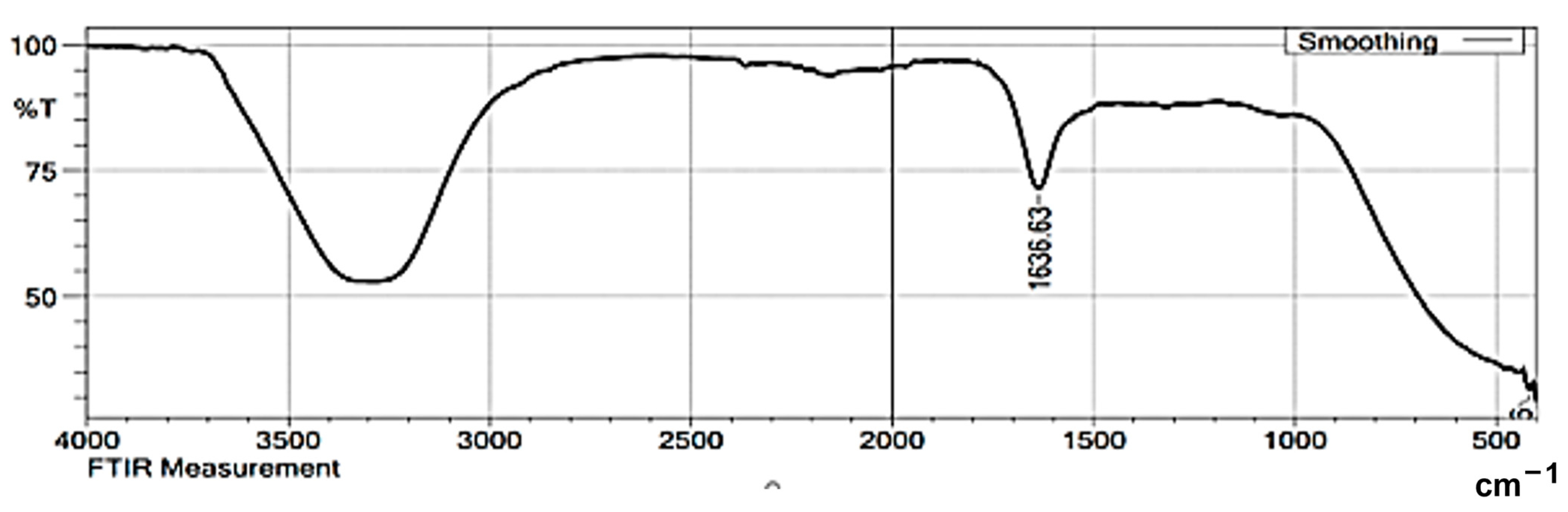
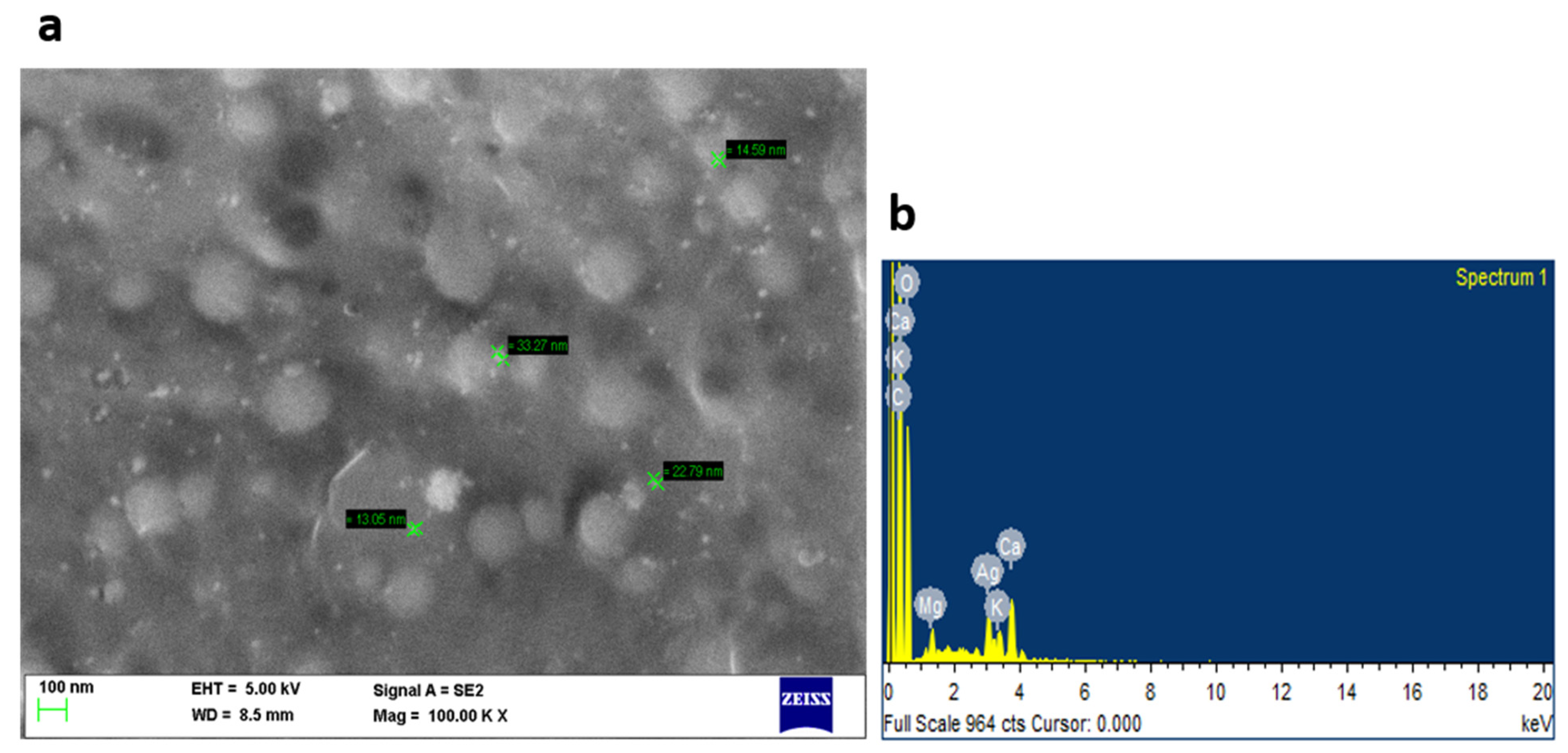
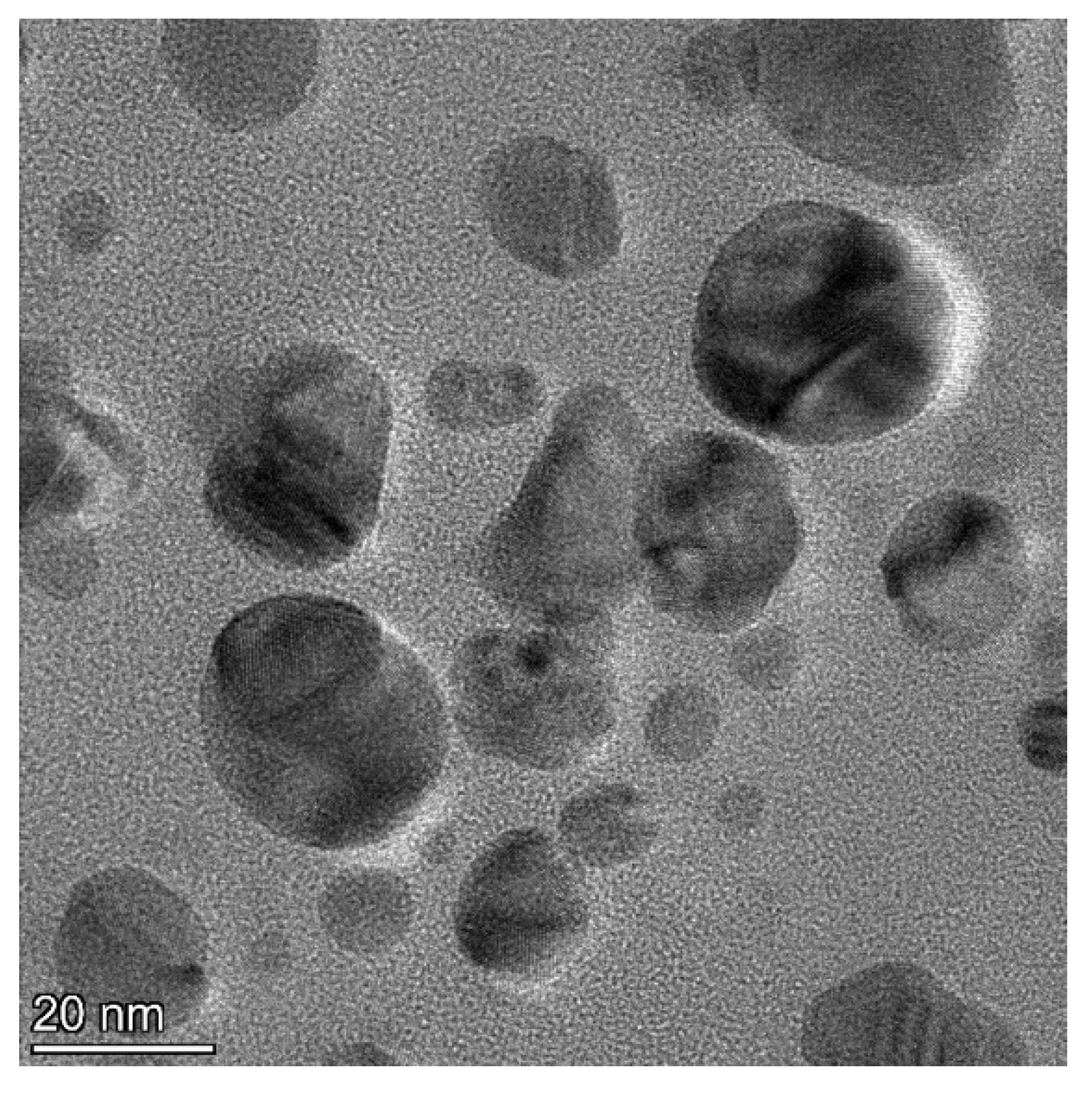
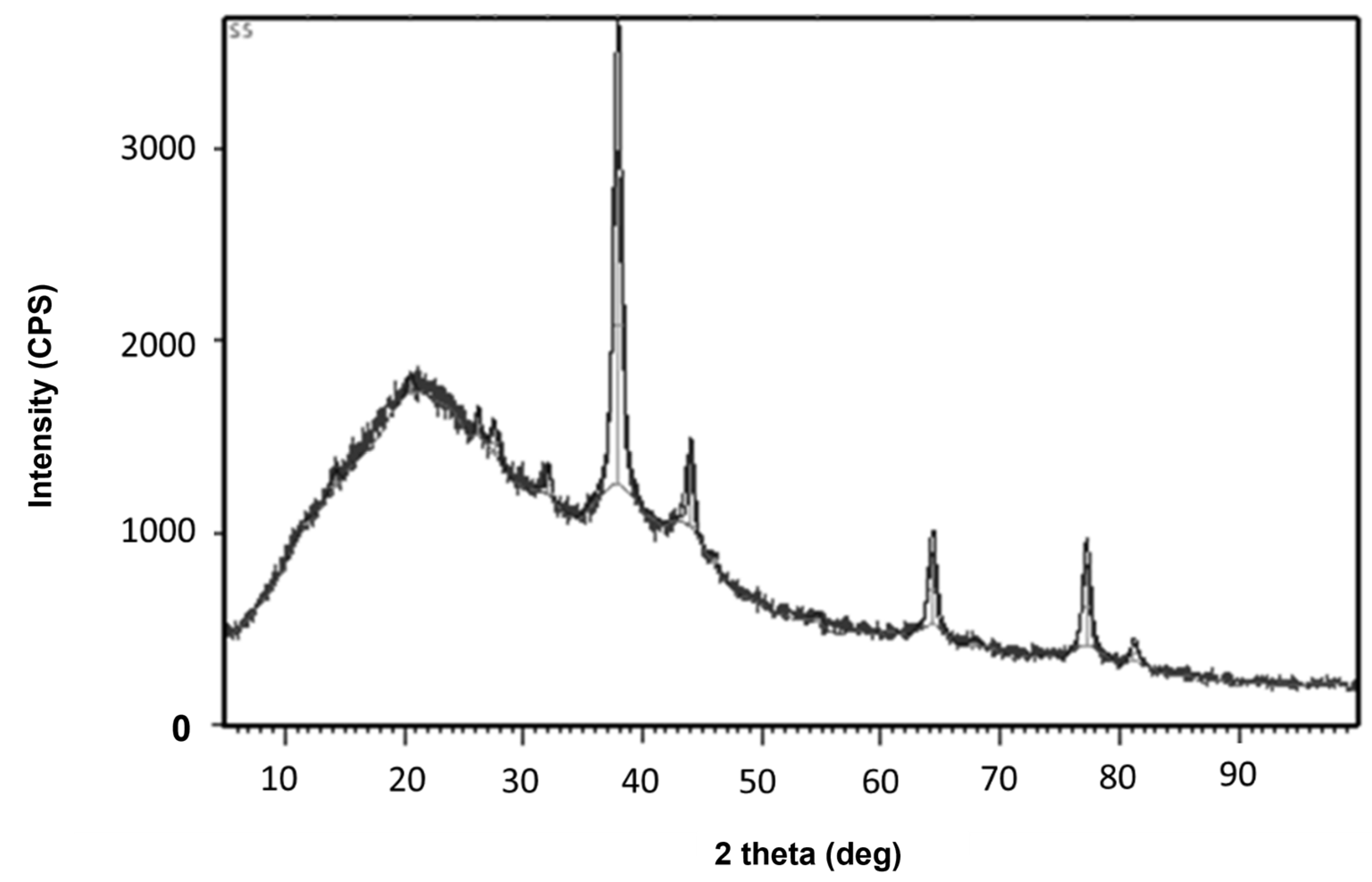
3.1. Characterization of Synthesized Silver Nanoparticles
3.2. Toxicity Studies on Earthworm and Zebrafish
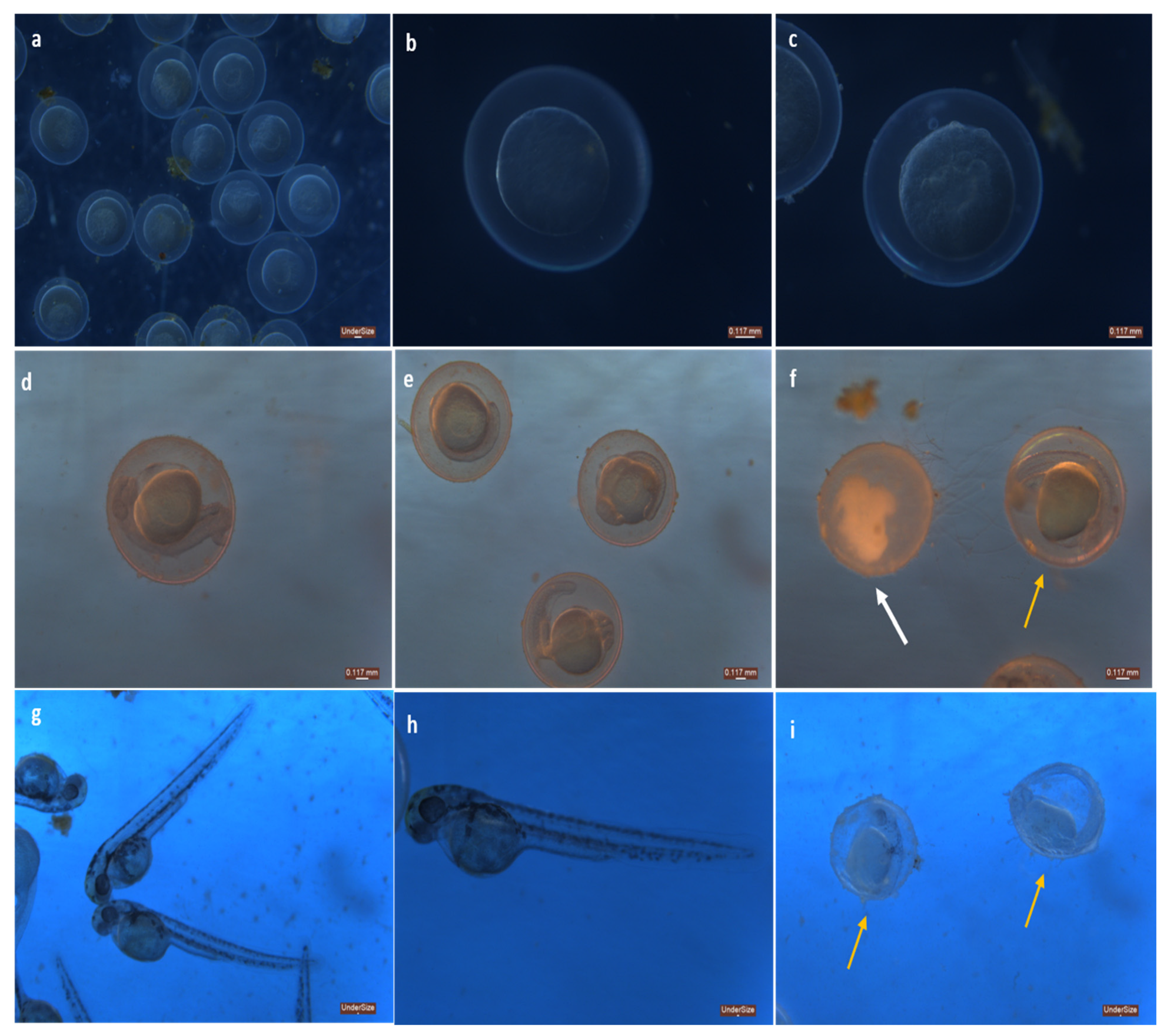
3.2.1. Phenotypical Changes
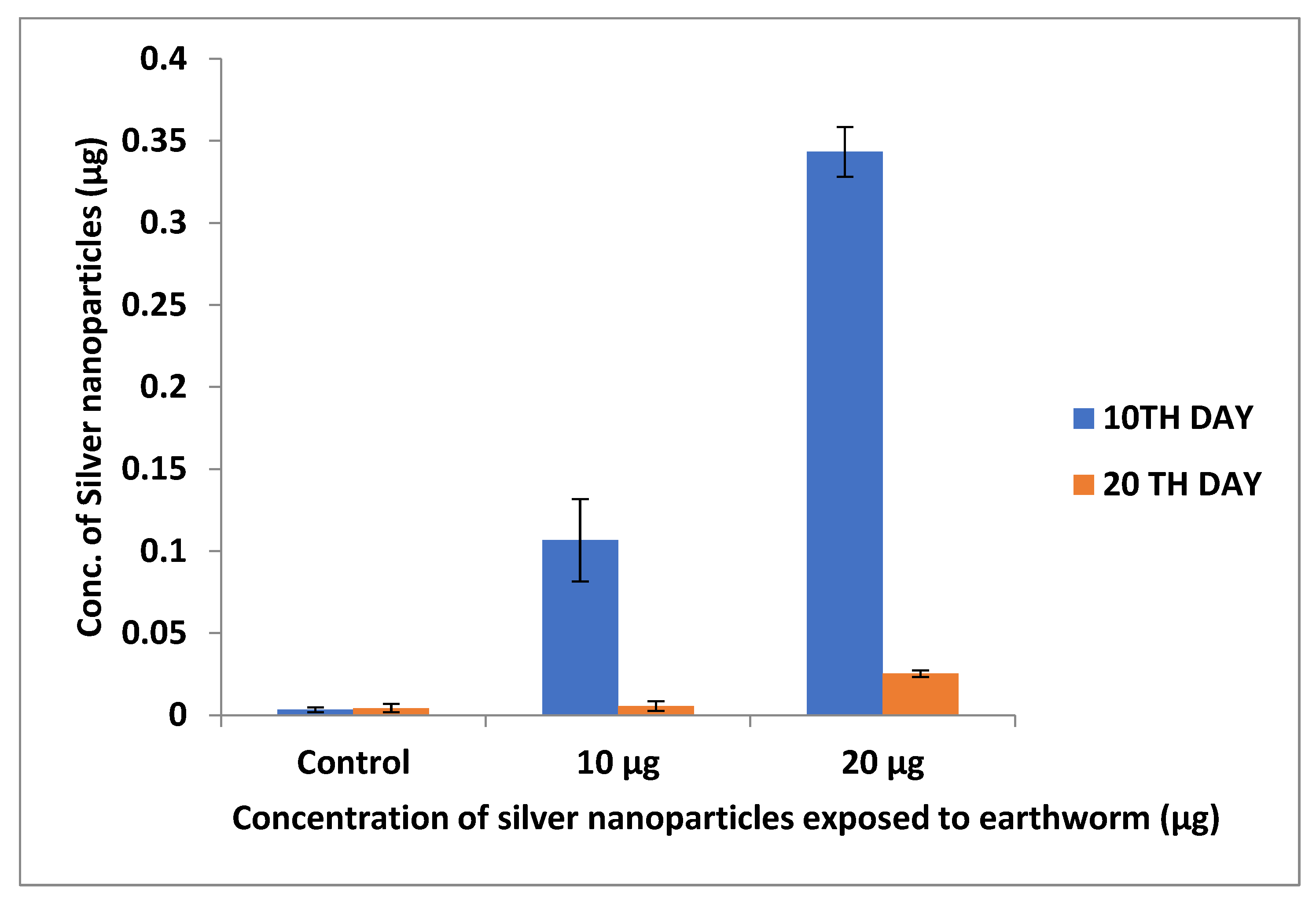
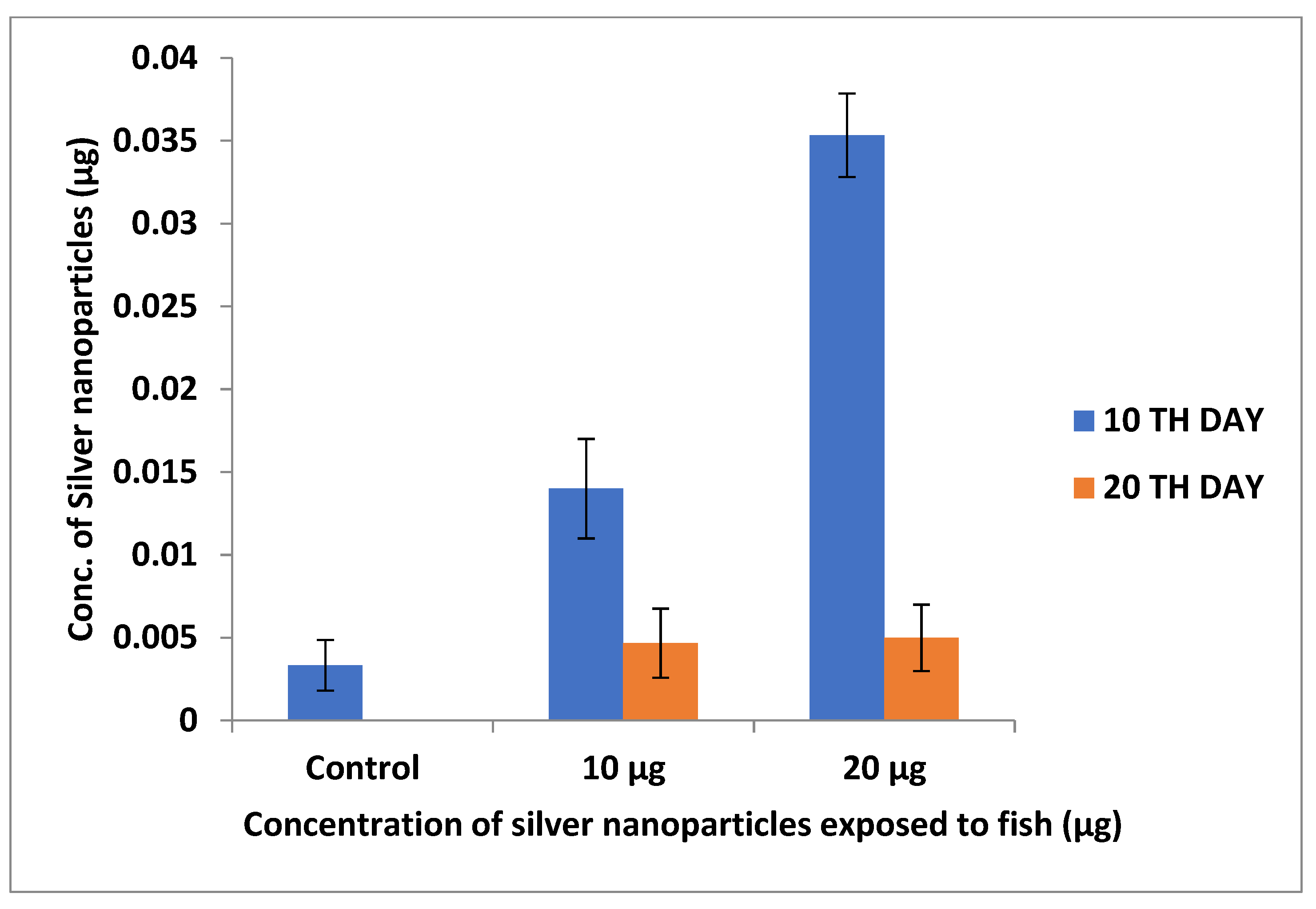
3.2.2. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP–OES)
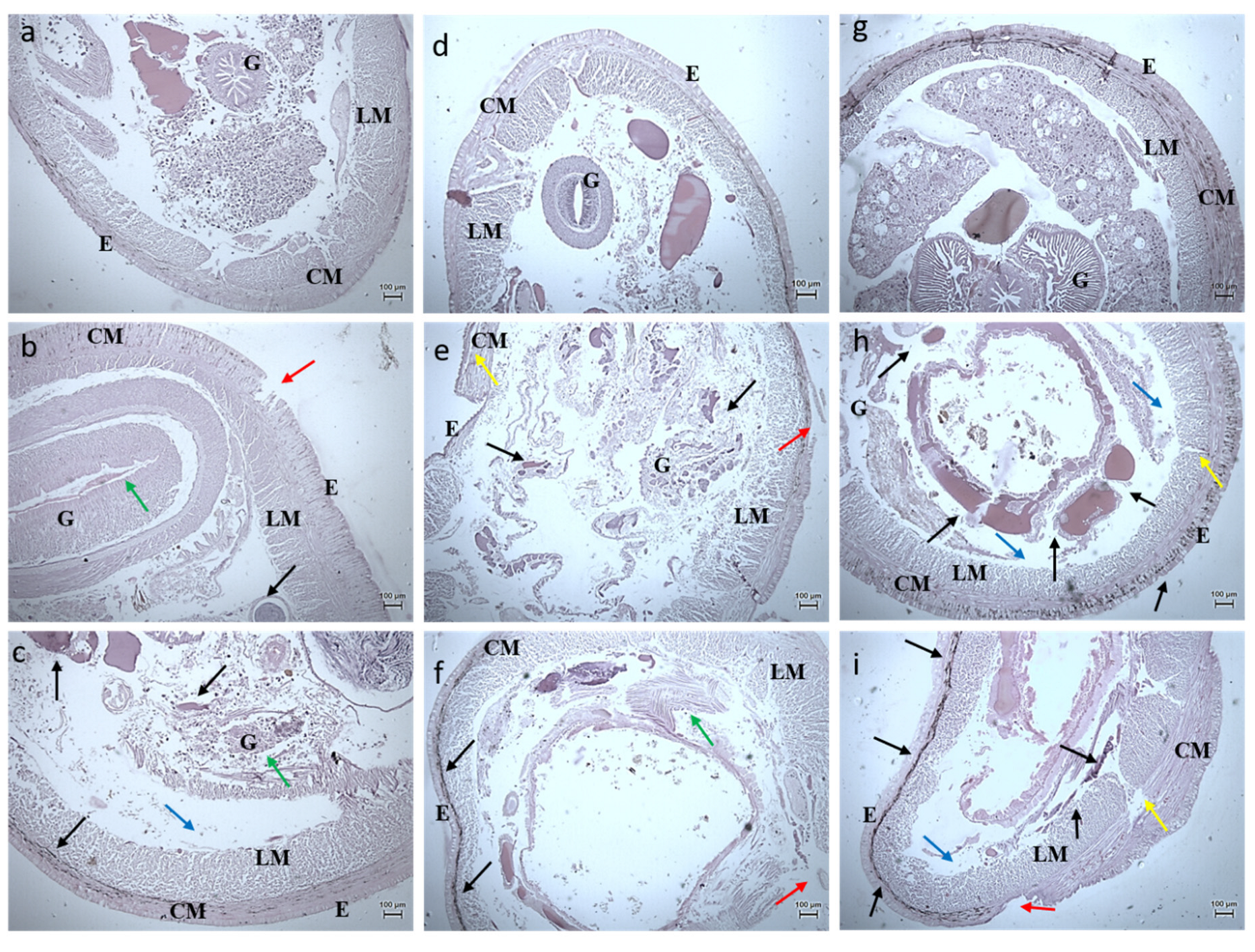
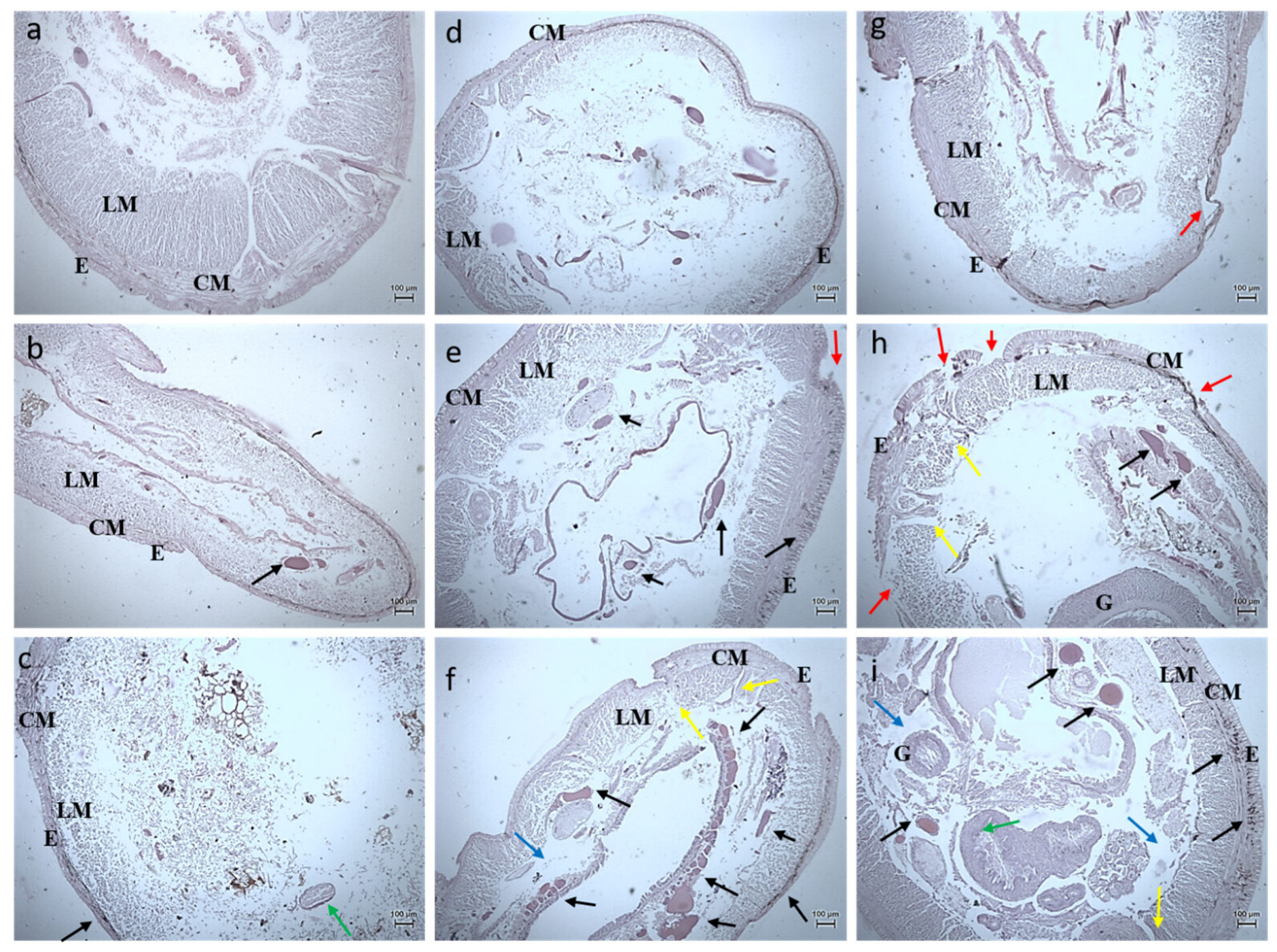
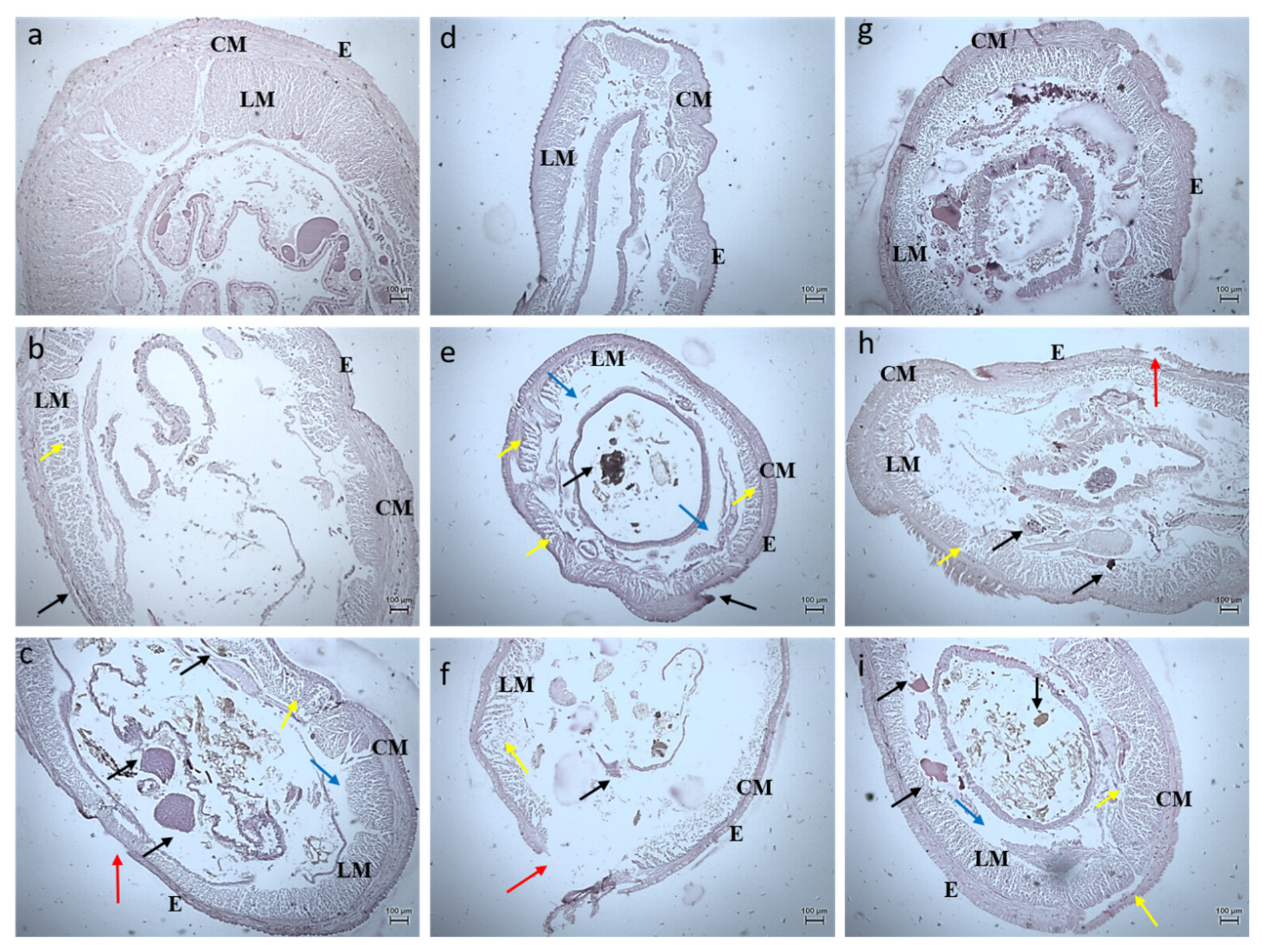
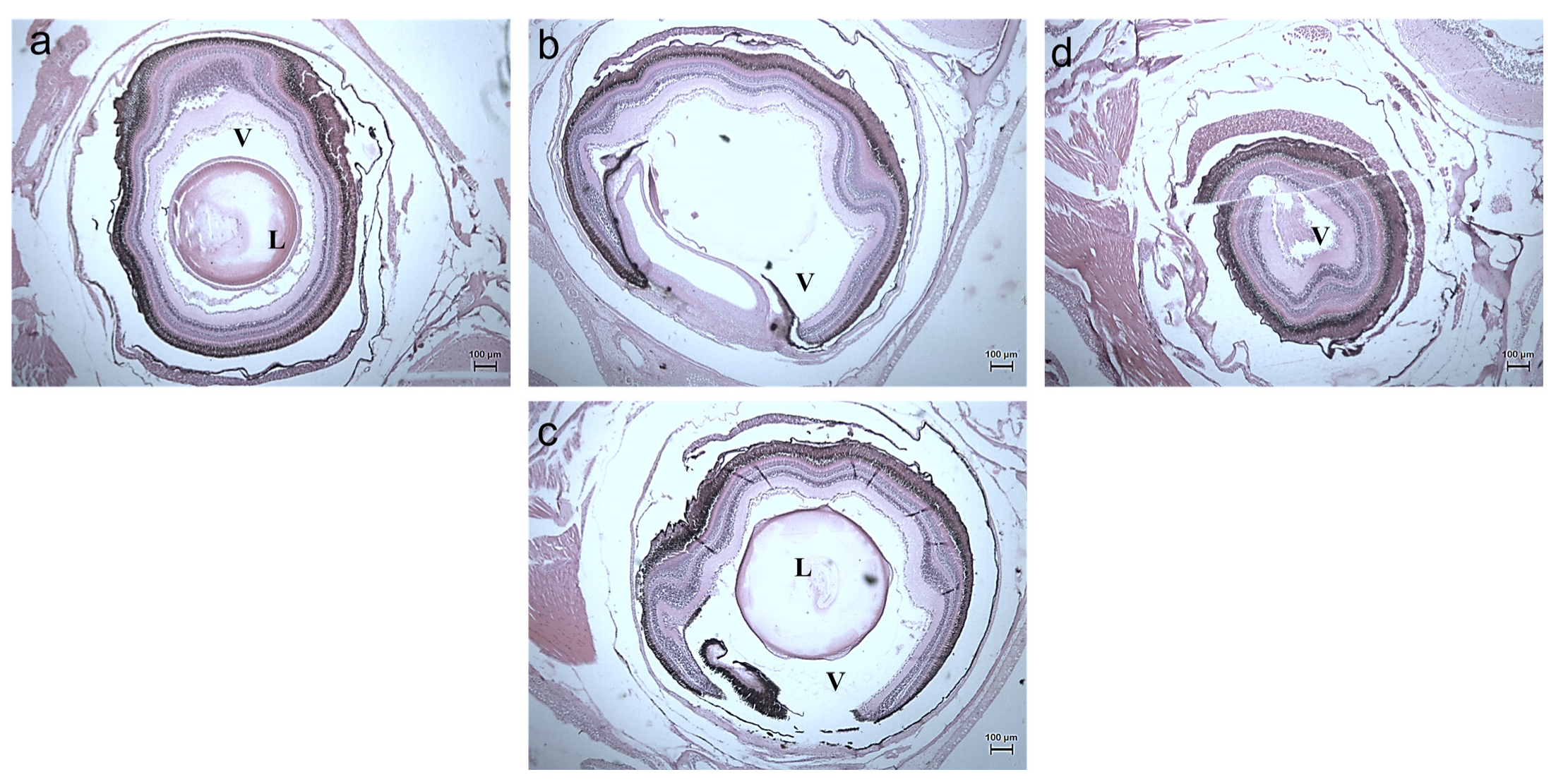
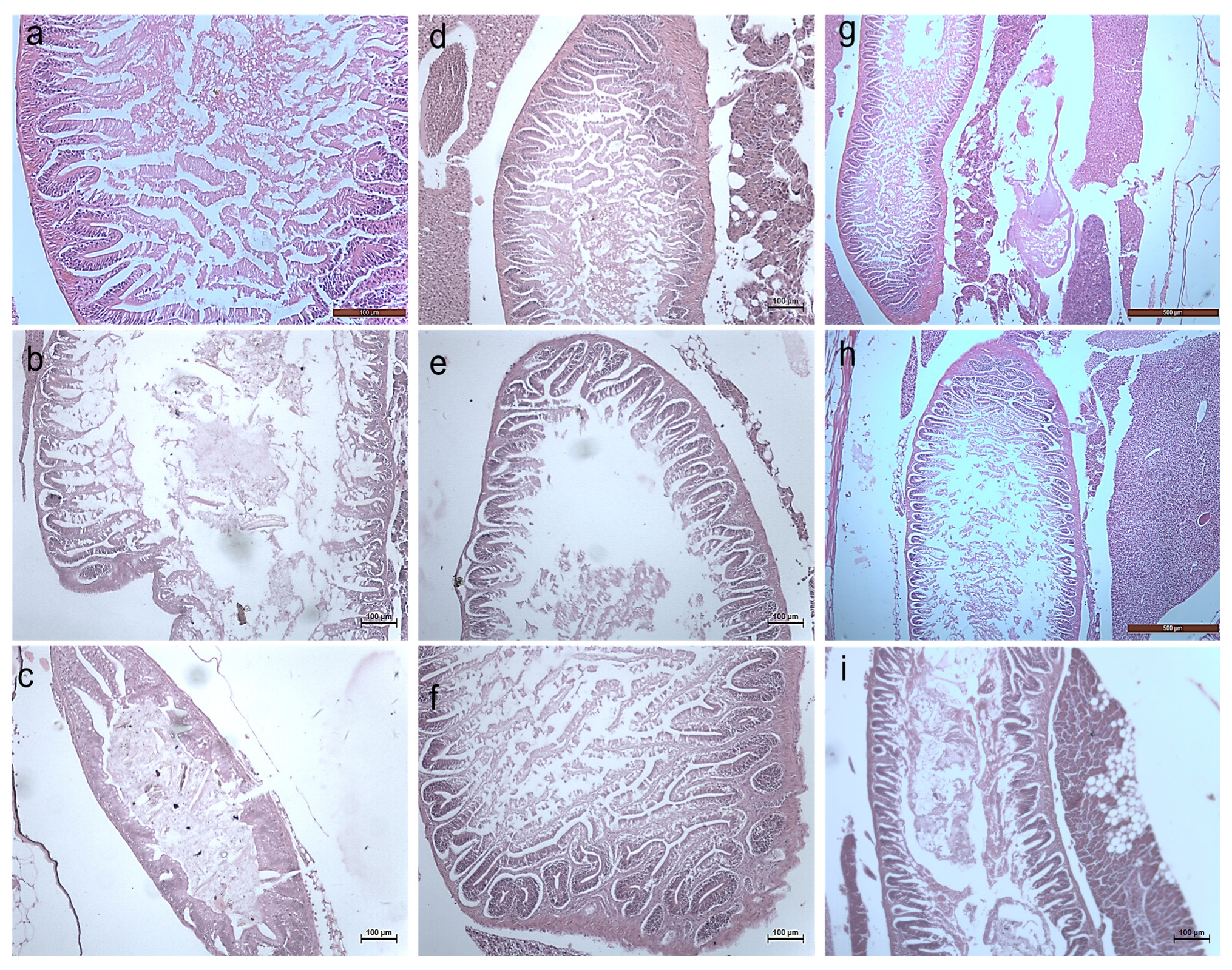
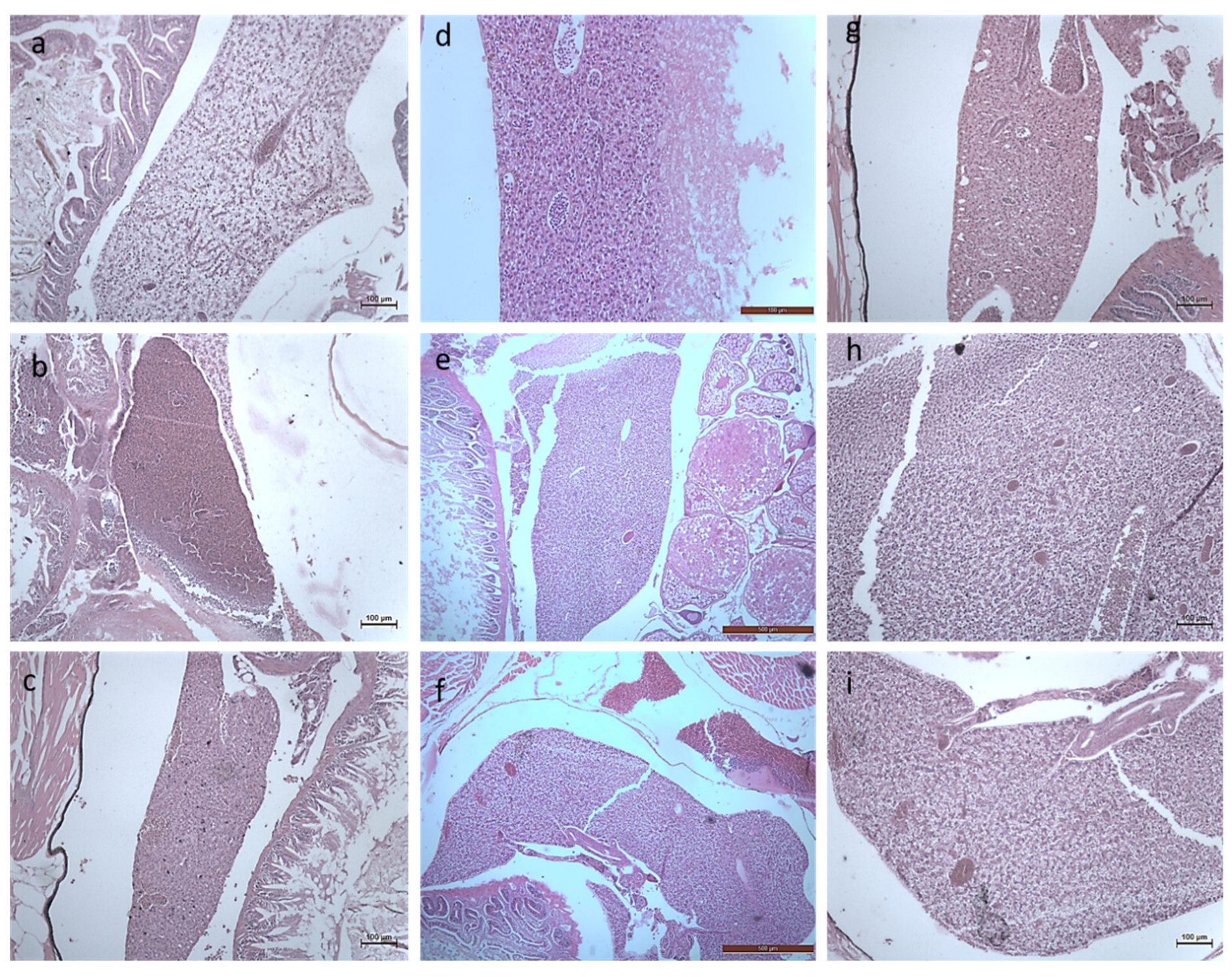
3.2.3. Histology Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, V.; Zareei, A.; Elkashif, A.; Maruthamuthu, M.K.; Chittiboyina, S.; Delisi, D.; Li, Z.; Cai, L.; Pol, V.G.; Seleem, M.N.; et al. Hierarchical micro/mesoporous copper structure with enhanced antimicrobial property via laser surface texturing. Adv. Mater. Interfaces 2020, 7, 1901890. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Al-Dahmash, N.D.; Ranjitsingh, A.J.A. Synthesis of silver nanoparticles using gum Arabic: Evaluation of its inhibitory action on Streptococcus mutans causing dental caries and endocarditis. J. Infect. Public Health 2021, 14, 324–330. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, G.; Chandrasekharan, S.; Koh, T.W.; Bhatnagar, S. Synthesis, characterization, antibacterial and wound healing efficacy of silver nanoparticles from Azadirachta indica. Front. Microbiol. 