The Use of Human Biomonitoring to Assess Occupational Exposure to PAHs in Europe: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy for Papers Concerning HBM of Occupational PAHs Exposure
2.2. Quality Scoring of the Articles Retrieved
3. Results
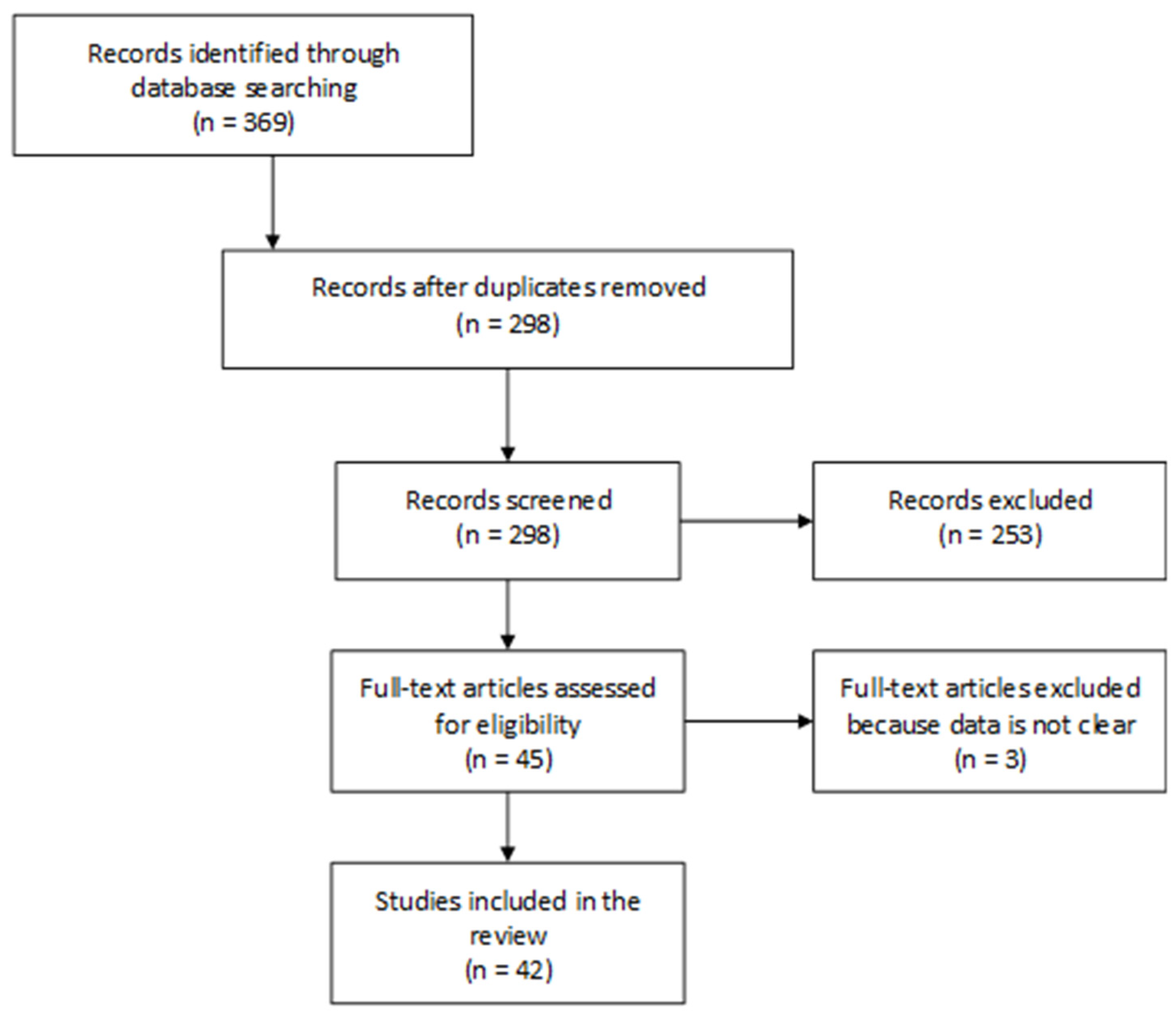
3.1. Literature Search and LaKind Scoring
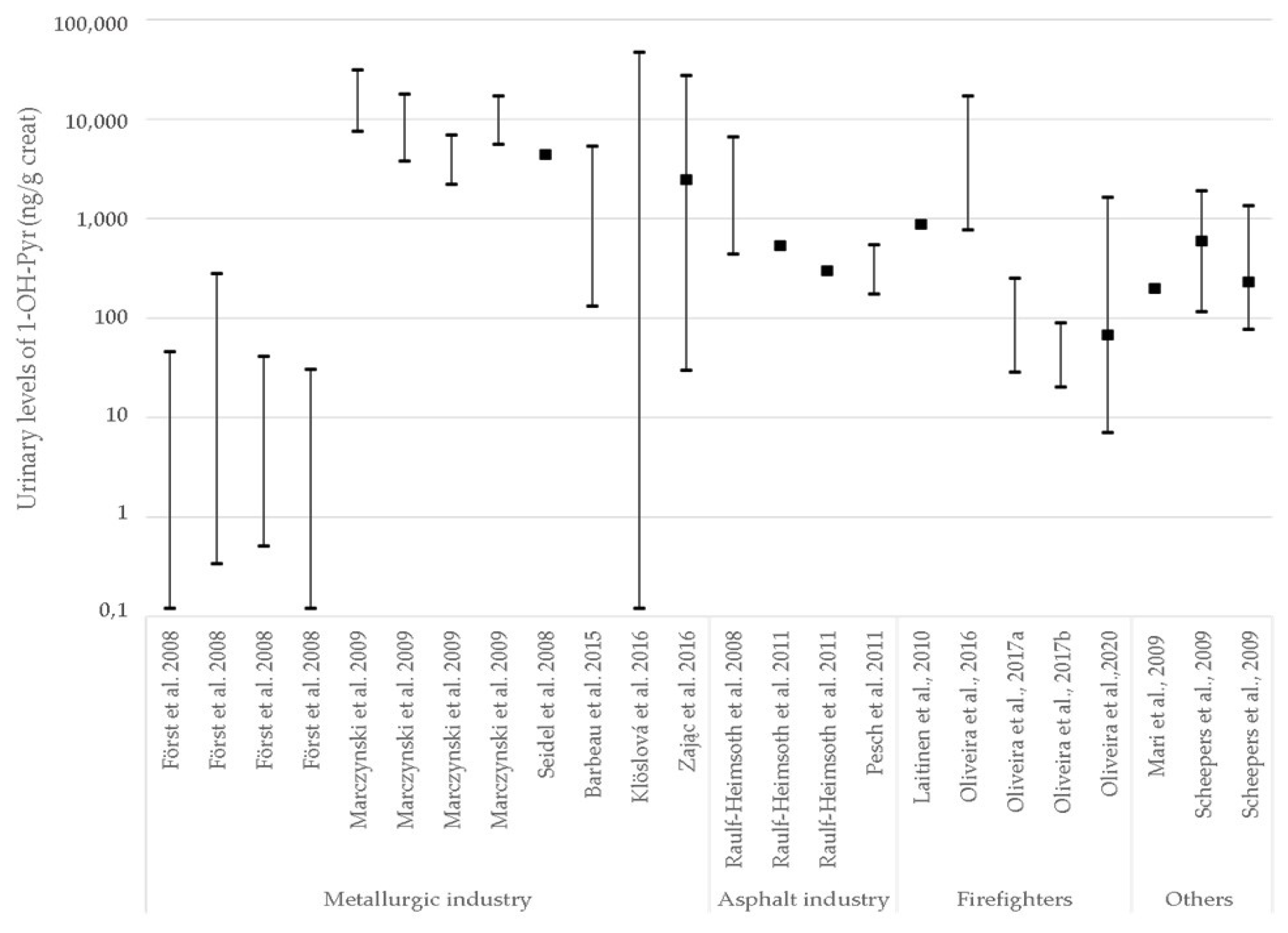
3.2. Exposure Biomarkers and Occupational Exposure (Limit) Values
3.3. Effect Biomarkers
4. Discussion
4.1. Strengths and Limitations of the Strategies Used for HBM of PAHs
4.2. HBM Added Value, Gaps and Needs for New Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Samburova, V.; Zielinska, B.; Khlystov, A. Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity? Toxics 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Cincinelli, A.; Martellini, T.; Malik, R.N. A review of PAH exposure from the combustion of biomass fuel and their less surveyed effect on the blood parameters. Environ. Sci. Pollut. Res. 2015, 22, 4076–4098. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Polycyclic aromatic hydrocarbons, carcinogenic (PAH) [MAK Value Documentation, 2012]. In The MAK-Collection for Occupational Health and Safety; Wiley-VCH: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Jongeneelen, F.J. A guidance value of 1-hydroxypyrene in urine in view of acceptable occupational exposure to polycyclic aromatic hydrocarbons. Toxicol. Lett. 2014, 231, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.G.; Saber, A.T.; Pedersen, J.E.; Pedersen, P.B.; Clausen, P.A.; Løhr, M.; Kermanizadeh, A.; Loft, S.; Ebbehøj, N.E.; Hansen, M.; et al. Assessment of polycyclic aromatic hydrocarbon exposure, lung function, systemic inflammation, and genotoxicity in peripheral blood mononuclear cells from firefighters before and after a work shift. Environ. Mol. Mutagen. 2018, 59, 539–548. [Google Scholar] [CrossRef]
