Pharmacokinetic Study of rhIL-18BP and Its Effect on Radiation-Induced Cytokine Changes in Mouse Serum and Intestine
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mice and Animal Care
2.3. PK Study
2.4. Cytokine Study
2.4.1. Irradiation and rhIL-18BP Treatment
2.4.2. Tissue Collection and Cytokine Multi-Plex Assay
2.5. Statistical Analysis
3. Results
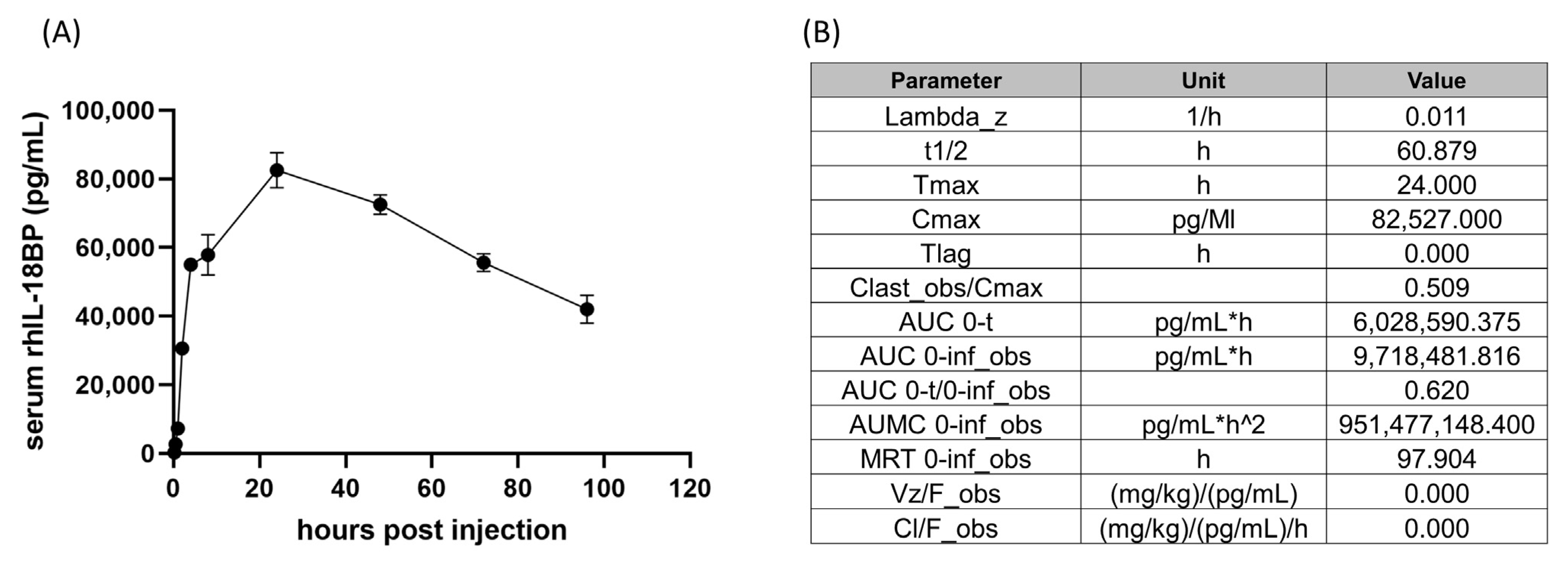
3.1. Pharmacokinetic Analysis of rhIL-18BP in CD2F1 Mice
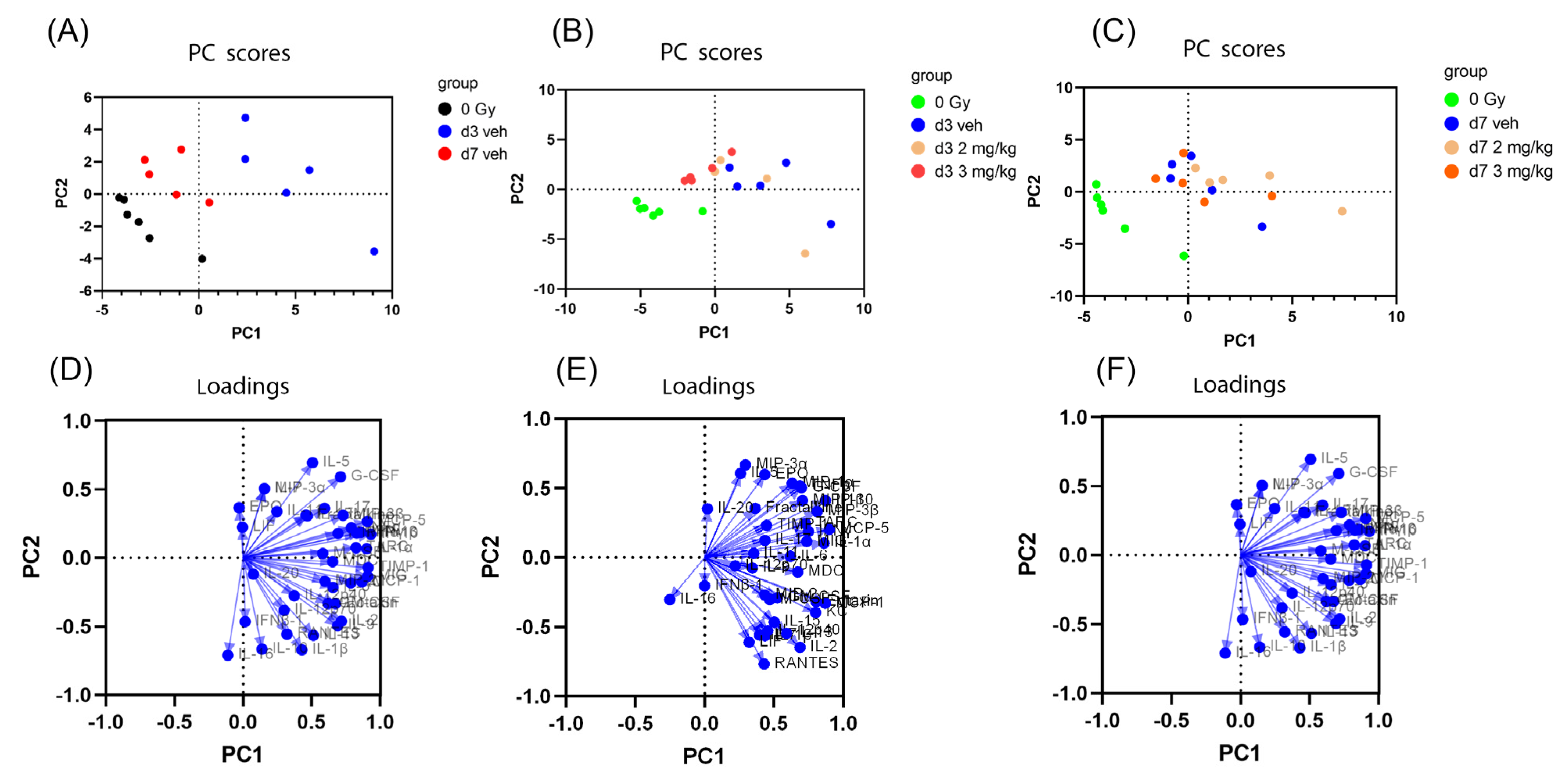
3.2. Principal Component Analysis of Mouse Serum Cytokines after Irradiation and IL-18BP Treatment
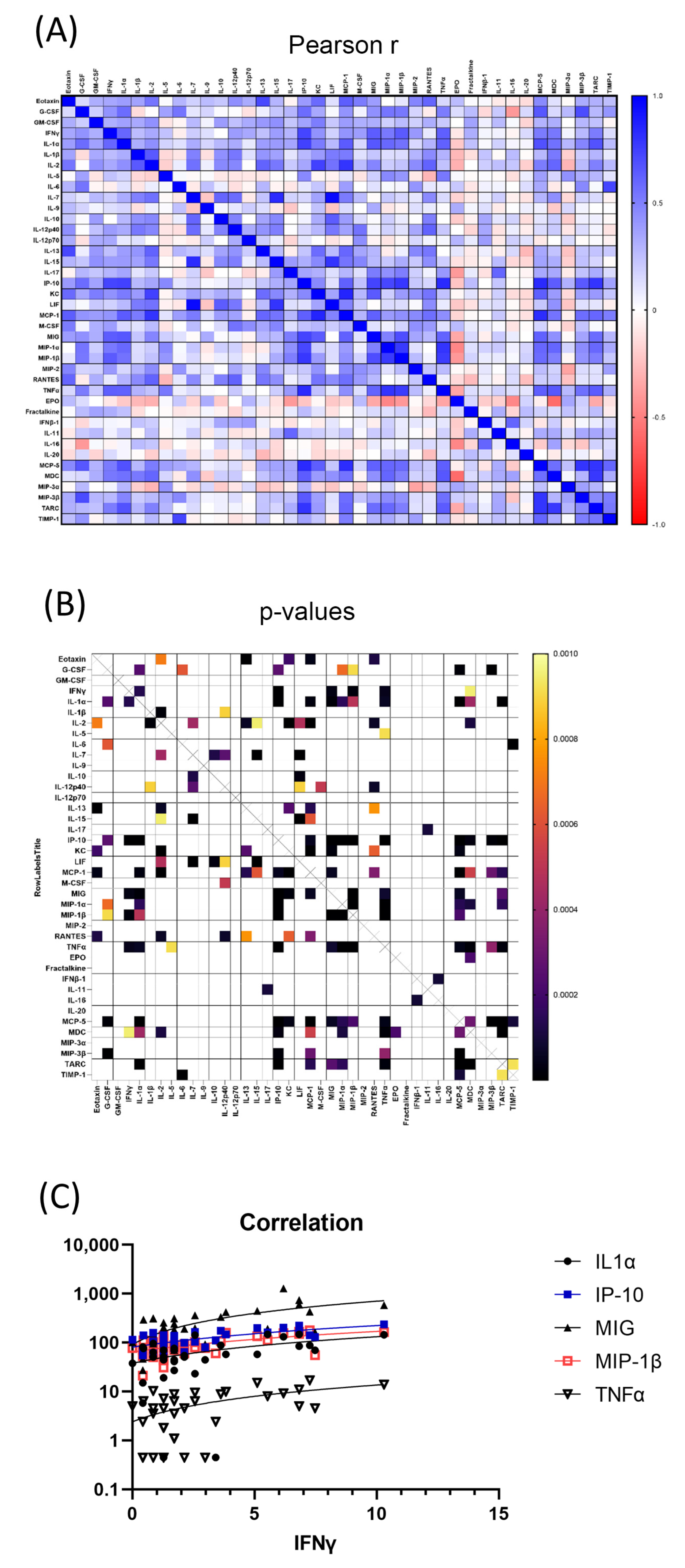
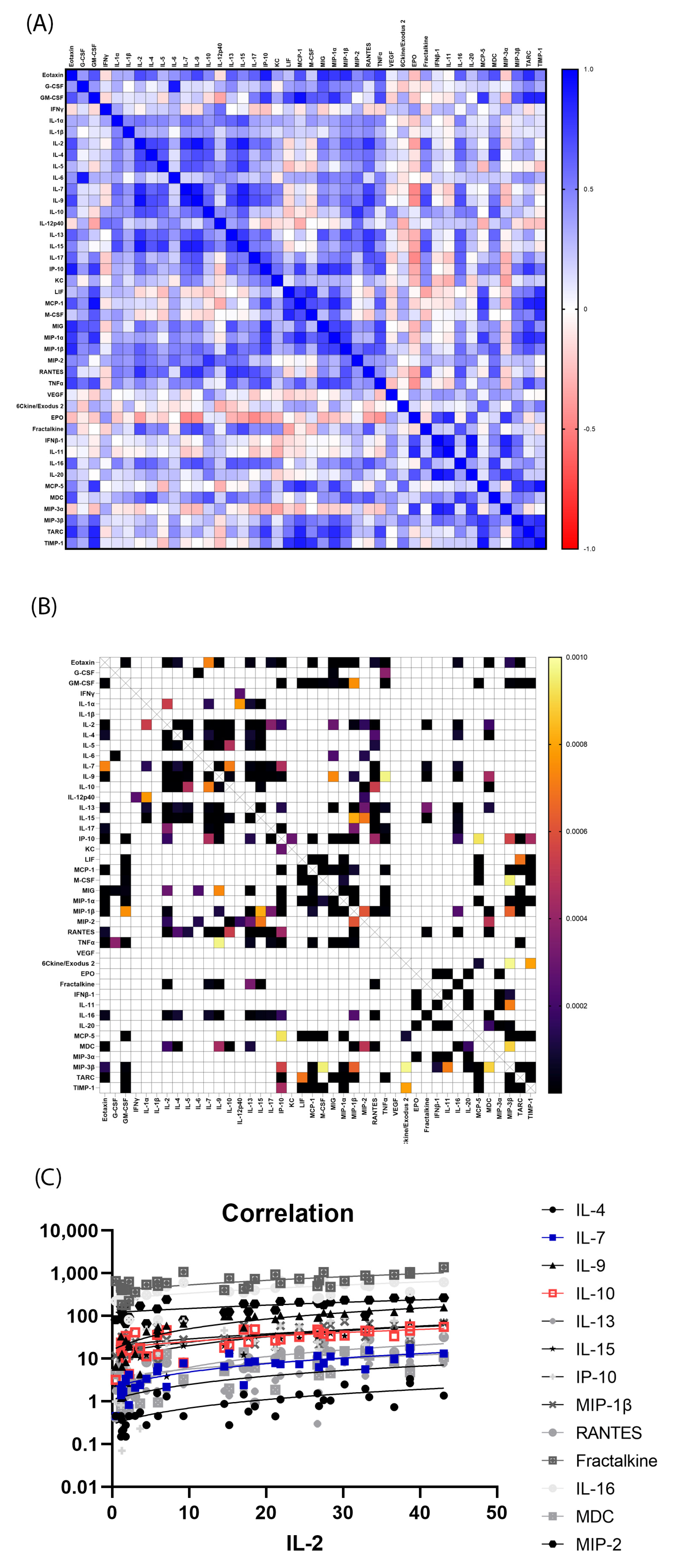
3.3. Significantly Correlated Serum Cytokines
3.4. Individual Mouse Serum Cytokine Changes after 9.0 Gy TBI
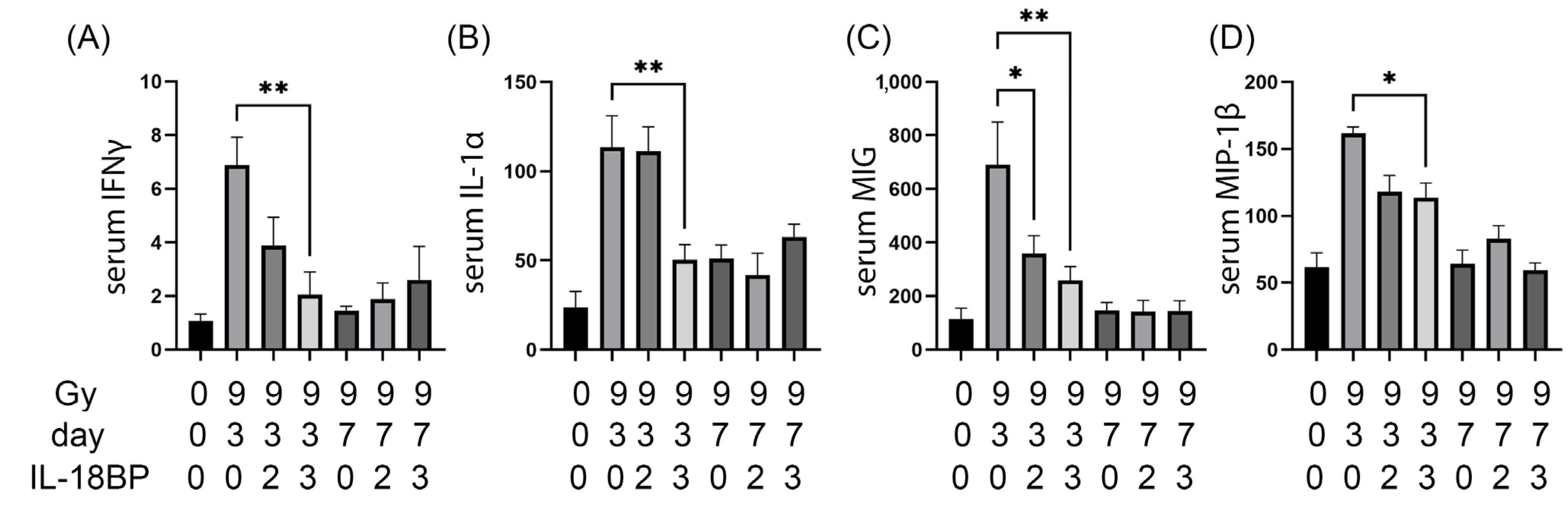
3.5. Serum Cytokines That Were Significantly Changed by rhIL-18BP Treatment after 9.0 Gy TBI
3.6. Principal Component Analysis of Mouse Intestinal Cytokines after Irradiation and rhIL-18BP Treatment
3.7. Significantly Correlated Mouse Intestinal Cytokines
3.8. Individual Mouse Intestinal Cytokine Changes after 9.0 Gy TBI
3.9. Intestinal Cytokines Significantly Changed by rhIL-18BP Treatment after 9.0 Gy TBI
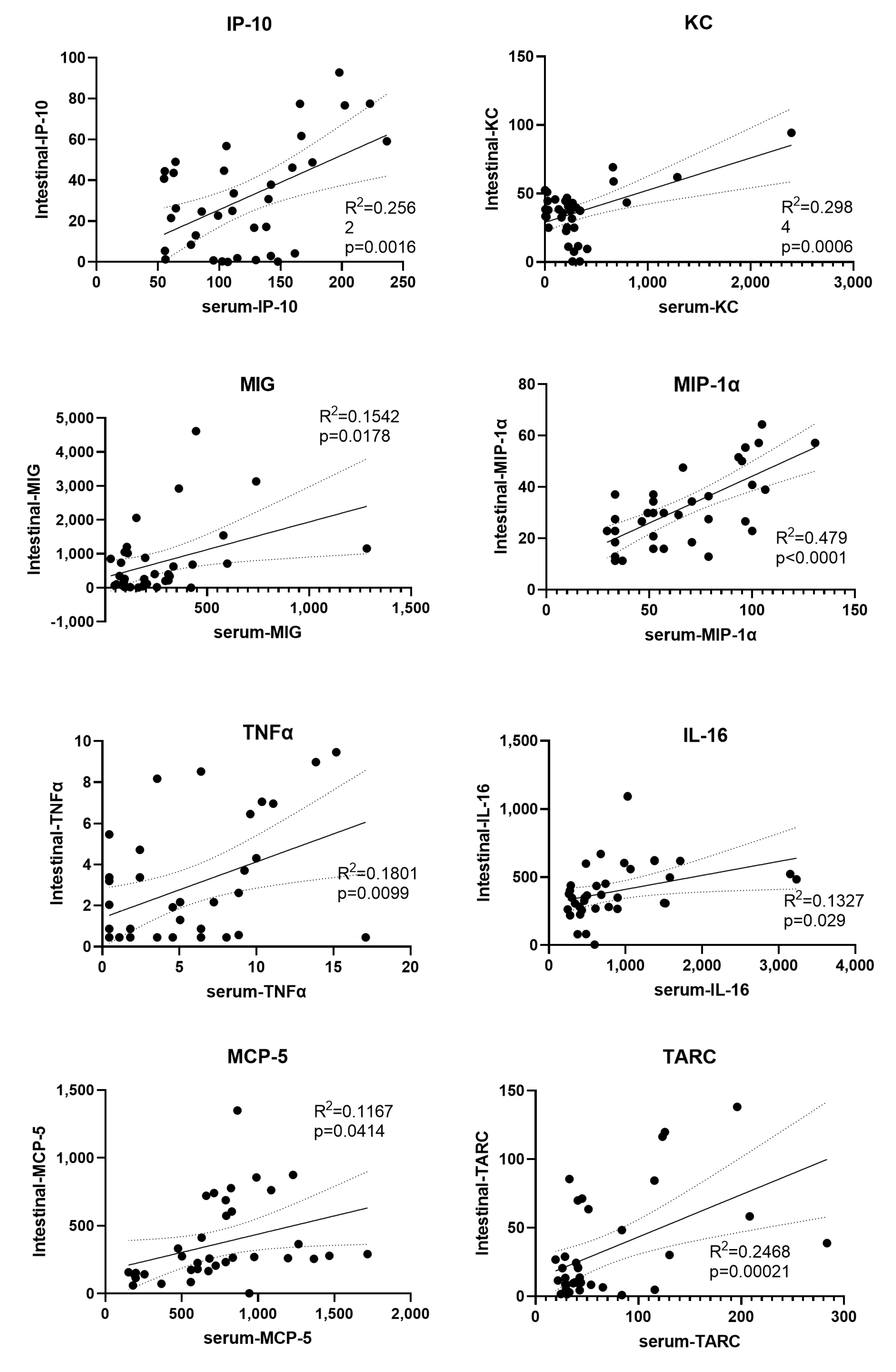
3.10. Significantly Correlated Intestinal and Serum Cytokines
4. Discussion
4.1. Pharmacokinetics of hIL-18BP in Human and Mice
4.2. Radiation-Induced Cytokine Storms
4.3. The Effect of rhIL-18BP on the Radiation-Induced Cytokine Changes
4.4. Correlated Cytokines between the Serum and Intestine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mettler, F.A., Jr.; Voelz, G.L. Major radiation exposure--what to expect and how to respond. N. Engl. J. Med. 2002, 346, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.N.; Stone, H.B.; Moulder, J.E.; Pellmar, T.C. Modulation of radiation injury. Science 2004, 304, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Okamura, H.; Nagata, K.; Komatsu, T.; Tamura, T. Purification of a factor which provides a costimulatory signal for gamma interferon production. Infect. Immun. 1993, 61, 64–70. [Google Scholar] [CrossRef]