2021, 12, 611560. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Shobana, N.; Suresh Kumar, S.; Narendrakumar, G. Production, Optimization and Characterisation of Chitosanase of Bacillus sp. and its Applications in Nanotechnology. J. Clust. Sci. 2019, 30, 607–620. [Google Scholar] [CrossRef]
- Samrot, A.V.; Shobana, N.; Jenna, R. Antibacterial and antioxidant activity of different staged ripened fruit of Capsicum annuum and its green synthesized silver nanoparticles. BioNanoScience 2018, 8, 632–646. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Burman, U.S.P.; Yamini, P.; Rabel, A.M. A Study on Toxicity of Chemically Synthesised Silver Nanoparticle on Eudrilus eugeniae. Toxicol. Environ. Health Sci. 2018, 10, 162–167. [Google Scholar] [CrossRef]
- Samrot, A.V.; Angalene, J.; Roshini, S.M.; Raji, P.; Stefi, S.M.; Preethi, R.; Selvarani, A.J.; Madankumar, A. Bioactivity and heavy metal removal using plant gum mediated green synthesized silver nanoparticles. J. Clust. Sci. 2019, 30, 1599–1610. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R.D. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Durán, M.; Yadav, A.; Gade, A.; Rai, M. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl. Microbiol. Biotechnol. 2011, 90, 1609–1624. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar]
- Messaoudi, O.; Bendahou, M. Biological synthesis of nanoparticles using endophytic microorganisms: Current development. In Nanotechnology and the Environment; IntechOpen: London, UK, 2020. [Google Scholar]
