1. Introduction
Nitrous oxide (N
2O) was initially used in medicine for its analgesic action and in industry as a propellant or an oxidant. In the last few few years, there has been an increase in the recreational consumption of N
2O by inhalation in the form of cartridges or cylinders via balloons which allow the gas to be heated. However, chronic N
2O consumption has dramatically increased in Europe [
1], and it can lead to neurological symptoms which can be sensory disorders such as paresthesia or motor disorders such as walking disorders [
2]. Although the neurological symptoms can be more severe with the development of a disabling motor handicap or combined subacute degeneration of the spinal cord, psychological disorders and thrombotic events have been reported [
1,
2,
3].
The literature links neurological toxicity to a functional inactivation of vitamin B12 by oxidation of its cobalt ion [
4,
5]. This oxidation prevents the vitamin B12 from acting as a cofactor for the methionine synthase [
4], which transform homocysteine into methionine. Vitamin B12 is also the cofactor of methylmalonyl-CoA mutase, but the impact of N
2O on this enzyme remains to be discussed [
5]. The methionine is involved in the formation of myelin. Thus, demyelination related to methionine deficiency could be responsible for the neurological disorders associated with N
2O consumption, including the combined sclerosis of the spinal cord. Indeed, a study on fruit bats exposed to N
2O for several weeks showed that bats supplemented with methionine showed a reduce number of neurological disorders after their exposure, which is contrary to the other groups of bats (without methionine supplementation) [
6]. Thus, this study showed the protective effect of methionine on the development of neurological disorders. Methionine supplementation could therefore help in the recovery of patients with an associated deficiency. However, no data in the literature have shown a strong link between the clinical severity of nitrous oxide intoxication and the severity of the associated clinical signs.
Hence, here, we aimed to study the correlation between the plasma methionine levels and clinical severity observed in N2O users. Indeed, the existence of a correlation between methionine and clinical severity could explain the pathophysiology of neurological disorders during nitrous oxide intoxication, and it could also be a therapeutic option to cure the neurological symptoms in a context of nitrous oxide consumption.
2. Materials and Methods
2.1. Patients Selection and Data Collection
We retrospectively collected clinical and biological data from patients who declared recent, chronic N
2O consumption (less than two weeks prior). The patients who consumed N
2O who were treated in Lille University Hospital between 2020 and 2022 were included in the study (declaration number: DEC21-356). In line with the regulations set out by the French National Data Protection Commission and international recommendations [
7], written informed consent was not required for this non-interventional retrospective study. All of the patients underwent both clinical and biological examinations, notably, measurements of their plasma amino acid levels as part of their routine care. Therefore, no additional tubes were collected for this study. The clinical evaluation included the Peripheral Neuropathy Disability score which was evaluated by a neurologist. The collected data (extracted from Lille University Hospital database) were clinical symptoms related to N
2O consumption and the biological results obtained at the day of consultation or at the first day of hospitalization.
2.2. Rationale of Study Group Composition
To unequivocally study the relation between the plasma methionine level and clinical severity, the following approach was used. The patients were divided into 4 groups based on the severity of their clinical symptoms (based on Peripheral Neuropathy Disability (PND score)): level 0: no symptoms, level 1: patients with distal sensory disorders without gait disorders, level 2: patients with walking disorders, but they could walk without help, and level 3: patients with walking disorders who needed help or bedridden patients.
2.3. Amino Acids Measurement
The plasma amino acids measurements, including methionine, were performed systematically. The blood samples were collected in heparin tubes. The plasma was aliquoted and then frozen and stored at −20 °C until the analysis. The amino acids measurement was performed as described previously [
8]. Briefly, proteins are precipitated by sulfosalicylic acid, and the internal standard is added. The amino acids were measured by high performance liquid chromatography on UFLC XR (Shimadzu, Kyoto, Japan) associated with tandem mass spectrometry using MRM on API 3200 Q TRAP (AB SCIEX, Framingham, MA, USA).
2.4. Homocysteine Measurement
A homocysteine measurement was performed systematically. The blood samples were collected in EDTA tubes. The plasma was aliquoted and then frozen and stored at −20 °C until the analysis. Homocysteine measurement was performed as described previously [
9]. Briefly, dithiothreitol was used to release homocysteine from plasma proteins. Then, the proteins were precipitated by acetonitrile in acidic environment, and the internal standard, homocysteine d4, was added. Homocysteine was measured by high performance liquid chromatography on UFLC XR (Shimadzu, Kyoto, Japan) associated with tandem mass spectrometry using MRM on API 3200 Q TRAP (AB SCIEX, Framingham, MA, USA). A plasma homocysteine increase was defined as >15 µmol/L.