- Xiao, M. The Role of Proinflammatory Cytokine Interleukin-18 in Radiation Injury. Health Phys. 2016, 111, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Carroll, H.P.; Paunovic, V.; Gadina, M. Signalling, inflammation and arthritis: Crossed signals: The role of interleukin-15 and -18 in autoimmunity. Rheumatology 2008, 47, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Ha, C.T.; Li, X.H.; Fu, D.; Moroni, M.; Fisher, C.; Arnott, R.; Srinivasan, V.; Xiao, M. Circulating interleukin-18 as a biomarker of total-body radiation exposure in mice, minipigs, and nonhuman primates (NHP). PLoS ONE 2014, 9, e109249. [Google Scholar] [CrossRef]
- Ha, C.T.; Li, X.; Fu, D.; Xiao, M. Circulating IL-18 Binding Protein (IL-18BP) and IL-18 as Dual Biomarkers of Total-Body Irradiation in Mice. Radiat. Res. 2016, 185, 375–383. [Google Scholar] [CrossRef]
- Aizawa, Y.; Akita, K.; Taniai, M.; Torigoe, K.; Mori, T.; Nishida, Y.; Ushio, S.; Nukada, Y.; Tanimoto, T.; Ikegami, H.; et al. Cloning and expression of interleukin-18 binding protein. FEBS Lett. 1999, 445, 338–342. [Google Scholar] [CrossRef]
- Novick, D.; Kim, S.H.; Fantuzzi, G.; Reznikov, L.L.; Dinarello, C.A.; Rubinstein, M. Interleukin-18 binding protein: A novel modulator of the Th1 cytokine response. Immunity 1999, 10, 127–136. [Google Scholar] [CrossRef]
- Kim, S.H.; Eisenstein, M.; Reznikov, L.; Fantuzzi, G.; Novick, D.; Rubinstein, M.; Dinarello, C.A. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc. Natl. Acad. Sci. USA 2000, 97, 1190–1195. [Google Scholar] [CrossRef]
- Gabay, C.; Fautrel, B.; Rech, J.; Spertini, F.; Feist, E.; Kotter, I.; Hachulla, E.; Morel, J.; Schaeverbeke, T.; Hamidou, M.A.; et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still’s disease. Ann. Rheum. Dis. 2018, 77, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Canna, S.W.; Girard, C.; Malle, L.; de Jesus, A.; Romberg, N.; Kelsen, J.; Surrey, L.F.; Russo, P.; Sleight, A.; Schiffrin, E.; et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J. Allergy Clin. Immunol. 