- Parida, S.; Mahalik, G. Green leafy vegetables used by seven tribes of Odisha, India. Plant Arch. 2020, 20, 1866–1871. [Google Scholar]
- PJ, J.C.; Saigeetha, S.; Samrot, A.V.; Ponniah, P.; Chakravarthi, S. Overview on toxicity of nanoparticles, it’s mechanism, models used in toxicity studies and disposal methods–A review. Biocatal. Agric. Biotechnol. 2021, 36, 102117. [Google Scholar]
- Samrot, A.V.; Jie, L.S.; Abirami, S.; Renitta, R.E.; Dhiva, S.; Prakash, P.; Saigeetha, S.; Shobana, N. Bioactivity and Plant Growth Stimulation Studies using Mangifera indica L Gum. J. Pure Appl. Microbiol. 2021, 15, 2073–2085. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Devanesan, S.; AlSalhi, M.S.; Rajasekar, A.; Maruthamuthu, M.K.; Babujanarthanam, R. Phytosynthesis of silver nanoparticles from Jatropha integerrima Jacq. flower extract and their possible applications as antibacterial and antioxidant agent. Saudi J. Biol. Sci. 2022, 29, 680–688. [Google Scholar] [CrossRef]
- Samrot, A.V.; SaiPriya, C.; Angalene, L.A.J.; Roshini, S.M.; Cypriyana, J.P.J.; Saigeetha, S.; Kumar, S. Evaluation of nanotoxicity of Araucaria heterophylla gum derived green synthesized silver nanoparticles on Eudrilus eugeniae and Danio rerio. J. Clust. Sci. 2019, 30, 1017–1024. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G.; et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. J. Vis. Exp. 2012, 69, e4196. [Google Scholar] [CrossRef]