- Brzeźnicki, S.; Jakubowski, M.; Czerski, B. Elimination of 1-hydroxypyrene after human volunteer exposure to polycyclic aromatic hydrocarbons. Environ. Mol. Mutagen. 1997, 70, 257–260. [Google Scholar] [CrossRef]
- Elovaara, E.; Heikkila, P.; Pyy, L.; Mutanen, P.; Riihimaki, V. Significance of dermal and respiratory uptake in creosote workers: Exposure to polycyclic aromatic hydrocarbons and urinary excretion of 1-hydroxypyrene. Occup. Environ. Med. 1995, 52, 196–203. [Google Scholar] [CrossRef]
- VanRooij, J.G.; Bodelier-Bade, M.M.; Jongeneelen, F.J. Estimation of individual dermal and respiratory uptake of polycyclic aromatic hydrocarbons in 12 coke oven workers. Occup. Environ. Med. 1993, 50, 623–632. [Google Scholar] [CrossRef]
- Van Rooij, J.G.; Van Lieshout, E.M.; Bodelier-Bade, M.M.; Jongeneelen, F.J. Effect of the reduction of skin contamination on the internal dose of creosote workers exposed to polycyclic aromatic hydrocarbons. Scand. J. Work. Environ. Health 1993, 19, 200–207. [Google Scholar] [CrossRef]
- Mitchell, C. Distribution and retention of benzo(a)pyrene in rats after inhalation. Toxicol. Lett. 1982, 11, 35–42. [Google Scholar] [CrossRef]
- Withey, J.R.; Shedden, J.; Law, F.C.P.; Abedini, S. Distribution of benzo[a]pyrene in pregnant rats following inhalation exposure and a comparison with similar data obtained with pyrene. J. Appl. Toxicol. 1993, 13, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Metabolism of chemical carcinogens. Carcinogenesis 2000, 21, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic Aromatic Hydrocarbons: From Metabolism to Lung Cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T. Xenobiotic-Metabolizing Enzymes Involved in Activation and Detoxification of Carcinogenic Polycyclic Aromatic Hydrocarbons. Drug Metab. Pharmacokinet. 2006, 21, 257–276. [Google Scholar] [CrossRef]
