1. Introduction
Herbal remedies have been recognized as powerful medicines and also effective methods for preserving and balancing human health since the ancient history of healthcare worldwide [
1,
2]. Triphala (TPL) is primarily based on the Sanskrit language, in which “Tri” means three and “Phala” means fruits. The TPL formula contains equal amounts of three dried fruits. These three different medicinal plants include
Emblica officinalis (Family Euphorbiaceae),
Terminalia bellirica (Family Combretaceae), and
Terminalia chebula (Family Combretaceae) [
3]. Recent reports indicate that TPL activates the immune system and enhances health and longevity [
4]. It has been shown that Gallic acid and ascorbic acid are the most prevalent in TPL, suggesting an abundance of anti-oxidative effects. Accordingly, TPL can prevent radiation-induced oxidative damage [
5,
6,
7]. Nowadays, the TPL formula is commonly used in many countries to treat various chronic illnesses such as anemia, asthma, and recurrent ulcers as a part of complementary and alternative medicines [
5,
6,
7]. In addition to the anti-oxidant, TPL also has a protective effect against human pancreatic cancer cells in both in vitro and in vivo models [
8].
Regardless of TPL efficiency, the safety of the continuous use of TPL is concerning. There are limited data about the long-term welfare of chronic TPL users. However, neither morbidity nor mortality is reported when a daily dose of 2,000 mg of TPL is administered for 4 weeks, in order to evaluate its effect on gut microbiota [
9]. The in vitro antimutagenic activity was studied as well, and the outcome indicated that the TPL formula is safe [
5,
10]. The aqueous and alcoholic extracts of TPL that dose up to 1750 mg/kg reveal no signs of acute oral toxicity.
In general, toxicology tests are an important part of pharmacological research and drug development. A major purpose of testing the toxicities in animals is to establish the drug’s safety prior to clinical use in humans [
11,
12,
13]. Usually, acute toxicity tests are conducted within the first 24 hours after a high dose of drug administration, in order to investigate any harmful effects that occur in the tested animals. Although some drugs do not exhibit major adverse effects in the course of acute toxicity testing, they may cause subacute or chronic toxicity, especially when the drugs accumulate in the internal organs at high concentrations [
12,
14,
15,
16]. Hence, chronic toxicity tests are always necessary to perform to evaluate the physiological, biochemical, hematological, and pathological safety of long-term drug use [
17]. The purpose of this study was to investigate both acute and chronic toxicities of the aqueous extract of Triphala in Sprague-Dawley rats. It was the aim of this study to demonstrate the safety of TPL in animals, serving as a primary step to support the long-term clinical use of TPL in the future.
4. Discussion
Herbal remedies from traditional medicinal plants are commonly used in the healthcare systems of developing and underdeveloped countries [
24,
25]. Some people use herbal remedies improperly and carelessly because they believe that all herbal medicines are safe since the remedies are derived from natural sources. Despite the fact that herbal medicines are widely used by healthcare personnel worldwide, there is still a lack of scientific data regarding the toxicities and side effects of certain herbal recipes [
26]. According to previous studies, several medicinal plants can lead to a variety of illnesses, organ toxicities, morbidity, and mortality in humans [
27]. In order to assure the safety of herbal products for human consumption, toxicity tests in animals and systematic studies are required to review the acute toxicities and define the safe dosage [
28].
In Ayurveda, a traditional system of medicine, Triphala is a powdered mixture of three fruits,
E. officinalis,
T. bellirica, and
T. chebula, and is used to treat numerous illnesses [
3,
29]. Each ingredient in Triphala was proven to be safe by acute and/or subacute toxicity testing in animals. For example, the methanolic extract of
E. officinalis fruit in Swiss albino mice [
30] and
T. chebula hydroalcoholic extracts in BALB/c mice [
31]. Furthermore, the aqueous extract from the dried fruits of
T. bellirica did not cause chronic toxicity in either female or male rats [
32]. In this study, we qualify herbs by implementing a control approach established by the Thai Herbal Pharmacopoeia (THP) [
33]. Gallic acid and ellagic acid are the most common compounds found in Triphala that are used as standards [
34]. However, the active compounds within plants might differ based on a variety of factors, such as the planting location and the cultivar [
35]. According to the OECD 425 guideline, the short-term use of TPL is highly recommended because there have never been reports of toxic signs from the short-term use of this recipe [
36]. In addition, data suggesting the safety of the long-term use of TPL is insufficient.