- Bhoopathy, S.; Inbakandan, D.; Thirugnanasambandam, R.; Kumar, C.; Sampath, P.; Bethunaickan, R.; Raguraman, V.; Vijayakumar, G.K. A comparative study on chitosan nanoparticle synthesis methodologies for application in aquaculture through toxicity studies. IET Nanobiotechnol. 2021, 15, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Bhavya, K.S.; Sahithya, C.S.; Sowmya, N. Evaluation of Toxicity of Chemically Synthesised Gold Nanoparticles Against Eudrilus eugeniae. J. Cluster Sci. 2018, 29, 1217–1225. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10(1), 1–10. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Mehta, B.K.; Chhajlani, M.; Shrivastava, B.D. 2017, April. Green synthesis of silver nanoparticles and their characterization by XRD. J. Phys. Conf. Ser. 2017, 836, 012050. [Google Scholar] [CrossRef]
- Ezemonye, L.I.N.; Ogeleka, D.F.; Okeimen, F.E. Toxicity of Neatex (industrial detergent) and Norust CR 486 (corrosion inhibitors) to earthworms (Aporrectodea longa) in naturally spiked soil. Afr. J. Biotechnol. 2006, 5, 1113–1117. [Google Scholar]
- Samrot, A.V.; Justin, C.; Padmanaban, S.; Burman, U. A study on the effect of chemically synthesized magnetite nanoparticles on earthworm: Eudrilus eugeniae. Appl. Nanosci. 2017, 7, 17–23. [Google Scholar] [CrossRef]
- Schlich, K.; Klawonn, T.; Terytze, K.; Hund-Rinke, K. Effects of silver nanoparticles and silver nitrate in the earthworm reproduction test. Environ. Toxicol. Chem. 2013, 32, 181–188. [Google Scholar] [CrossRef]