2017, 139, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, W.; Hull, L.; Wang, L.; Yu, T.; Xiao, M. IL-18 binding protein (IL-18BP) as a novel radiation countermeasure after radiation exposure in mice. Sci. Rep. 2020, 10, 18674. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Bacchi, M.; Bertolino, M. Pharmacokinetics of IL-18 binding protein in healthy volunteers and subjects with rheumatoid arthritis or plaque psoriasis. Eur. J. Drug Metab. Pharmacokinet. 2006, 31, 109–116. [Google Scholar] [CrossRef]
- Schaue, D.; Kachikwu, E.L.; McBride, W.H. Cytokines in radiobiological responses: A review. Radiat. Res. 2012, 178, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.I.; Cassatt, D.R.; Hollingsworth, B.A.; Satyamitra, M.M.; Tadesse, Y.S.; Taliaferro, L.P.; Winters, T.A.; DiCarlo, A.L. Commonalities Between COVID-19 and Radiation Injury. Radiat. Res. 2021, 195, 1–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Unverdorben, F.; Richter, F.; Hutt, M.; Seifert, O.; Malinge, P.; Fischer, N.; Kontermann, R.E. Pharmacokinetic properties of IgG and various Fc fusion proteins in mice. MAbs 2016, 8, 120–128. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, Z.; Ma, S.; Liu, X. Radiotherapy and Cytokine Storm: Risk and Mechanism. Front. Oncol. 2021, 11, 670464. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Zhao, L.; Davis, M.A.; Lubman, D.M.; Lawrence, T.S.; Kong, F.M. Radiation produces differential changes in cytokine profiles in radiation lung fibrosis sensitive and resistant mice. J. Hematol. Oncol. 2009, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Lierova, A.; Jelicova, M.; Nemcova, M.; Proksova, M.; Pejchal, J.; Zarybnicka, L.; Sinkorova, Z. Cytokines and radiation-induced pulmonary injuries. J. Radiat. Res. 2018, 59, 709–753. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Grace, M.B.; Parekh, V.I.; Whitnall, M.H.; Landauer, M.R. Effects of genistein administration on cytokine induction in whole-body gamma irradiated mice. Int. Immunopharmacol. 2009, 9, 1401–1410. [Google Scholar] [CrossRef]