- Barbeau, D.; Lutier, S.; Bonneterre, V.; Persoons, R.; Marques, M.; Herve, C.; Maitre, A. Occupational exposure to polycyclic aromatic hydrocarbons: Relations between atmospheric mixtures, urinary metabolites and sampling times. Int. Arch. Occup. Environ. Health 2015, 88, 1119–1129. [Google Scholar] [CrossRef]
- Li, Z.; Sandau, C.D.; Romanoff, L.C.; Caudill, S.P.; Sjodin, A.; Needham, L.L.; Patterson, D.G. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ. Res. 2008, 107, 320–331. [Google Scholar] [CrossRef]
- Motorykin, O.; Schrlau, J.; Jia, Y.; Harper, B.; Harris, S.; Harding, A.; Stone, D.; Kile, M.; Sudakin, D.; Simonich, S.L.M. Determination of parent and hydroxy PAHs in personal PM2.5 and urine samples collected during Native American fish smoking activities. Sci. Total Environ. 2015, 505, 694–703. [Google Scholar] [CrossRef]
- Urbancova, K.; Lankova, D.; Rossner, P.; Rossnerova, A.; Svecova, V.; Tomaniova, M.; Veleminsky, M.; Sram, R.J.; Hajslova, J.; Pulkrabova, J. Evaluation of 11 polycyclic aromatic hydrocarbon metabolites in urine of Czech mothers and newborns. Sci. Total Environ. 2016, 577, 212–219. [Google Scholar] [CrossRef]
- Klotz, K.; Weiß, T.; Zobel, M.; Bury, D.; Breuer, D.; Werner, S.; Sucker, K.; Zschiesche, W.; Göen, T.; Brüning, T.; et al. Validity of different biomonitoring parameters in human urine for the assessment of occupational exposure to naphthalene. Arch. Toxicol. 2019, 93, 2185–2195. [Google Scholar] [CrossRef]
- Granella, M.; Clonfero, E. Urinary excretion of 1-pyrenol in automotive repair workers. Int. Arch. Occup. Environ. Health 1993, 65, 241–245. [Google Scholar] [CrossRef]