- van der Ploeg, M.J.; Handy, R.D.; Waalewijn-Kool, P.L.; van den Berg, J.H.; Herrera Rivera, Z.E.; Bovenschen, J.; Molleman, B.; Baveco, J.M.; Tromp, P.; Peters, R.J.; et al. Effects of silver nanoparticles (NM-300K) on Lumbricus rubellus earthworms and particle characterization in relevant test matrices including soil. Environ. Toxicol. Chem. 2014, 33, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Bourdineaud, J.P.; Štambuk, A.; Šrut, M.; Radić Brkanac, S.; Ivanković, D.; Lisjak, D.; Sauerborn Klobučar, R.; Dragun, Z.; Bačić, N.; Klobučar, G.I. Gold and silver nanoparticles effects to the earthworm Eisenia fetida–the importance of tissue over soil concentrations. Drug Chem. Toxicol. 2021, 44, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Tilton, S.C.; Zaikova, T.; Richman, E.; Waters, K.M.; Hutchison, J.E.; Tanguay, R.L. Surface functionalities of gold nanoparticles impact embryonic gene expression responses. Nanotoxicology 2013, 7, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef]
- Di Gioacchino, M.; Petrarca, C.; Lazzarin, F.; Di Giampaolo, L.; Sabbioni, E.; Boscolo, P.; Mariani-Costantini, R.; Bernardini, G. Immunotoxicity of nanoparticles. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. S1), 65S–71S. [Google Scholar]
- Geffroy, B.; Ladhar, C.; Cambier, S.; Treguer-Delapierre, M.; Brèthes, D.; Bourdineaud, J.P. Impact of dietary gold nanoparticles in zebrafish at very low contamination pressure: The role of size, concentration and exposure time. Nanotoxicology 2012, 6, 144–160. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, L.; Su, M.; Zhao, X.; Hu, R.; Yu, X.; Hong, J.; Liu, D.; Xu, B.; Zhu, Y.; et al. Mechanism of TiO2 nanoparticle-induced neurotoxicity in zebrafish (Danio rerio). Environ. Toxicol. 2016, 31, 163–175. [Google Scholar] [CrossRef]