- Li, X.; Cui, W.; Hull, L.; Smith, J.T.; Kiang, J.G.; Xiao, M. Effects of Low-to-Moderate Doses of Gamma Radiation on Mouse Hematopoietic System. Radiat. Res. 2018, 190, 612–622. [Google Scholar] [CrossRef]
- Farrugia, M.; Baron, B. The role of TNF-alpha in rheumatoid arthritis: A focus on regulatory T cells. J. Clin. Transl. Res. 2016, 2, 84–90. [Google Scholar] [CrossRef]
- Adegbola, S.O.; Sahnan, K.; Warusavitarne, J.; Hart, A.; Tozer, P. Anti-TNF Therapy in Crohn’s Disease. Int. J. Mol. Sci. 2018, 19, 2244. [Google Scholar] [CrossRef]
- Bendele, A.M.; Chlipala, E.S.; Scherrer, J.; Frazier, J.; Sennello, G.; Rich, W.J.; Edwards, C.K. Combination benefit of treatment with the cytokine inhibitors interleukin-1 receptor antagonist and PEGylated soluble tumor necrosis factor receptor type I in animal models of rheumatoid arthritis. Arthritis Rheum. 2000, 43, 2648–2659. [Google Scholar] [CrossRef]
- Lieben, L. Autoinflammatory diseases: Free IL-18 causes macrophage activation syndrome. Nat. Rev. Rheumatol. 2018. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Singh, V.K.; Fatanmi, O.O.; Singh, P.K.; Whitnall, M.H. Role of radiation-induced granulocyte colony-stimulating factor in recovery from whole body gamma-irradiation. Cytokine 2012, 58, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Farese, A.M.; Brown, C.R.; Smith, C.P.; Gibbs, A.M.; Katz, B.P.; Johnson, C.S.; Prado, K.L.; MacVittie, T.J. The ability of filgrastim to mitigate mortality following LD50/60 total-body irradiation is administration time-dependent. Health Phys. 2014, 106, 39–47. [Google Scholar] [CrossRef]
- Marques, R.E.; Guabiraba, R.; Russo, R.C.; Teixeira, M.M. Targeting CCL5 in inflammation. Expert Opin. Ther. Targets 2013, 17, 1439–1460. [Google Scholar] [CrossRef]