- Santella, R.M.; Hemminki, K.; Tang, D.L.; Paik, M.; Ottman, R.; Young, T.L.; Savela, K.; Vodickova, L.; Dickey, C.; Whyatt, R.; et al. Polycy-clic aromatic hydrocarbon-DNA adducts in white blood cells and urinary 1-hydroxypyrene in foundry workers. Cancer Epidemiol Biomarkers Prev. 1993, 2, 59–62. [Google Scholar] [PubMed]
- Bentsen, R. The effect of dust-protective respirator mask and the relevance of work category on urinary 1-hydroxypyrene concentration in PAH exposed electrode paste plant workers. Ann. Occup. Hyg. 1998, 42, 135–144. [Google Scholar] [CrossRef]
- Jongeneelen, F.J.; Anzion, R.B.M.; Scheepers, P.T.J.; Bos, R.P.; Henderson, P.T.; Nijenhuis, E.H.; Veenstra, S.J.; Brouns, R.M.E.; Winkes, A. 1-hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments *. Ann. Occup. Hyg. 1988, 32, 35–43. [Google Scholar] [CrossRef]
- Quinlan, R.; Kowalczyk, G.; Gardiner, K.; Calvert, I.; Hale, K.; Walton, S. Polycyclic aromatic hydrocarbon exposure in coal liquefaction workers: The value of urinary 1-hydroxypyrene excretion in the development of occupational hygiene control strategies. Ann. Occup. Hyg. 1995, 39, 329–346. [Google Scholar] [CrossRef]
- Viau, C. The toxicokinetics of pyrene and its metabolites in rats. Toxicol. Lett. 1999, 108, 201–207. [Google Scholar] [CrossRef]
- Kroese, E.D.; Muller, J.J.A.; Mohn, G.R.; Dortant, P.M.; Wester, P.W. Tumorigenic Effects in Wistar Rats Orally Administered Benzo[a]pyrene for Two Years (Gavage Studies): Implications for Human Cancer Risks Associated with Oral Exposure to Polycyclic Aromatic Hydrocarbons; (658603 010); National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2001; Available online: http://www.rivm.nl/bibliotheek/rapporten/658603010.pdf (accessed on 28 March 2022).