- Xia, G.; Liu, T.; Wang, Z.; Hou, Y.; Dong, L.; Zhu, J.; Qi, J. The effect of silver nanoparticles on zebrafish embryonic development and toxicology. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1116–1121. [Google Scholar] [CrossRef]
- Kim, K.T.; Zaikova, T.; Hutchison, J.E.; Tanguay, R.L. Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol. Sci. 2013, 133, 275–288. [Google Scholar] [CrossRef]
- Rizzo, L.Y.; Golombek, S.K.; Mertens, M.E.; Pan, Y.; Laaf, D.; Broda, J.; Jayapaul, J.; Möckel, D.; Subr, V.; Hennink, W.E.; et al. In vivo nanotoxicity testing using the zebrafish embryo assay. J. Mater. Chem. B 2013, 1, 3918–3925. [Google Scholar] [CrossRef]
- Samrot, A.V.; Shobana, N.; Sathiyasree, M.; Thirugnanasambandam, R.; Visvanathan, S.; Mohanty, B.K.; Sabesan, G.S.; Dhiva, S. Toxicity evaluation of SPIONs on Danio rerio embryonic development. Mater. Today Proc. 2022, 59, 1555–1560. [Google Scholar] [CrossRef]
- Sibiya, A.; Gopi, N.; Jeyavani, J.; Mahboob, S.; Al-Ghanim, K.A.; Sultana, S.; Mustafa, A.; Govindarajan, M.; Vaseeharan, B. Comparative toxicity of silver nanoparticles and silver nitrate in freshwater fish Oreochromis mossambicus: A multi-biomarker approach. Comp. Biochem. Physiol. Part C Toxicol. 2022, 259, 109391. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Lv, Y.; Kou, G.; Liu, Y.; Liu, Y.; Chen, Y.; Wu, X.; Yang, F.; Luo, J.; Yang, X. Silver nanoparticles induce developmental toxicity via oxidative stress and mitochondrial dysfunction in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 243, 113993. [Google Scholar] [CrossRef] [PubMed]