2.5. Statistical Analysis
The values are expressed as mean ± SD, mean ± SEM, or median ± IQR, as indicated in the figure legends. Statistical differences in the clinical and biological parameters were assessed using the Mann–Whitney U or Student t test for continuous variables. Values of p < 0.05 were considered to be statistically significant.
3. Results
3.1. Patients Characteristics
The clinical and biological data of the patients are showed in
Table 1. Overall, ninety-three patients were divided over the four groups of neurological severity as follows: level 0: forty-four patients (five women and thirty-nine men), level 1: sixteen patients (three women and thirteen men), level 2: twenty-seven patients (eight women and nineteen men), and level 3: six patients (two women and four men). Thus, we notice a male predominance in all of the groups, and globally, a young age, with a mean age ranging from 20 to 26 years depending on the groups.
3.2. Decrease in Plasma Methionine Is Associated to Clinical Severity in Case of N2O Consumption
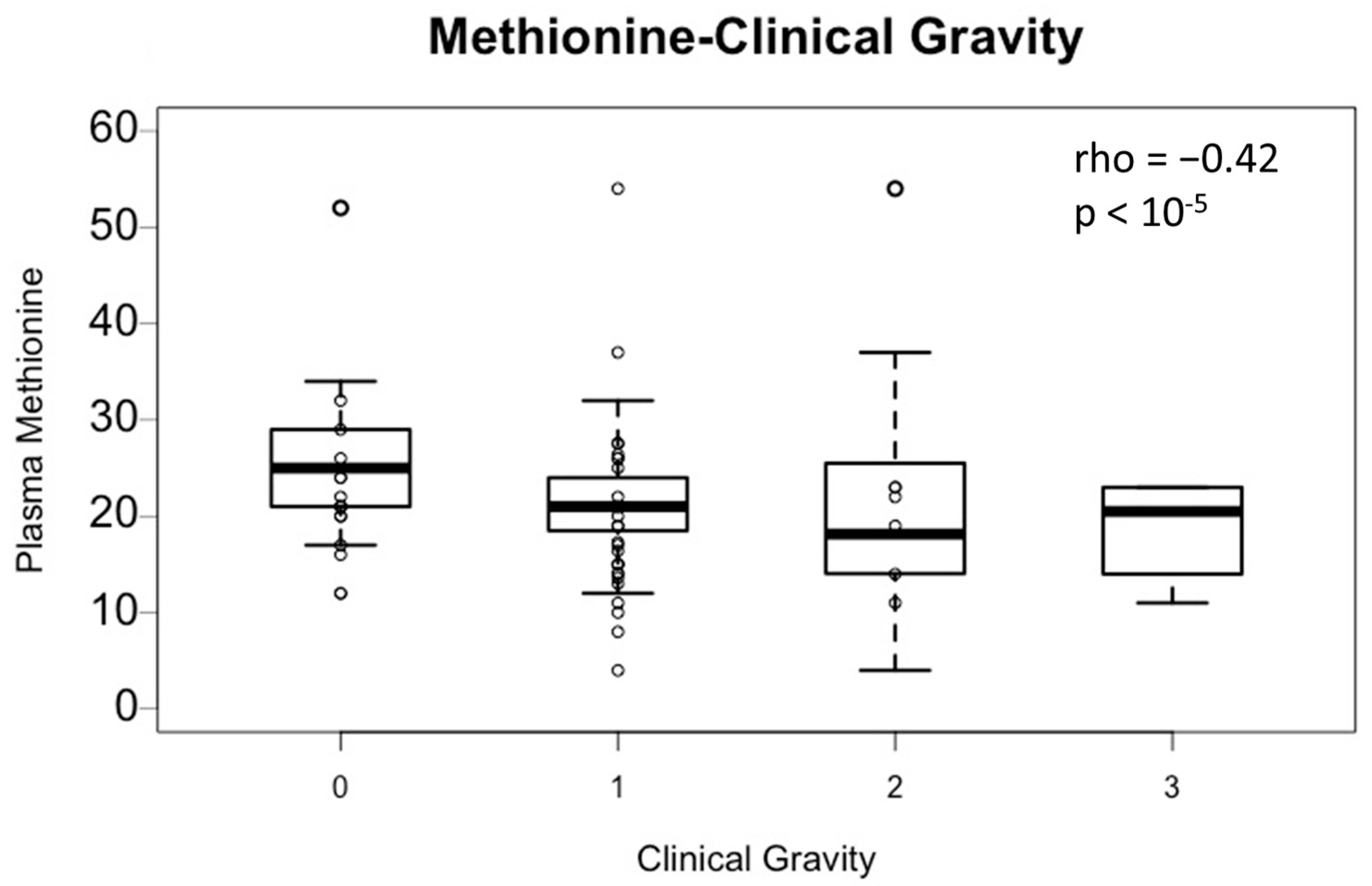
The association between plasma methionine and clinical severity (levels 0, 1, 2, and 3) was assessed (
Figure 1). Plasma methionine is significantly correlated with the clinical severity (Spearman coefficient: −0.42;
p-value < 10
−5). Thus, plasma methionine decreases as the clinical severity increases (group 0: mean = 25.5 ± 6.3 μmol/L, group 1: mean = 21.1 ± 5.4 μmol/L, group 2: mean = 20.0 ± 9.9 μmol/L, and group 3: mean = 18.7 ± 5.1 μmol/L). However, the average methionine level in the four groups is within physiological values (N: 16–23 µmol/L), so there is no quantitative methionine deficiency observed here.