Before the study of the efficacy of herbal products in clinical trials, the testing of acute and chronic toxicities in animals is usually a primary step to determine the appropriate dose and duration of product administration [
37]. Considering the dose of Triphala for chronic toxicity tests in rats, the 1200 mg/kg concentration of Triphala was selected as an ideal concentration in this study because it is the dosage at which the anti-inflammatory and anti-nociceptive effects were shown to be effective in a previous study [
38]. The doses of 600 mg/kg and 2400 mg/kg were selected as the lowest and highest concentrations to investigate the chronic toxicity of TPL. Based on this research, an acute toxicity test and a 270-day chronic toxicity study were performed on experimental animals to assess the safety profile of an aqueous solution extract of Triphala.
The acute oral toxicity test was used to evaluate the adverse effects following a large dose of drug administration within 24 hours. The use of a single oral administration of TPL at 5000 mg/kg was selected in this study because 2000 mg/kg was preliminarily tested and did not cause any symptoms of toxicity or death. The results demonstrated that a high dose of the aqueous solution of TPL did not result in death or severe organ toxicity. Although a slight difference in body weight between the control and treatment groups was observed, this finding was not statistically significant (
Table 1). The internal organs, including the brain, lungs, heart, liver, kidney, spleen, stomach, duodenum, small intestine, and sex organs were weighed, gross, and thoroughly microscopically examined, revealing further that the TPL did not induce tissue damage. Therefore, a single oral dose of TPL was not toxic to rats in terms of body weight or internal organs, with a decrease in sex organs. Testicular weight varies with age in rodents—this is dependent on species, strain, nutrition, and living conditions [
39], and the increased testicular weights are related to the growth of testicular cells, with a response in total sperm production rate and seminiferous tube size [
40,
41]. In the case of chronic toxicity, however, the parameter related to reproductive organs should be carefully evaluated. In addition, further research on the reproductive toxicity of Triphala in rats is considered necessary.
Chronic toxicity tests are carried out for 9 to 12 months and investigate whether repetitive exposure to substances can cause toxic signs that might not exhibit toxicity immediately. According to WHO guidelines, the duration of substance administration in animal testing should be based on the estimated duration of clinical use in humans [
37]. Changes in general behaviors and body weight are one of the most important indicators for the onset of substance-induced toxicity. These changes include the signs that animals continue losing their body weight so that they may be unable to survive [
32,
42,
43]. From the results of this study, with the chronic administration of TPL for 270 days, the mean body weights of both male and female rats showed no significant change between the control and TPL-treated groups. It is now confirmed that TPL is safe for long-term use in rats.
Alterations in internal organ weight have long been recognized as a sensitive marker of drug-induced organ changes. In toxicological experiments, organ weights are compared between the treated and untreated animals [
44]. From the results, the assessment of the gross pathology of the TPL-treated groups revealed no significant deformity, and the findings were comparable to those of the control group (
Table 3 and
Table 4). There were some minor statistically significant changes in the TPL-treated animals, but these values still remained within the normal range [
45,
46]. In chronic toxicity studies with an increase or decrease in internal organ weight, this may be attributed to the changed body weight of the individual rats, which causes their internal organs to be different from the control [
47]. Based on the principles and procedures of OECD Test Guideline 452, we conclude in this study that TPL is non-toxic. However, hematology, blood chemistries, and histopathological examinations on female rats (heart, liver, spleen, adrenal gland, and kidney) and male rats (liver, pancreas, kidney, testis, and epididymis) should be considered intently.