- Piryani, S.O.; Kam, A.Y.F.; Vu, U.T.; Chao, N.J.; Doan, P.L. CCR5 Signaling Promotes Murine and Human Hematopoietic Regeneration following Ionizing Radiation. Stem Cell Rep. 2019, 13, 76–90. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, A.M.; Demirel, O.; Hooibrink, B.; Brandts, C.H.; Nolte, M.A. Interferon-gamma impairs proliferation of hematopoietic stem cells in mice. Blood 2013, 121, 3578–3585. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, A.M.; Voermans, C.; Nolte, M.A. Impact of interferon-gamma on hematopoiesis. Blood 2014, 124, 2479–2486. [Google Scholar] [CrossRef] [PubMed]


| Cytokines | 0 Gy Concentration | 9 Gy d3 veh Concentration | p Value d3 veh vs. 0 Gy | 9 Gy d7 veh Concentration | p Value 9 Gy d7 veh vs. 0 Gy | |
|---|---|---|---|---|---|---|
| 1 | MCP-1 | 13.31 ± 1.97 | 495.87 ± 194.8 | p < 0.05 | 138.79 ± 32.75 | n.s |
| 2 | MDC | 325.67 ± 42.59 | 513.38 ± 72.71 | p < 0.05 | 187.58 ± 22.82 | n.s |
| 3 | MIP-3β | 178.38 ± 24.48 | 320.74 ± 11.59 | p < 0.01 | 283.41 ± 34.49 | p < 0.05 |
| 4 | IL-17 | 3.12 ± 0.37 | 8.16 ± 1.56 | p < 0.01 | 3.33 ± 0.89 | n.s |
| 5 | MIG | 112.85 ± 42.23 | 690.56 ± 159.29 | p < 0.01 | 146.93 ± 29.25 | n.s |
| 6 | MIP-1α | 46.67 ± 7.3 | 95.7 ± 10.46 | p < 0.01 | 42.61 ± 4.72 | n.s |
| 7 | TARC | 29.73 ± 3.86 | 132.74 ± 31.44 | p < 0.01 | 43.26 ± 6.11 | n.s |
| 8 | IL-5 | 4.75 ± 1.59 | 23.37 ± 3.52 | p < 0.001 | 18.5 ± 0.94 | p < 0.01 |
| 9 | IL-1α | 23.77 ± 9.04 | 113.51 ± 17.61 | p < 0.001 | 51.19 ± 7.54 | n.s |
| 10 | TNFα | 2.16 ± 0.83 | 12.25 ± 1.44 | p < 0.001 | 2.67 ± 1.56 | n.s |
| 11 | TIMP-1 | 1627.59 ± 330.01 | 4850.59 ± 707.44 | p < 0.001 | 1611.3 ± 190.79 | n.s |
| 12 | MCP-5 | 196.13 ± 14.01 | 1112.96 ± 140.57 | p < 0.0001 | 672.18 ± 64 | p < 0.01 |
| 13 | G-CSF | 378.82 ± 213.87 | 12,031.13 ± 1317.17 | p < 0.0001 | 7820.85 ± 1429.67 | p < 0.001 |
| 14 | IFNγ | 1.07 ± 0.26 | 6.88 ± 1.04 | p < 0.0001 | 1.45 ± 0.17 | n.s |
| 15 | IP-10 | 66.32 ± 6.74 | 190.45 ± 19.34 | p < 0.0001 | 104.05 ± 10 | n.s |
| 16 | MIP-1β | 61.82 ± 10.59 | 161.69 ± 4.78 | p < 0.0001 | 64.12 ± 10.51 | n.s |
| 17 | IL-16 | 1957.48 ± 396.85 | 935.04 ± 162.28 | n.s | 429.42 ± 86.09 | p < 0.01 |
| 18 | MIP-3α | 80.5 ± 23.53 | 206.4 ± 44.29 | n.s | 343.82 ± 55.68 | p < 0.01 |
| 19 | EPO | 246.74 ± 32.09 | 340.36 ± 30.09 | n.s | 791.16 ± 110.13 | p < 0.001 |