3.3. Decrease in Plasma Methionine in Case of N2O Consumption Is Related to Homocysteine-Methionine Transformation
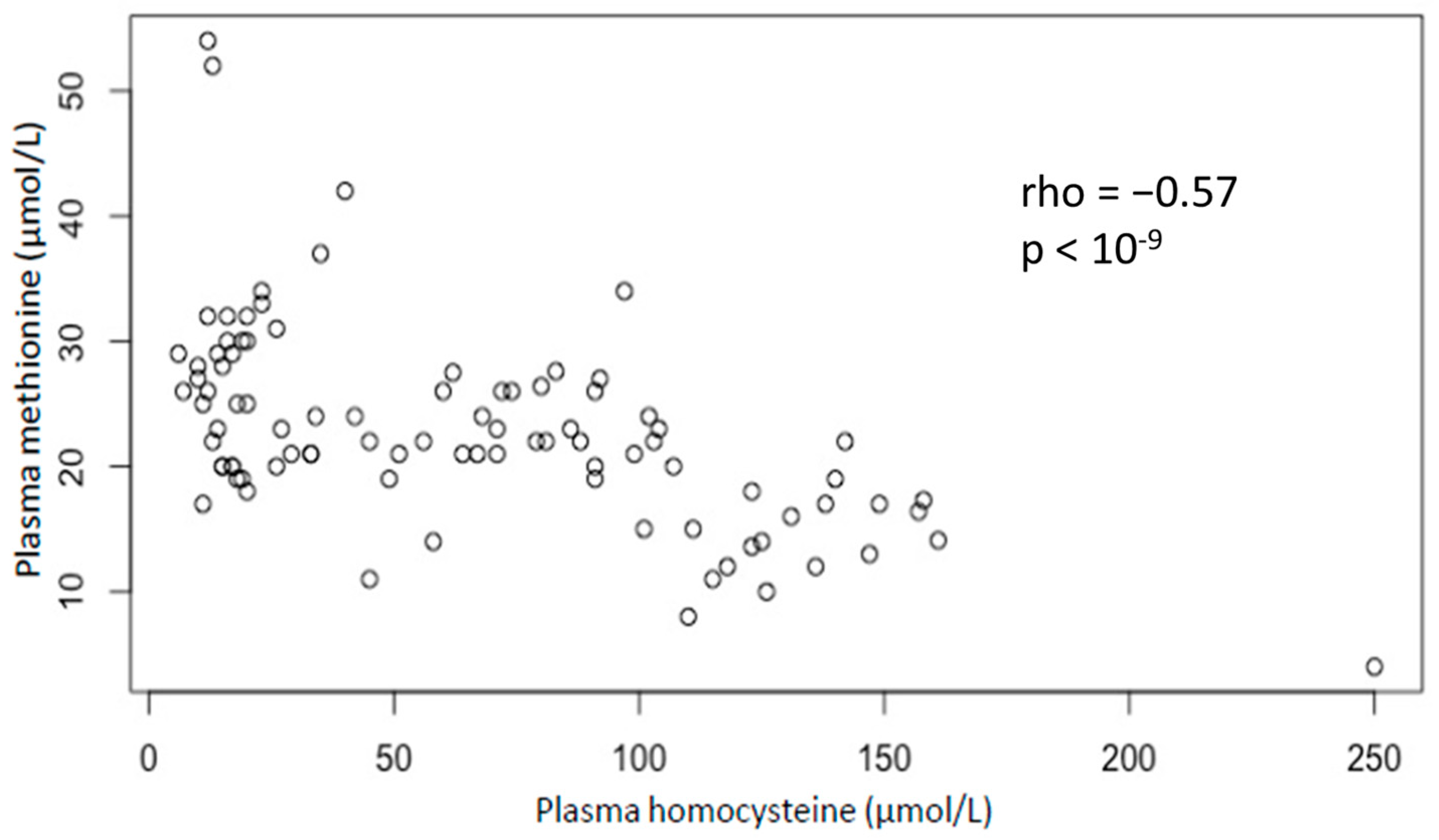
The association between plasma methionine and homocysteine was assessed (
Figure 2). There is a significant inverse correlation between