Adsorption of Diclofenac Sodium by Aged Degradable and Non-Degradable Microplastics: Environmental Effects, Adsorption Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Aging of MPs
2.3. Characterization of MPs
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Characteristic Analysis of Aged MPs
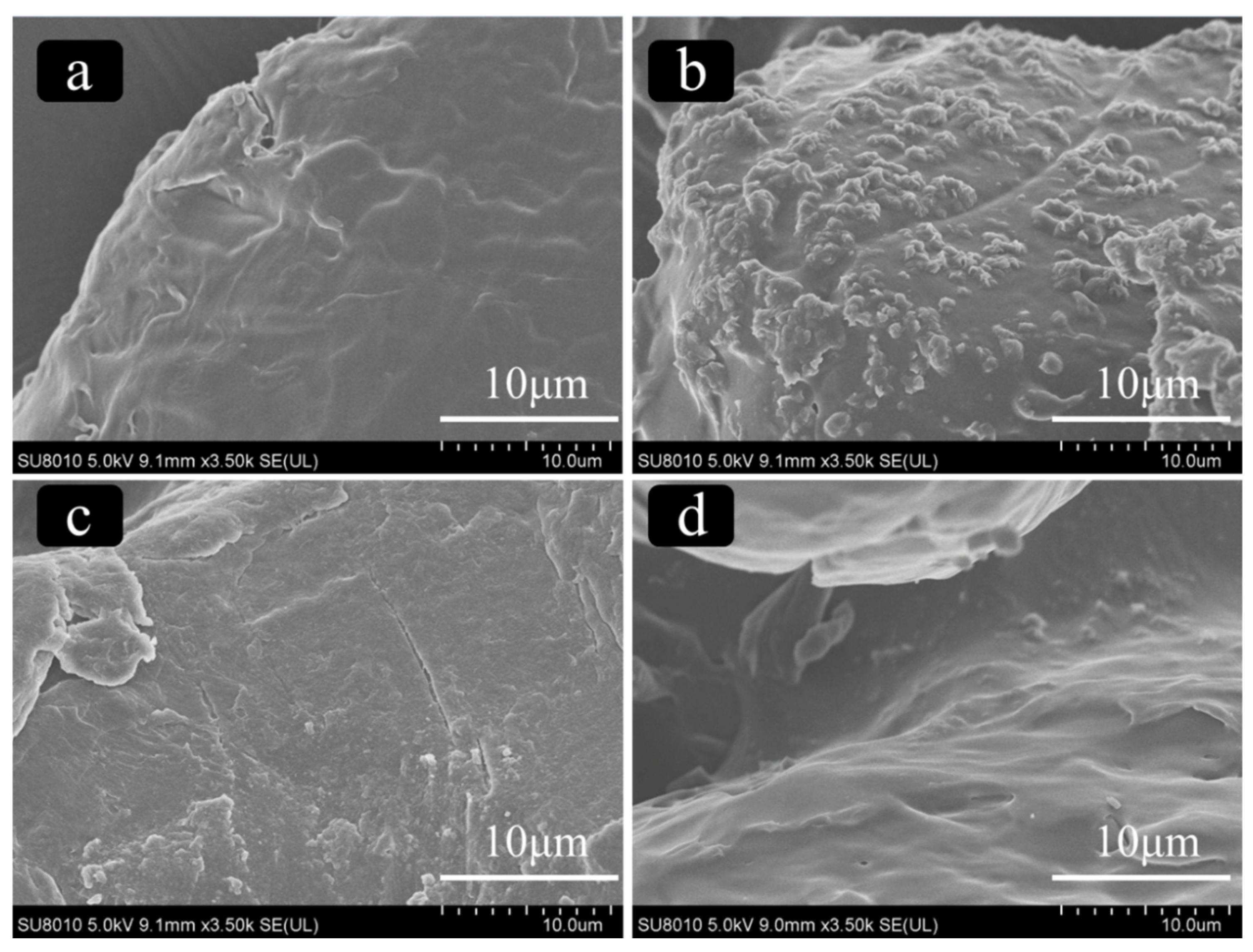
3.1.1. SEM Analysis
3.1.2. FTIR and EDS Analysis
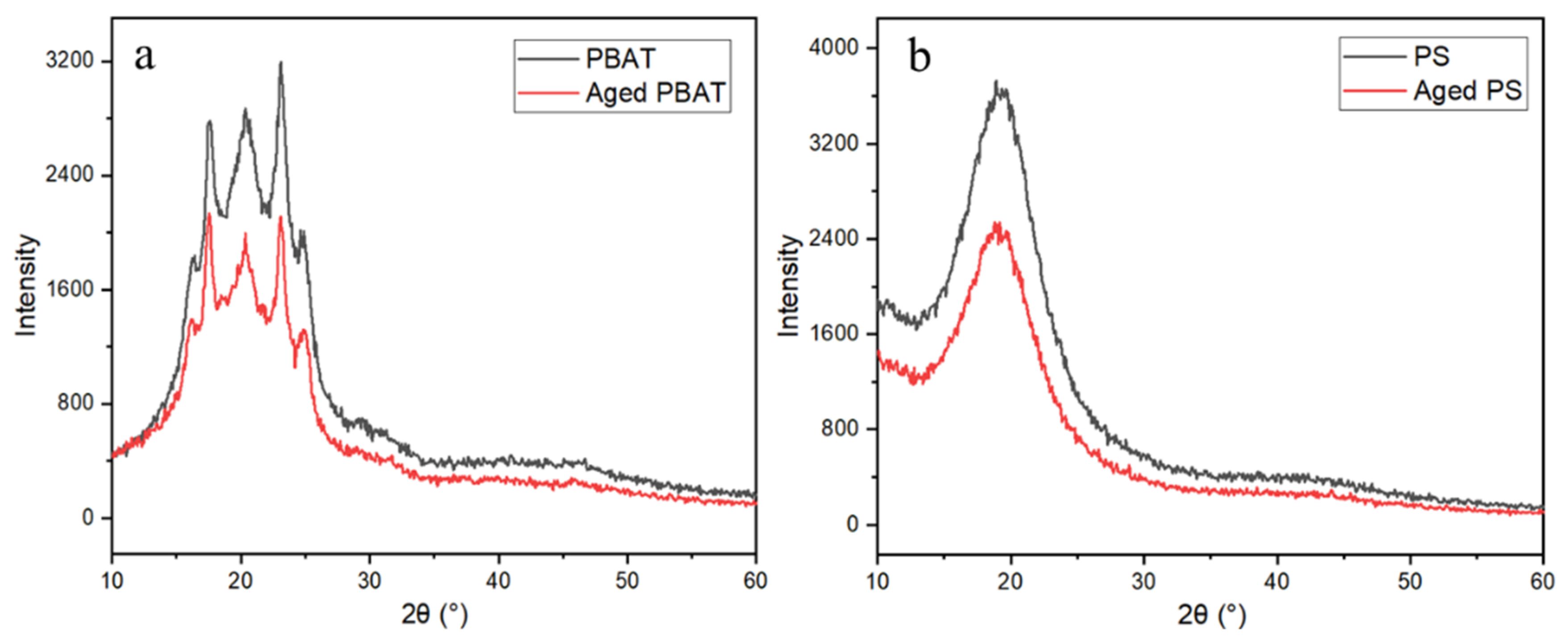
3.1.3. Zeta Potential and XRD Analysis
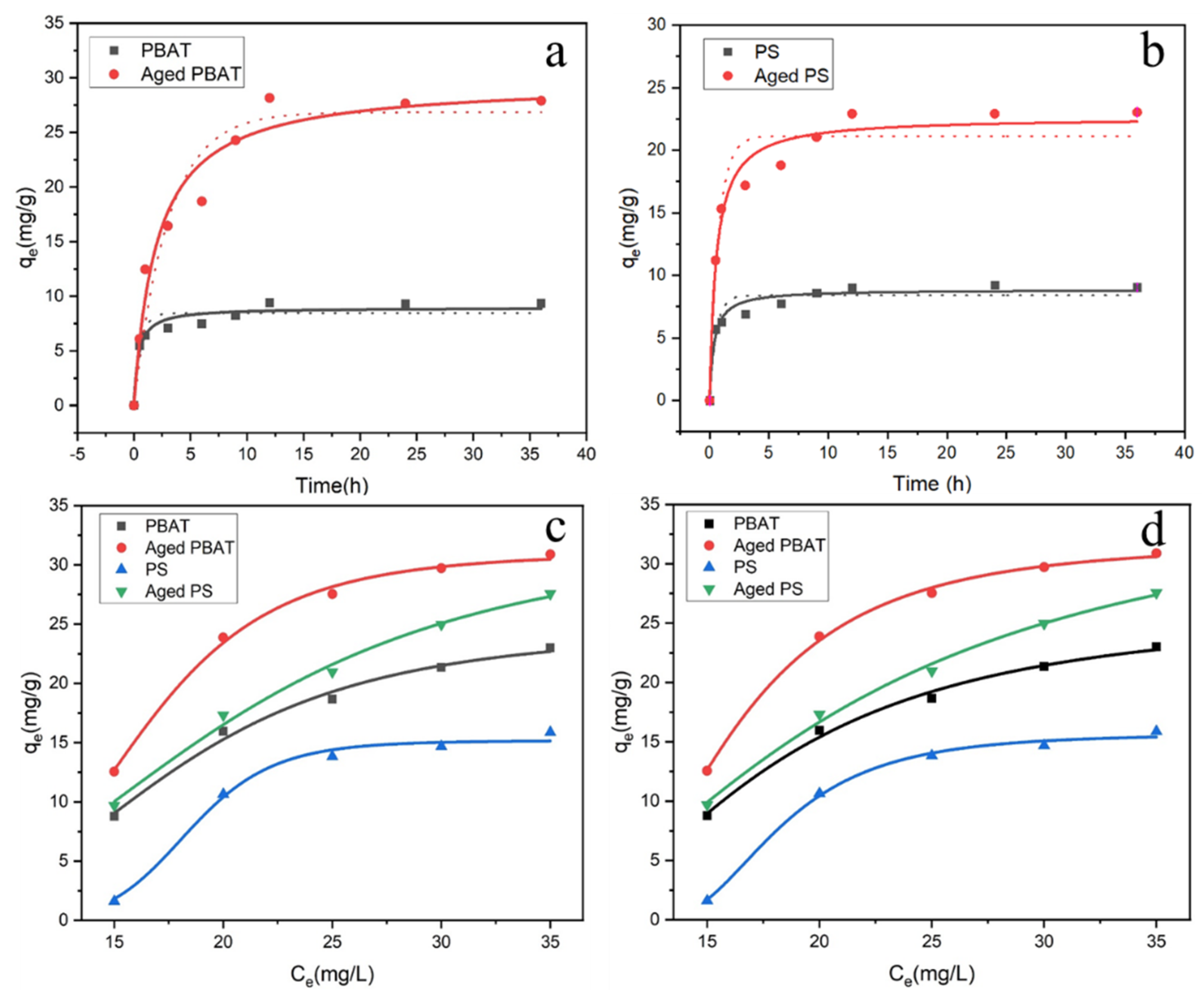
3.2. Adsorption Kinetics
3.3. Adsorption Isotherm
3.4. Effects of Environmental Factors on Adsorption
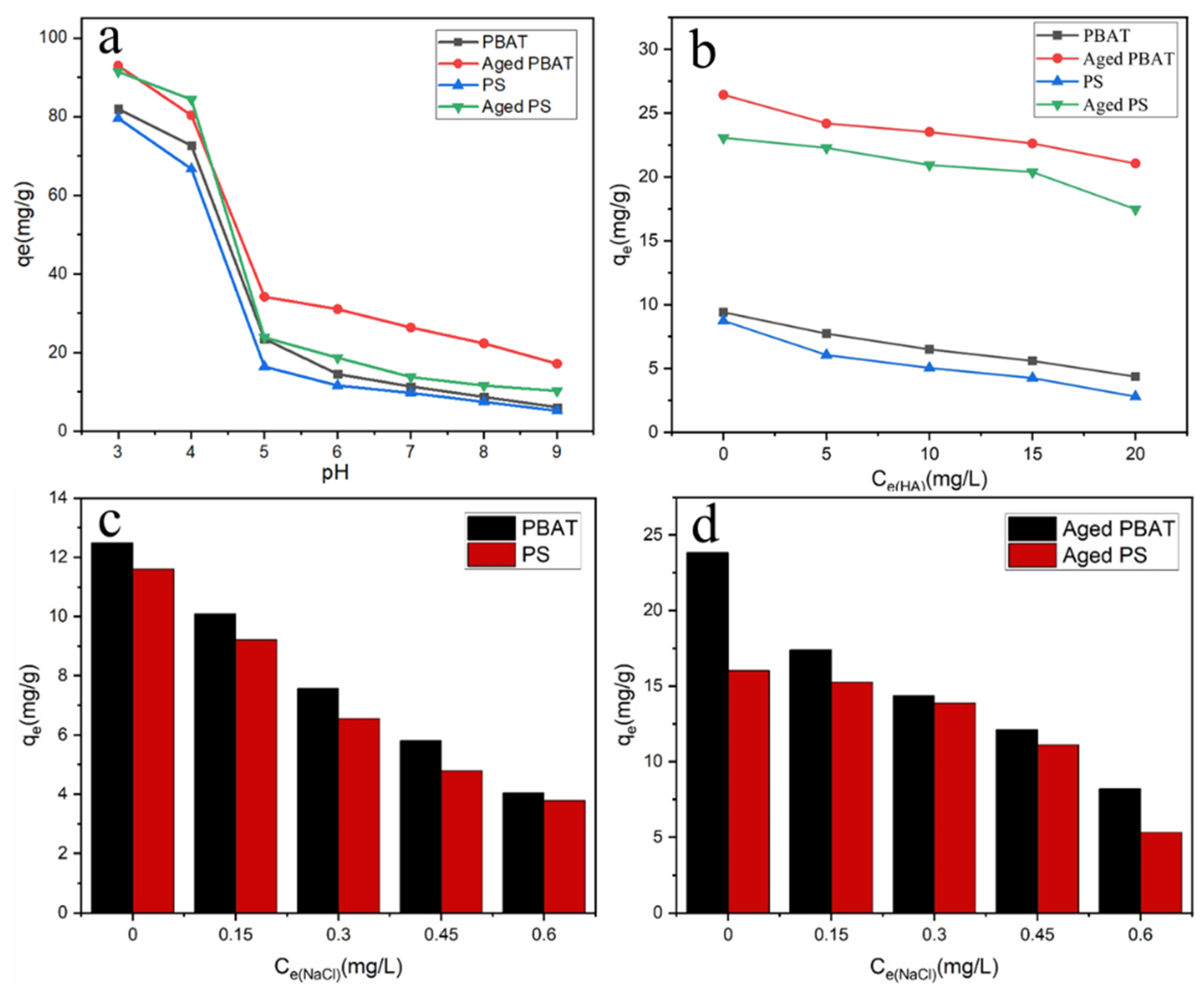
3.4.1. Effect of pH
3.4.2. Effect of HA
3.4.3. Effect of Ionic Strength
3.5. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorensen, R.M.; Jovanović, B. From nanoplastic to microplastic: A bibliometric analysis on the presence of plastic particles in the environment. Mar. Pollut. Bull. 2020, 163, 111926. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2019, 384, 121193. [Google Scholar] [CrossRef] [PubMed]
- Kaposi, K.L.; Mos, B.; Kelaher, B.P.; Dworjanyn, S.A. Ingestion of Microplastic Has Limited Impact on a Marine Larva. Environ. Sci. Technol. 2013, 48, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qin, Z.; Fei, J.; Ding, D.; Sun, H.; Wang, J.; Yin, X. Surfactant-induced adsorption of Pb(II) on the cracked structure of microplastics. J. Colloid Interface Sci. 2022, 621, 91–100. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M. Microplastics in aquatic environments: A review on occurrence, distribution, toxic effects, and implications for human health. Sci. Total Environ. 2021, 780, 146551. [Google Scholar] [CrossRef]