To determine direct cellular damage to the internal organs, blood chemistry and hematology are applied to look for tissue injury or stress responses. Blood transports numerous nutrients and foreign substances in the body, therefore it is a sensitive target for toxic compounds and an important index of physiological and pathological states [
48,
49]. As a result, blood components such as red blood cells, white blood cells, platelets, and hemoglobin will be firstly impaired by the toxic substance [
50]. According to the hematological and other parameters, regional characteristics and local environmental factors may influence blood profile variations, including temperature [
51,
52]. In chronic toxicity, hematological parameters varied greatly between extracts, doses, and genders, with the predominant alteration being a decrease in RBC, Hb, and HCT levels, along with a decrease in the MCV index. This clinical condition was observed in males treated with Triphala 2400 mg/kg and satellite groups. However, these alterations lacked a dose-response correlation and may be the consequence of individual variation within the normal range [
45,
53,
54]. Moreover, TPL caused leukopenia, especially in neutrophils. In a previous study, TPL could stimulate neutrophil function without increasing the number of neutrophils in mice [
55]. Furthermore, TPL significantly increased in lymphocytes, including cytotoxic T and B lymphocytes [
56], which resigned results consistent with this study, but the effect was seen only in male rats.
A clinical blood chemistry analysis was conducted to quantify any toxic effects on pancreatic function (glucose), liver function (AST, ALT, ALP, total protein, albumin, and bilirubin), and renal function (BUN and creatinine). In general, rats with serum glucose exceeding 200 mg/dL are classified as “hyperglycemic” [
54,
57]. Thus, the significantly higher blood sugar level observed in the TPL-treated male rats is still within normal ranges, suggesting no destruction of pancreatic islets. Additionally, TPL was not directly toxic or the cause of hepatotoxin-induced toxicity in the liver, as shown by the absence of alteration in AST, ALT, and ALP levels [
58,
59]. Toxic substances can directly access the kidneys because of the high volume of blood that flows through the organ. The renal tubules could become obstructed with a wide variety of toxins. Among the most reliable indications of renal disease are alterations in creatinine and BUN levels [
60]. In this study, BUN (in females and males) and creatinine (in females) were significantly changed. However, these values changed marginally and remained within the normal range [
45,
46,
61,
62,
63]. Therefore, Triphala was not toxic to the kidneys. In addition, total protein levels in the blood are a preliminary indicator of normal protein status in the normally functioning kidney and liver [
64]. Total protein in male rats was slightly decreased but remained within the normal range. The incidence of delayed toxic effects or the possibility of reverse effects was assessed additionally in the satellite group [
23,
65]. Triphala was given to the rats in the satellite group at a dose of 2400 mg/kg/day for 270 days and the rats were kept alive for 28 more days. Significant increases in glucose and BUN levels were detected, but no hazardous symptoms were found. Interestingly, the elevated level of BUN and creatinine in female rats was within a normal range. Taken together, this recipe is safe for rats according to the chronic toxicity test in satellite rats. These blood chemistry results suggested that the histopathological examination of females (kidney), males (pancreas and kidney), and the male satellite group (pancreas and kidney) should be carefully assessed. From the results of the chronic toxicity tests, including general behaviors, body, internal organ weight, gross pathology, hematology, and blood chemistries, it could be concluded that TPL had no toxic effect in rats. However, the histopathological examination should be further examined to confirm the toxicity of TPL.