| 20 | Eotaxin | 826.05 ± 160.13 | 1472.6 ± 361.65 | n.s | 1357.4 ± 222.35 | n.s |
| 21 | GM-CSF | 3.96 ± 1.67 | 6.76 ± 3.06 | n.s | 2.24 ± 1.09 | n.s |
| 22 | IL-1β | 3.79 ± 0.45 | 3.81 ± 0.53 | n.s | 2.85 ± 0.69 | n.s |
| 23 | IL-2 | 3.25 ± 0.8 | 6.16 ± 2.31 | n.s | 2.04 ± 0.95 | n.s |
| 24 | IL-3 | n.d | n.d | n.s | n.d | n.s |
| 25 | IL-4 | n.d | n.d | n.s | n.d | n.s |
| 26 | IL-6 | 6.42 ± 3.28 | 26.44 ± 12.7 | n.s | 18.32 ± 7.34 | n.s |
| 27 | IL-7 | 0.51 ± 0.03 | 1.1 ± 0.64 | n.s | 0.45 ± 0 | n.s |
| 28 | IL-9 | 6.27 ± 1.33 | 11.23 ± 3.97 | n.s | 5.48 ± 2.08 | n.s |
| 29 | IL-10 | 5.96 ± 1.9 | 4.3 ± 1.28 | n.s | 2.63 ± 0.84 | n.s |
| 30 | IL-12p40 | 1.52 ± 1.06 | 2.88 ± 1.18 | n.s | 2.3 ± 1.26 | n.s |
| 31 | IL-12p70 | 2.47 ± 2.02 | 2.38 ± 1.09 | n.s | 0.82 ± 0.37 | n.s |
| 32 | IL-13 | 43.27 ± 5.81 | 48.95 ± 10.74 | n.s | 48.22 ± 6.14 | n.s |
| 33 | IL-15 | 3.84 ± 1.58 | 27.39 ± 13.8 | n.s | 2.54 ± 0.27 | n.s |
| 34 | KC | 12.45 ± 4.9 | 812.75 ± 408.45 | n.s | 241.88 ± 62.98 | n.s |
| 35 | LIF | 0.73 ± 0.28 | 0.82 ± 0.31 | n.s | 0.45 ± 0 | n.s |
| 36 | M-CSF | 7.16 ± 1.83 | 11.87 ± 1.41 | n.s | 7.77 ± 1.55 | n.s |
| 37 | MIP-2 | 141.3 ± 2.91 | 147.92 ± 2.69 | n.s | 138.06 ± 1.44 | n.s |
| 38 | RANTES | 19.32 ± 2.81 | 21.6 ± 7.15 | n.s | 22.69 ± 4.77 | n.s |
| 39 | VEGF | n.d | 0.86 ± 0.32 | n.s | n.d | n.s |
| 40 | 6Ckine/Exodus 2 | a.d | a.d | n.s | a.d | n.s |
| 41 | Fractalkine | 201.36 ± 19.64 | 370.76 ± 119.56 | n.s | 250 ± 43.94 | n.s |
| 42 | IFNβ-1 | 138.23 ± 59.63 | 75.54 ± 9.65 | n.s | 48.13 ± 1.62 | n.s |
| 43 | IL-11 | 25.75 ± 3.47 | 39.79 ± 11.55 | n.s | 28.57 ± 19.57 | n.s |
| 44 | IL-20 | 78.37 ± 18.03 | 81.86 ± 11.61 | n.s | 67.13 ± 11.64 | n.s |
| Cytokines | 0 Gy Concentrations | 9 Gy d3 veh Concentrations | p Value d3 veh vs. 0 Gy | 9 Gy d7 veh Concentrations | p Value 9 Gy d7 veh vs. 0 Gy | |
|---|---|---|---|---|---|---|
| 1 | M-CSF | 4.39 ± 0.56 | 9.02 ± 0.6 | p < 0.05 | 6.48 ± 1.03 | n.s |
| 2 | TIMP-1 | 176.41 ± 20.02 | 610.07 ± 152.39 | p < 0.05 | 282.09 ± 53.98 | n.s |