| Parameter Analyzed | Control | 10 µg | 20 µg | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Color change | Brownish black | Brownish black | Brownish black | Brownish pink | Brownish black | Brownish pink |
| Behavioral change | Nil | Nil | Nil | Nil | Nil | Nil |
| Conc. | Before | 10th day | 20th day | 30th day |
|---|---|---|---|---|
| Control | 10 | 22 | 27 | 51 |
| 10 µg | 10 | 17 | 22 | 14 |
| 20 µg | 10 | 17 | 13 | 9 |
| Parameter Analyzed | Control | 10 µg | 20 µg | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Color change | Nil | Nil | Nil | Yellowish tint in the gills and fins | Nil | Yellowish tint in the gills and fins |
| Behavioral change | Active | Active | Active | Weak and slow | Active | Weak and slow |
| Growth | Normal | Normal | Normal | Inhibition in growth | Normal | Inhibition in growth |
| Conc. | Before | 10th Day | 20th Day | 30th Day |
|---|---|---|---|---|
| Control | 10 | 10 | 10 | 10 |
| 10 µg | 10 | 10 | 9 | 9 |
| 20 µg | 10 | 9 | 8 | 8 |
| Control | 10 µg | 20 µg | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0th h | 24 hpf | 48 hpf | 0th h | 24 hpf | 48 hpf | 0th h | 24 hpf | 48 hpf | |
| Hatching | NA | NA | +++ | NA | NA | ++ | NA | NA | + |
| Coagulated embryos | - | + | - | - | + | ++ | - | + | ++ |
| Somite formation | + | + | + | + | + | + | + | + | + |