- European Commission; Directorate-General for Employment; Social Affairs and Inclusion; Heederik, D.; Papameletiou, D.; Bolt, H.; Klein, C.L. SCOEL/REC/404 Polycyclic Aromatic Hydrocarbon Mixtures Containing Benzoapyrene (PAH): Recommendation from the from the Scientific Committee on Occupational Exposure Limits; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Alhamdow, A.; Lindh, C.; Albin, M.; Gustavsson, P.; Tinnerberg, H.; Broberg, K. Early markers of cardiovascular disease are associated with occupational exposure to polycyclic aromatic hydrocarbons. Sci. Rep. 2017, 7, 9426. [Google Scholar] [CrossRef]
- Alshaarawy, O.; Zhu, M.; Ducatman, A.; Conway, B.; Andrew, M.E. Polycyclic aromatic hydrocarbon biomarkers and serum markers of inflammation. A positive association that is more evident in men. Environ. Res. 2013, 126, 98–104. [Google Scholar] [CrossRef]
- European Commission. Scientific Committee on Food Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Iodine (Brussels). SCF/CS/NUT/GEN/18 Final. 2003. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_out171_en.pdf (accessed on 28 March 2022).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 1–556. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Chemical agents and related occupations. IARC Monogr. Eval. Carcinog Risks Hum. 2012, 100, 9–562. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Some chemicals present in industrial and consumer products, food and drinking water. IARC Monogr. Eval. Carcinog. Risks Hum. 2013, 101, 9–549. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans.Outdoor Air Pollution. IARC Monogr. Eval. Carcinog. Risks Hum. 2016, 109, 9–444. [Google Scholar]
- Lee, S.; Hong, S.; Liu, X.; Kim, C.; Jung, D.; Yim, U.H.; Shim, W.J.; Khim, J.S.; Giesy, J.P.; Choi, K. Endocrine disrupting potential of PAHs and their alkylated analogues associated with oil spills. Environ. Sci. Process. Impacts 2017, 19, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- ECHA Substance Information. Polycyclic-Aromatic Hydrocarbons (PAH). Available online: https://echa.europa.eu/fr/substance-information/-/substanceinfo/100.239.209 (accessed on 5 April 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- LaKind, J.S.; Sobus, J.R.; Goodman, M.; Barr, D.B.; Fürst, P.; Albertini, R.J.; Arbuckle, T.E.; Schoeters, G.; Tan, Y.-M.; Teeguarden, J.; et al. A proposal for assessing study quality: Biomonitoring, Environmental Epidemiology, and Short-lived Chemicals (BEES-C) instrument. Environ. Int. 2014, 73, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, J.; Mäkelä, M.; Mikkola, J.; Huttu, I. Fire fighting trainers’ exposure to carcinogenic agents in smoke diving simulators. Toxicol. Lett. 2010, 192, 61–65. [Google Scholar] [CrossRef]
- Lutier, S.; Maître, A.; Bonneterre, V.; Bicout, D.J.; Marques, M.; Persoons, R.; Barbeau, D. Urinary elimination kinetics of 3-hydroxybenzo(a)pyrene and 1-hydroxypyrene of workers in a prebake aluminum electrode production plant: Evaluation of diuresis correction methods for routine biological monitoring. Environ. Res. 2016, 147, 469–479. [Google Scholar] [CrossRef]
- Barbeau, D.; Persoons, R.; Marques, M.; Hervé, C.; Laffitte-Rigaud, G.; Maitre, A. Relevance of urinary 3-hydroxybenzo(a)pyrene and 1-hydroxypyrene to assess exposure to carcinogenic polycyclic aromatic hydrocarbon mixtures in metallurgy workers. Ann. Occup. Hyg. 2014, 58, 579–590. [Google Scholar] [CrossRef]
- Barbeau, D.; Lutier, S.; Choisnard, L.; Marques, M.; Persoons, R.; Maitre, A. Urinary trans-anti-7,8,9,10-tetrahydroxy-7,8,9,10-tetrahydrobenzo(a)pyrene as the most relevant biomarker for assessing carcinogenic polycyclic aromatic hydrocarbons exposure. Environ. Int. 2018, 112, 147–155. [Google Scholar] [CrossRef]