| 3 | RANTES | 22.74 ± 3.48 | 9.14 ± 2.35 | p < 0.01 | 2.97 ± 1.81 | p < 0.0001 |
| 4 | GM-CSF | 1.72 ± 0.81 | 18.5 ± 4.01 | p < 0.01 | 0.45 ± 0 | n.s |
| 5 | LIF | 3.16 ± 0.33 | 16.14 ± 1.82 | p < 0.001 | 4.76 ± 0.42 | n.s |
| 6 | MCP-1 | 23 ± 5.98 | 97.03 ± 20.24 | p < 0.001 | 15.5 ± 1.63 | n.s |
| 7 | IL-2 | 30.45 ± 5.16 | 16.05 ± 4.4 | n.s | 5.36 ± 3.96 | p < 0.05 |
| 8 | IL-4 | 1.73 ± 0.35 | 0.93 ± 0.19 | n.s | 0.27 ± 0.06 | p < 0.05 |
| 9 | IL-5 | 1.14 ± 0.19 | 0.64 ± 0.23 | n.s | 0.4 ± 0.08 | p < 0.05 |
| 10 | IL-9 | 123.77 ± 17.15 | 67.88 ± 19.32 | n.s | 24.11 ± 15.9 | p < 0.05 |
| 11 | IL-13 | 6.2 ± 1.1 | 3.58 ± 0.7 | n.s | 2.04 ± 0.27 | p < 0.05 |
| 12 | MIP-1β | 49 ± 7.27 | 45.38 ± 9.36 | n.s | 17.61 ± 2.29 | p < 0.05 |
| 13 | MDC | 14.12 ± 1.38 | 6.92 ± 2.54 | n.s | 5.29 ± 0.97 | p < 0.05 |
| 14 | Eotaxin | 324.26 ± 15.66 | 346.38 ± 101.8 | n.s | 34.57 ± 21.06 | n.s |
| 15 | G-CSF | 2.02 ± 0.22 | 4.89 ± 1.69 | n.s | 2.92 ± 0.5 | n.s |
| 16 | IFNγ | 4.55 ± 0.53 | 3.75 ± 1.14 | n.s | 4.18 ± 0.8 | n.s |
| 17 | IL-1α | 121.84 ± 13.94 | 96.47 ± 12.64 | n.s | 81.4 ± 6.21 | n.s |
| 18 | IL-1β | 15.91 ± 2.34 | 11.06 ± 2.21 | n.s | 8.28 ± 0.27 | n.s |
| 19 | IL-3 | b.d | b.d | n.s | b.d | n.s |
| 20 | IL-6 | 3.6 ± 0.14 | 5.83 ± 1.74 | n.s | 4.86 ± 0.58 | n.s |
| 21 | IL-7 | 10.41 ± 2.52 | 6.26 ± 1.71 | n.s | 3.13 ± 1.23 | n.s |
| 22 | IL-10 | 47.89 ± 2.93 | 27.25 ± 6.85 | n.s | 26.5 ± 2.31 | n.s |
| 23 | IL-12p40 | 11.11 ± 2.34 | 5.21 ± 1.52 | n.s | 10.88 ± 2.03 | n.s |
| 24 | IL-12p70 | b.d | b.d | n.s | b.d | n.s |
| 25 | IL-15 | 47.12 ± 10.27 | 22.24 ± 5.35 | n.s | 16.72 ± 4.65 | n.s |
| 26 | IL-17 | 16.82 ± 4.53 | 16.67 ± 4.94 | n.s | 2.35 ± 1.26 | n.s |
| 27 | IP-10 | 37.03 ± 4.84 | 50.28 ± 14.45 | n.s | 5.37 ± 3.34 | n.s |
| 28 | KC | 41.05 ± 3.5 | 43.7 ± 14.93 | n.s | 16.55 ± 3.56 | n.s |
| 29 | MIG | 715.16 ± 171.53 | 1302.87 ± 524.66 | n.s | 106.75 ± 74.75 | n.s |
| 30 | MIP-1α | 25.79 ± 4.29 | 45.27 ± 6.09 | n.s | 25.08 ± 3.46 | n.s |
| 31 | MIP-2 | 215.85 ± 25.28 | 175.98 ± 25.08 | n.s | 160.72 ± 13.27 | n.s |