plasma methionine and homocysteine (Spearman coefficient: −0.57;
p-value < 10
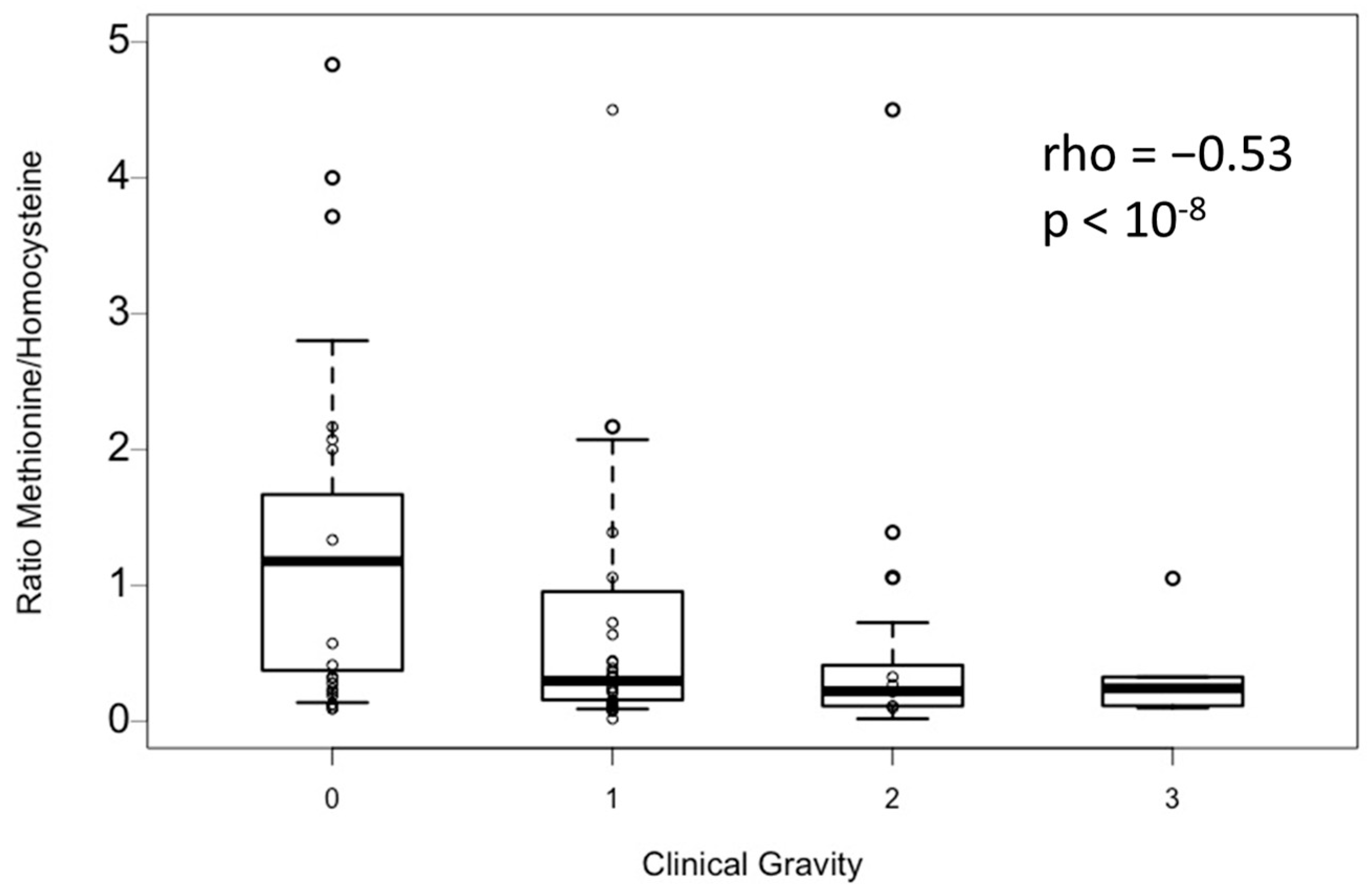
−9). In addition, the methionine-to-homocysteine ratio is significantly correlated with clinical severity homocysteine (Spearman coefficient: −0.53;
p-value < 10
−8) (
Figure 3).