- Barbeau, D.; Lutier, S.; Marques, M.; Persoons, R.; Maitre, A. Comparison of gaseous polycyclic aromatic hydrocarbon metabolites according to their specificity as biomarkers of occupational exposure: Selection of 2-hydroxyfluorene and 2-hydroxyphenanthrene. J. Hazard. Mater. 2017, 332, 185–194. [Google Scholar] [CrossRef]
- Forster, K.; Preuss, R.; Rossbach, B.; Bruning, T.; Angerer, J.; Simon, P. 3-Hydroxybenzo[a]pyrene in the urine of workers with occupational exposure to polycyclic aromatic hydrocarbons in different industries. Occup. Environ. Med. 2008, 65, 224–229. [Google Scholar] [CrossRef]
- Klöslová, Z.; Drímal, M.; Balog, K.; Koppová, K.; Dubajová, J. The Relations between Polycyclic Aromatic Hydrocarbons Exposure and 1-OHP Levels as a Biomarker of the Exposure. Cent. Eur. J. Public Health 2016, 24, 302–307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marczynski, B.; Pesch, B.; Wilhelm, M.; Rossbach, B.; Preuss, R.; Hahn, J.-U.; Rabstein, S.; Raulf-Heimsoth, M.; Seidel, A.; Rihs, H.-P.; et al. Occupational exposure to polycyclic aromatic hydrocarbons and DNA damage by industry: A nationwide study in Germany. Arch. Toxicol. 2009, 83, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Seidel, A.; Spickenheuer, A.; Straif, K.; Rihs, H.-P.; Marczynski, B.; Scherenberg, M.; Dettbarn, G.; Angerer, J.; Wilhelm, M.; Brüning, T.; et al. New Biomarkers of Occupational Exposure to Polycyclic Aromatic Hydrocarbons. J. Toxicol. Environ. Health Part A 2008, 71, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Raulf-Heimsoth, M.; Angerer, J.; Pesch, B.; Marczynski, B.; Hahn, J.U.; Spickenheuer, A.; Preuss, R.; Rühl, R.; Rode, P.; Brüning, T. Biological Monitoring as a Useful Tool for the Detection of a Coal-Tar Contamination in Bitumen-Exposed Workers. J. Toxicol. Environ. Health Part A 2008, 71, 746–750. [Google Scholar] [CrossRef]
- Raulf-Heimsoth, M.; Marczynski, B.; Spickenheuer, A.; Pesch, B.; Welge, P.; Rühl, R.; Bramer, R.; Kendzia, B.; Heinze, E.; Angerer, J.; et al. Bitumen workers handling mastic versus rolled asphalt in a tunnel: Assessment of exposure and biomarkers of irritation and genotoxicity. Arch. Toxicol. 2011, 85, 81–87. [Google Scholar] [CrossRef]
- Rihs, H.-P.; Spickenheuer, A.; Heinze, E.; Pesch, B.; Raulf-Heimsoth, M.; Angerer, J.; Brüning, T. Modulation of urinary polycyclic aromatic hydrocarbon metabolites by enzyme polymorphisms in workers of the German Human Bitumen Study. Arch. Toxicol. 2011, 85, 73–79. [Google Scholar] [CrossRef]
- Marczynski, B.; Raulf-Heimsoth, M.; Spickenheuer, A.; Pesch, B.; Kendzia, B.; Mensing, T.; Engelhardt, B.; Lee, E.-H.; Schindler, B.K.; Heinze, E.; et al. DNA adducts and strand breaks in workers exposed to vapours and aerosols of bitumen: Associations between exposure and effect. Arch. Toxicol. 2011, 85, 53–64. [Google Scholar] [CrossRef]
- Pesch, B.; Spickenheuer, A.; Kendzia, B.; Schindler, B.K.; Welge, P.; Marczynski, B.; Rihs, H.-P.; Raulf-Heimsoth, M.; Angerer, J.; Brüning, T. Urinary metabolites of polycyclic aromatic hydrocarbons in workers exposed to vapours and aerosols of bitumen. Arch. Toxicol. 2011, 85, 29–39. [Google Scholar] [CrossRef]
- Lotz, A.; Pesch, B.; Dettbarn, G.; Raulf, M.; Welge, P.; Rihs, H.-P.; Breuer, D.; Gabriel, S.; Hahn, J.-U.; Brüning, T.; et al. Metabolites of the PAH diol epoxide pathway and other urinary biomarkers of phenanthrene and pyrene in workers with and without exposure to bitumen fumes. Int. Arch. Occup. Environ. Health 2016, 89, 1251–1267. [Google Scholar] [CrossRef]
- Fostinelli, J.; Madeo, E.; Toraldo, E.; Sarnico, M.; Luzzana, G.; Tomasi, C.; De Palma, G. Environmental and biological monitoring of occupational exposure to polynuclear aromatic hydrocarbons during highway pavement construction in Italy. Toxicol. Lett. 2018, 298, 134–140. [Google Scholar] [CrossRef]