| 32 | TNFα | 2.95 ± 0.86 | 4.88 ± 1.46 | n.s | 0.88 ± 0.3 | n.s |
| 33 | VEGF | 35.16 ± 7.69 | 17.33 ± 6.47 | n.s | 38.02 ± 4.49 | n.s |
| 34 | 6Ckine/Exodus 2 | 1246.48 ± 159.41 | 4785.37 ± 3818.81 | n.s | 821.86 ± 239.75 | n.s |
| 35 | EPO | 93.9 ± 35.11 | 37.71 ± 21.7 | n.s | 147.72 ± 34.56 | n.s |
| 36 | Fractalkine | 938.65 ± 115.42 | 520.75 ± 171.04 | n.s | 529.81 ± 103.02 | n.s |
| 37 | IFNβ-1 | 252.83 ± 44.12 | 92.82 ± 19.95 | n.s | 318.49 ± 72.79 | n.s |
| 38 | IL-11 | 631.25 ± 124.99 | 193.75 ± 49.54 | n.s | 785.42 ± 195.99 | n.s |
| 39 | IL-16 | 640.47 ± 93.76 | 387.31 ± 118.71 | n.s | 334.19 ± 33.16 | n.s |
| 40 | IL-20 | 39.94 ± 4.59 | 21.38 ± 2.34 | n.s | 38.9 ± 7.28 | n.s |
| 41 | MCP-5 | 126.93 ± 14.83 | 447.78 ± 156.78 | n.s | 204 ± 35.6 | n.s |
| 42 | MIP-3α | 5.11 ± 0.94 | 2.39 ± 0.31 | n.s | 9.17 ± 2.05 | n.s |
| 43 | MIP-3β | 76.22 ± 10.04 | 90.66 ± 19.28 | n.s | 84.96 ± 9.44 | n.s |
| 44 | TARC | 21.57 ± 2.48 | 59.78 ± 23.06 | n.s | 8.88 ± 0.63 | n.s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.; Hull, L.; Zizzo, A.; Wang, L.; Lin, B.; Zhai, M.; Xiao, M. Pharmacokinetic Study of rhIL-18BP and Its Effect on Radiation-Induced Cytokine Changes in Mouse Serum and Intestine. Toxics 2023, 11, 35. https://doi.org/10.3390/toxics11010035
Cui W, Hull L, Zizzo A, Wang L, Lin B, Zhai M, Xiao M. Pharmacokinetic Study of rhIL-18BP and Its Effect on Radiation-Induced Cytokine Changes in Mouse Serum and Intestine. Toxics. 2023; 11(1):35. https://doi.org/10.3390/toxics11010035
Chicago/Turabian StyleCui, Wanchang, Lisa Hull, Alex Zizzo, Li Wang, Bin Lin, Min Zhai, and Mang Xiao. 2023. "Pharmacokinetic Study of rhIL-18BP and Its Effect on Radiation-Induced Cytokine Changes in Mouse Serum and Intestine" Toxics 11, no. 1: 35. https://doi.org/10.3390/toxics11010035
APA StyleCui, W., Hull, L., Zizzo, A., Wang, L., Lin, B., Zhai, M., & Xiao, M. (2023). Pharmacokinetic Study of rhIL-18BP and Its Effect on Radiation-Induced Cytokine Changes in Mouse Serum and Intestine. Toxics, 11(1), 35. https://doi.org/10.3390/toxics11010035