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Mandal, S.; Xing, B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020, 261, 114089. [Google Scholar] [CrossRef]
- Huang, D.; Tao, J.; Cheng, M.; Deng, R.; Chen, S.; Yin, L.; Li, R. Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. J. Hazard. Mater. 2020, 407, 124399. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Chen, Q.; Kalogerakis, N.; Ji, R.; Ma, Y. Interactions between microplastics and organic pollutants: Effects on toxicity, bioaccumulation, degradation, and transport. Sci. Total Environ. 2020, 748, 142427. [Google Scholar] [CrossRef]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives. J. Hazard. Mater. 2020, 405, 123913. [Google Scholar] [CrossRef]
- Budhiraja, V.; Urh, A.; Horvat, P.; Krzan, A. Synergistic Adsorption of Organic Pollutants on Weathered Polyethylene Microplastics. Polymers 2022, 14, 2674. [Google Scholar] [CrossRef]
- Vroom, R.J.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Weniger, A.-K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Stenger, K.; Wikmark, O.; Bezuidenhout, C.; Molale-Tom, L. Microplastics pollution in the ocean: Potential carrier of resistant bacteria and resistance genes. Environ. Pollut. 2021, 291, 118130. [Google Scholar] [CrossRef]
- Polman, E.M.; Gruter, G.-J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2020, 753, 141953. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.-H.; Huang, Y.; Wang, H. Comprehensive evaluation of pharmaceuticals and personal care products (PPCPs) in typical highly urbanized regions across China. Environ. Pollut. 2015, 204, 223–232. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, H.; Xu, H.; Liu, Z.; Kan, G.; Yu, K.; Jiang, J. Adsorption behaviors of triclosan by non-biodegradable and biodegradable microplastics: Kinetics and mechanism. Sci. Total Environ. 2022, 842, 156832. [Google Scholar] [CrossRef]
- Fang, S.; Yu, W.; Li, C.; Liu, Y.; Qiu, J.; Kong, F. Adsorption behavior of three triazole fungicides on polystyrene microplastics. Sci. Total Environ. 2019, 691, 1119–1126. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Qin, X. Adsorption of diclofenac onto goethite: Adsorption kinetics and effects of pH. Chemosphere 2017, 180, 373–378. [Google Scholar] [CrossRef]
- Onesios, K.M.; Yu, J.T.; Bouwer, E.J. Biodegradation and removal of pharmaceuticals and personal care products in treatment systems: A review. Biodegradation 2009, 20, 441–466. [Google Scholar] [CrossRef] [PubMed]
- Chandan, J.S.; Zemedikun, D.T.; Thayakaran, R.; Byne, N.; Dhalla, S.; Acosta-Mena, D.; Gokhale, K.M.; Thomas, T.; Sainsbury, C.; Subramanian, A.; et al. Nonsteroidal Antiinflammatory Drugs and Susceptibility to COVID-19. Arthritis Rheumatol. 2020, 73, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Comber, S.; Gardner, M.; Sörme, P.; Leverett, D.; Ellor, B. Active pharmaceutical ingredients entering the aquatic environment from wastewater treatment works: A cause for concern? Sci. Total Environ. 