Hence, the decrease in plasma methionine during nitrous oxide intoxication seems to be linked to the decrease in methionine synthase activity, which confirms the action of nitrous oxide on this enzyme.
4. Discussion
Our study is the first to show, statistically, in a human cohort, the decrease in plasma methionine related to clinical severity in patients who chronically consume nitrous oxide. The main limitation of our study is that only a few patients are in the level 3 of clinical severity; indeed, a larger number of patients would have been more appropriate for the interpretation of the results. The decrease in plasma methionine could be used also as a blood marker, but also, on the other hand, it can be used to understand the underlying mechanisms.
Our study is consistent with the data in the literature, where the patients were administered nitrous oxide and therefore showed a rapid decrease in plasma methionine, which recovered after they stopped consuming it [
10].
The same phenomenon was observed for plasma homocysteine, and it correlates perfectly with the methionine levels found. Indeed, the homocysteine/methionine ratio also correlates with clinical severity, which is consistent with a remethylation disorder and a disruption of one-carbon metabolism [
11]. These changes in one-carbon metabolism have clinical consequences related to changes in many essential metabolic processes [
12,
13]. Hence, these metabolic changes could explain the severity of the neurological signs associated with demyelination.
However, the decrease in plasma methionine cannot be imputed as the only mechanism involved in the pathophysiology of the neurological disorders in nitrous oxide consumption. Indeed, even if our study shows an inverse correlation between plasma methionine levels and clinical severity, there is no quantitative methionine deficiency observed here. Hence, the inhibition of methionine synthase, which leads to a plasma methionine decrease, seems to play a role in the neurological severity during nitrous oxide intoxication, but it is probably not the only factor involved in the occurrence of these disorders. On the other hand, there are no reports in the literature of such severe and rapid onset clinical signs in patients with methionine or cobalamin deficiency. Additional factors, such as oxidative stress [
14] or changes in transmethylation [
11], must explain the pathophysiology, but this requires further studies for it to be understood.
In addition, there are few therapeutic indications for the use methionine. Indeed, it is used as a dietary supplement especially in hair loss. Additionally, methionine is currently used in a clinical trial as a treatment of Pulmonary Alveolar Proteinosis with MARS (methionyl-tRNA synthetase) mutation [
15]. Thus, there is not much information concerning its toxicity. However, methionine seems to be hepatotoxic [
16] and potentially neurotoxic in case of the over-dosage of it. Therefore, we should be careful concerning the potential use of methionine in nitrous oxide consumption, which needs further clinical investigation. Moreover, other pathophysiological mechanisms probably need to be identified in order to find potential therapeutic targets.
5. Conclusions
Our results showed that there is an association between plasma methionine and clinical severity of nitrous oxide consumption. Our results also showed that this decrease in methionine is related to a disturbance of homocysteine metabolism. However, the inhibition of the methionine synthase cannot be attributed as the only pathophysiological mechanism involved in the occurrence of neurological disorders in N2O consumers because there is no quantitative deficit in plasma methionine observed here. Thus, other pathophysiological mechanisms must be explored.