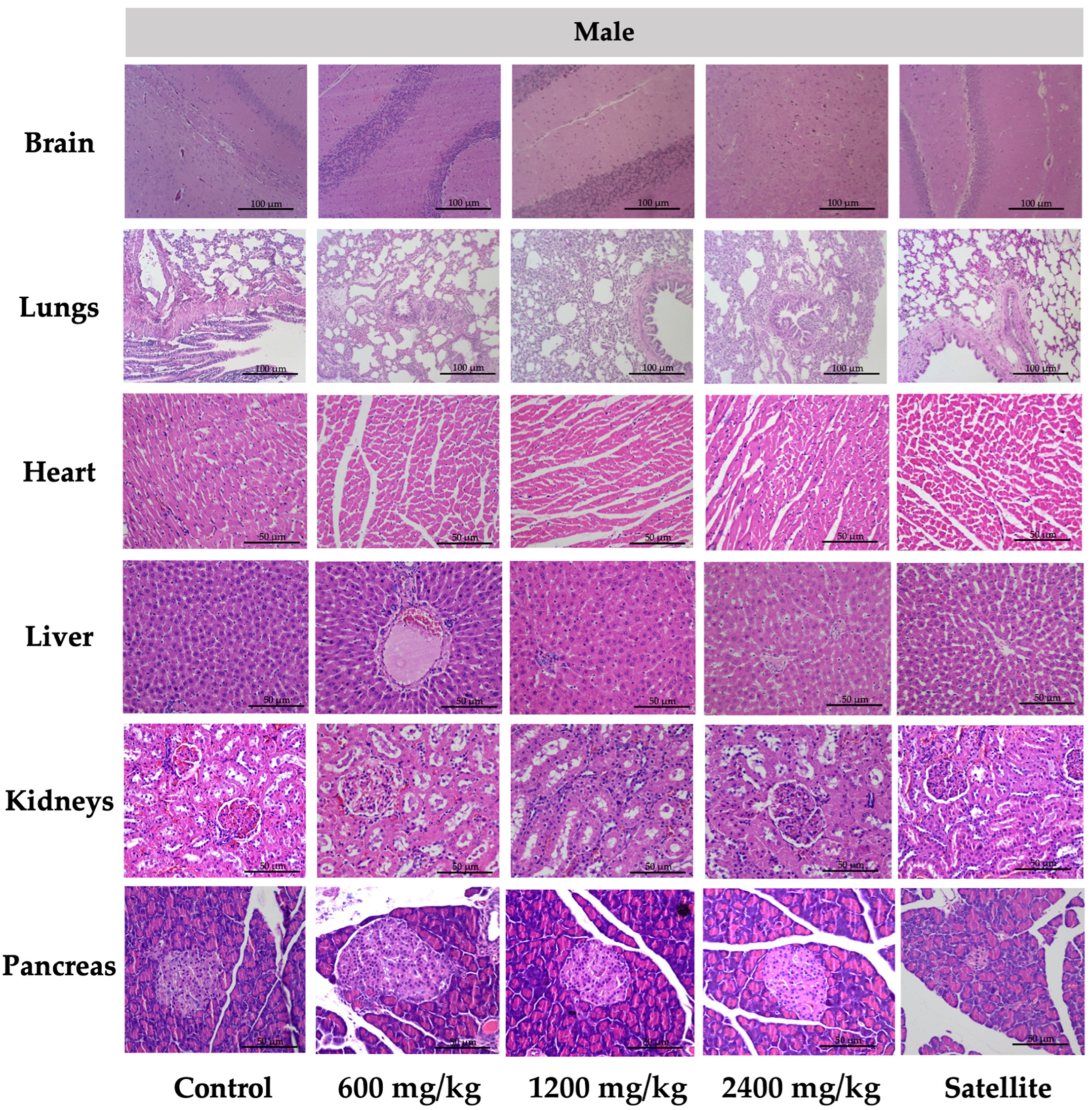
Histopathological examinations of internal organs were conducted on all animals to confirm the safety of TPL. There was no indication of toxicity and no histological abnormalities found in any of the internal organs of the rats that were treated with TPL, especially female rats (heart, liver, spleen, adrenal gland, and kidney), male rats (liver, pancreas, kidney, testis, and epididymis), and the male satellite group (pancreas and kidney).
Figure 2 and
Figure 3 represent the vital internal organs. From this study, the rats’ dose of Triphala at 2400 mg/kg could be calculated to be a human dose of approximately 23,000 mg/60 kg body weight [
66]. Consequently, the chronic toxicity test of TPL was considered safe.