2018, 613–614, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, T.; Zhang, X.; Huang, J.; Xia, B.; Jiang, J.; Chen, M.; Dong, W.; Wang, Y. Ti–Dopamine Hybrid Nanoparticles with UV-Blocking and Durable Poly(butylene adipate-co-terephthalate) Materials. ACS Appl. Polym. Mater. 2022, 4, 1314–1322. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, A.; Subbiah, S.; Anderson, T.A.; Green, M.J.; Zhao, X.; Cañas-Carrell, J.E. Sorption of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics. Sci. Total Environ. 2020, 715, 136974. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2019, 392, 123705. [Google Scholar] [CrossRef]
- Mannarswamy, A.; Munson-McGee, S.H.; Steiner, R.; Andersen, P.K. D-optimal experimental designs for Freundlich and Langmuir adsorption isotherms. Chemom. Intell. Lab. Syst. 2009, 97, 146–151. [Google Scholar] [CrossRef]
- Du, H.; Zhang, Y.; Jiang, H.; Wang, H. Adsorption of rhodamine B on polyvinyl chloride, polystyrene, and polyethylene terephthalate microplastics in aqueous environments. Environ. Technol. Innov. 2022, 27, 102495. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Y.; Huang, M.; Fu, S.; Hao, Y.; Hu, S.; Lai, D.; Zhao, L. Adsorption behaviors and mechanisms of antibiotic norfloxacin on degradable and nondegradable microplastics. Sci. Total Environ. 2021, 807, 151042. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. The sorption kinetics and isotherms of sulfamethoxazole with polyethylene microplastics. Mar. Pollut. Bull. 2018, 131, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Liu, G.; Zhang, Z.; Wu, H.; Cui, B.; Bai, J.; Zhang, W. Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicol. Environ. Saf. 2019, 173, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Czech, B.; Oleszczuk, P. Sorption of diclofenac and naproxen onto MWCNT in model wastewater treated by H2O2 and/or UV. Chemosphere 2016, 149, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.M.; Philip, L. Sorption of pharmaceutical compounds and nutrients by various porous low cost adsorbents. J. Environ. Chem. Eng. 2020, 9, 104916. [Google Scholar] [CrossRef]
- Landry, K.; Boyer, T.H. Diclofenac removal in urine using strong-base anion exchange polymer resins. Water Res. 2013, 47, 6432–6444. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; He, H.; Cheng, X.; Ma, T.; Hu, J.; Yang, S.; Li, S.; Zhang, L. Adsorption behavior and mechanism of 9-Nitroanthracene on typical microplastics in aqueous solutions. Chemosphere 2019, 245, 125628. [Google Scholar] [CrossRef]
- Torres, F.G.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; De-La-Torre, G.E. Sorption of chemical contaminants on degradable and non-degradable microplastics: Recent progress and research trends. Sci. Total Environ. 2020, 757, 143875. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Wang, F.; Yang, H.; Liu, L. Adsorption of tetracyclines onto polyethylene microplastics: A combined study of experiment and molecular dynamics simulation. Chemosphere 2020, 265, 129133. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.; Ma, S.; Guo, X.; Zhu, L. High temperature depended on the ageing mechanism of microplastics under different environmental conditions and its effect on the distribution of organic pollutants. Water Res. 2020, 174, 115634. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Wang, K.; Wang, K.; Kou, Y.; Wang, T.; Guo, C.; Wang, W.; Wang, J. Adsorption of Diclofenac Sodium by Aged Degradable and Non-Degradable Microplastics: Environmental Effects, Adsorption Mechanisms. Toxics 2023, 11, 24. https://doi.org/10.3390/toxics11010024
Liang S, Wang K, Wang K, Kou Y, Wang T, Guo C, Wang W, Wang J. Adsorption of Diclofenac Sodium by Aged Degradable and Non-Degradable Microplastics: Environmental Effects, Adsorption Mechanisms. Toxics. 2023; 11(1):24. https://doi.org/10.3390/toxics11010024
Chicago/Turabian StyleLiang, Siqi, Kangkang Wang, Kefu Wang, Yuli Kou, Tao Wang, Changyan Guo, Wei Wang, and Jide Wang. 2023. "Adsorption of Diclofenac Sodium by Aged Degradable and Non-Degradable Microplastics: Environmental Effects, Adsorption Mechanisms" Toxics 11, no. 1: 24. https://doi.org/10.3390/toxics11010024
APA StyleLiang, S., Wang, K., Wang, K., Kou, Y., Wang, T., Guo, C., Wang, W., & Wang, J. (2023). Adsorption of Diclofenac Sodium by Aged Degradable and Non-Degradable Microplastics: Environmental Effects, Adsorption Mechanisms. Toxics, 11(1), 24. https://doi.org/10.3390/toxics11010024