Author Contributions
Conceptualization, G.G. and E.G.; methodology, G.G. and E.G.; software, G.G. and E.G.; validation, G.G., E.G., C.D., S.D., M.J. and C.T.; formal analysis, G.G. and E.G.; investigation G.G. and E.G.; resources, G.G. and E.G.; data curation G.G. and E.G.; writing—original draft preparation, G.G. and E.G.; writing—review and editing, G.G. and E.G.; visualization, G.G. and E.G.; supervision, G.G.; project administration, G.G.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Monitoring Centre for Drugs and Drug Addiction. Recreational Use of Nitrous Oxide: A Growing Concern for Europe; EMCDDA: Lisbon, Portugal, 2022. [Google Scholar]
- Garakani, A.; Jaffe, R.J.; Savla, D.; Welch, A.K.; Protin, C.A.; Bryson, E.O.; McDowell, D.M. Neurologic, Psychiatric, and Other Medical Manifestations of Nitrous Oxide Abuse: A Systematic Review of the Case Literature. Am. J. Addict. 2016, 25, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Vollenbrock, S.E.; Fokkema, T.M.; Leijdekkers, V.J.; Vahl, A.C.; Konings, R.; van Nieuwenhuizen, R.C. Nitrous Oxide Abuse Associated with Severe Thromboembolic Complications. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 656–657. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Osborne, M.L.; Kolhouse, J.F.; Binder, M.J.; Podell, E.R.; Utley, C.S.; Abrams, R.S.; Allen, R.H. Nitrous Oxide Has Multiple Deleterious Effects on Cobalamin Metabolism and Causes Decreases in Activities of Both Mammalian Cobalamin-Dependent Enzymes in Rats. J. Clin. Investig. 1981, 67, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Grzych, G.; Gernez, E.; Deheul, S.; Kim, I. Methylmalonic Acid: Specific Marker of Chronic Nitrous Oxide Abuse? La Rev. Médecine Interne 2022, 43, 197–198. [Google Scholar] [CrossRef] [PubMed]
- van der Westhuyzen, J.; Fernandes-Costa, F.; Metz, J. Cobalamin Inactivation by Nitrous Oxide Produces Severe Neurological Impairment in Fruit Bats: Protection by Methionine and Aggravation by Folates. Life Sci. 1982, 31, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Borovecki, A.; Borovecki, A.; Mlinaric, A.; Mlinaric, A.; Horvat, M.; Horvat, M.; Smolcic, V.S.; Smolcic, V.S.; Smolcic, V.S. Informed Consent and Ethics Committee Approval in Laboratory Medicine. Biochem. Medica 2018, 28, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Filee, R.; Schoos, R.; Boemer, F. Evaluation of Physiological Amino Acids Profiling by Tandem Mass Spectrometry. JIMD Rep. 2013, 13, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Grzych, G.; Douillard, C.; Lannoy, J.; Joncquel Chevalier Curt, M. Very High Plasma Homocysteine without Malnutrition or Inherited Disorder. Clin. Chem. 2020, 66, 1468–1469. [Google Scholar] [CrossRef] [PubMed]
- Ermens, A.A.M.; Refsum, H.; Rupreht, J.; Spijkers, L.J.M.; Guttormsen, A.B.; Lindemans, J.; Ueland, P.M.; Abels, J. Monitoring Cobalamin Inactivation during Nitrous Oxide Anesthesia by Determination of Homocysteine and Folate in Plasma and Urine. Clin. Pharmacol. Ther. 1991, 49, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Burgess, K.; Bennett, C.; Mosnier, H.; Kwatra, N.; Bethel, F.; Jadavji, N.M. The Antioxidant Role of One-Carbon Metabolism on Stroke. Antioxidants 2020, 9, 1141. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Rodriguez-Guéant, R.-M.; Oussalah, A.; Jeannesson, E.; Wahl, D.; Ziuly, S.; Guéant, J.-L. Cardiovascular Manifestations of Intermediate and Major Hyperhomocysteinemia Due to Vitamin B12 and Folate Deficiency and/or Inherited Disorders of One-Carbon Metabolism: A 3.5-Year Retrospective Cross-Sectional Study of Consecutive Patients. Am. J. Clin. Nutr. 2021, 113, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.G.; Ossani, G.; Monserrat, A.J.; Boveris, A. Oxidative Damage: The Biochemical Mechanism of Cellular Injury and Necrosis in Choline Deficiency. Exp. Mol. Pathol. 2010, 88, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Comisso, M.; Hadchouel, A.; de Blic, J.; Mirande, M. Mutations in MARS Identified in a Specific Type of Pulmonary Alveolar Proteinosis Alter Methionyl-TRNA Synthetase Activity. FEBS J. 2018, 285, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.; Caro, P.; Sanchez, I.; Naudi, A.; Jove, M.; Portero-Otin, M.; Lopez-Torres, M.; Pamplona, R.; Barja, G. Effect of Methionine Dietary Supplementation on Mitochondrial Oxygen Radical Generation and Oxidative DNA Damage in Rat Liver and Heart. J. Bioenerg. Biomembr. 2009, 41, 309–321. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).