| Heartbeat | NA | NA | ++ | NA | NA | ++ | NA | NA | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shobana, N.; Prakash, P.; Samrot, A.V.; Saigeetha, S.; Sathiyasree, M.; Thirugnanasambandam, R.; Visvanathan, S.; Mohanty, B.K.; Sabesan, G.S.; Dhiva, S.; et al. Evaluation of the Toxic Effect of Bauhinia purpurea Mediated Synthesized Silver Nanoparticles against In-vitro and In-vivo Models. Toxics 2023, 11, 9. https://doi.org/10.3390/toxics11010009
Shobana N, Prakash P, Samrot AV, Saigeetha S, Sathiyasree M, Thirugnanasambandam R, Visvanathan S, Mohanty BK, Sabesan GS, Dhiva S, et al. Evaluation of the Toxic Effect of Bauhinia purpurea Mediated Synthesized Silver Nanoparticles against In-vitro and In-vivo Models. Toxics. 2023; 11(1):9. https://doi.org/10.3390/toxics11010009
Chicago/Turabian StyleShobana, Nagarajan, Pandurangan Prakash, Antony V. Samrot, Subramanian Saigeetha, Mahendran Sathiyasree, Rajendran Thirugnanasambandam, Sridevi Visvanathan, Basanta Kumar Mohanty, Gokul Shankar Sabesan, Shanmugaboopathi Dhiva, and et al. 2023. "Evaluation of the Toxic Effect of Bauhinia purpurea Mediated Synthesized Silver Nanoparticles against In-vitro and In-vivo Models" Toxics 11, no. 1: 9. https://doi.org/10.3390/toxics11010009
APA StyleShobana, N., Prakash, P., Samrot, A. V., Saigeetha, S., Sathiyasree, M., Thirugnanasambandam, R., Visvanathan, S., Mohanty, B. K., Sabesan, G. S., Dhiva, S., Remya, R. R., Pachiyappan, S., & Wilson, S. (2023). Evaluation of the Toxic Effect of Bauhinia purpurea Mediated Synthesized Silver Nanoparticles against In-vitro and In-vivo Models. Toxics, 11(1), 9. https://doi.org/10.3390/toxics11010009