- Persoons, R.; Roseau, L.; Petit, P.; Hograindleur, C.; Montlevier, S.; Marques, M.; Ottoni, G.; Maitre, A. Towards a recommended biomonitoring strategy for assessing the occupational exposure of roofers to PAHs. Toxicol. Lett. 2020, 324, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Campo, L.; Fustinoni, S.; Consonni, D.; Pavanello, S.; Kapka, L.; Siwinska, E.; Mielzyňska, D.; Bertazzi, P. Urinary carcinogenic 4–6 ring polycyclic aromatic hydrocarbons in coke oven workers and in subjects belonging to the general population: Role of occupational and environmental exposure. Int. J. Hyg. Environ. Health 2014, 217, 231–238. [Google Scholar] [CrossRef]
- Zając, J.; Gomółka, E.; Maziarz, B.; Szot, W. Occupational Exposure to Polycyclic Aromatic Hydrocarbons in Polish Coke Plant Workers. Ann. Occup. Hyg. 2016, 60, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Zając, J.; Gomółka, E.; Szot, W. Urinary 1-hydroxypyrene in occupationally-exposed and non-exposed individuals in Silesia, Poland. Ann. Agric. Environ. Med. 2018, 25, 625–629. [Google Scholar] [CrossRef]
- Klotz, K.; Zobel, M.; Schäferhenrich, A.; Hebisch, R.; Drexler, H.; Göen, T. Suitability of several naphthalene metabolites for their application in biomonitoring studies. Toxicol. Lett. 2018, 298, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hebisch, R.; Karmann, J.; Schäferhenrich, A.; Göen, T.; Berger, M.; Poppek, U.; Roitzsch, M. Inhalation and dermal exposure of workers during timber impregnation with creosote and subsequent processing of impregnated wood. Environ. Res. 2020, 181, 108877. [Google Scholar] [CrossRef]
- Gjesteland, I.; Hollund, B.E.; Kirkeleit, J.; Daling, P.; Bråtveit, M. Biomonitoring of Benzene and Effect of Wearing Respirators during an Oil Spill Field Trial at Sea. Ann. Work Expo. Health 2018, 62, 1033–1039. [Google Scholar] [CrossRef]
- Mari, M.; Schuhmacher, M.; Domingo, J.L. Levels of metals and organic substances in workers at a hazardous waste incinerator: A follow-up study. Int. Arch. Occup. Environ. Health 2009, 82, 519–528. [Google Scholar] [CrossRef]
- Mari, M.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Body burden monitoring of dioxins and other organic substances in workers at a hazardous waste incinerator. Int. J. Hyg. Environ. Health 2013, 216, 728–734. [Google Scholar] [CrossRef]
- Laitinen, J.; Mäkelä, M.; Mikkola, J.; Huttu, I. Firefighters’ multiple exposure assessments in practice. Toxicol. Lett. 2012, 213, 129–133. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Alves, M.J.; Fernandes, A.; Teixeira, J.P.; Delerue-Matos, C.; Pereira, M.D.C.; Morais, S. Firefighters’ exposure biomonitoring: Impact of firefighting activities on levels of urinary monohydroxyl metabolites. Int. J. Hyg. Environ. Health 2016, 219, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Slezakova, K.; Magalhães, C.P.; Fernandes, A.; Teixeira, J.P.; Delerue-Matos, C.; Pereira, M.D.C.; Morais, S. Individual and cumulative impacts of fire emissions and tobacco consumption on wildland firefighters’ total exposure to polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2017, 334, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Slezakova, K.; Alves, M.J.; Fernandes, A.; Teixeira, J.P.; Delerue-Matos, C.; do Carmo Pereira, M.; Morais, S. Polycyclic aromatic hydrocarbons at fire stations: Firefighters’ exposure monitoring and biomonitoring, and assessment of the contribution to total internal dose. J. Hazard. Mater. 2017, 323, 184–194. [Google Scholar] [CrossRef]
- Andersen, M.H.G.; Saber, A.T.; Pedersen, P.B.; Loft, S.; Hansen, M.; Koponen, I.K.; Pedersen, J.E.; Ebbehøj, N.; Nørskov, E.-C.; Clausen, P.A.; et al. Cardiovascular health effects following exposure of human volunteers during fire extinction exercises. Environ. Health 2017, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.G.; Saber, A.T.; Clausen, P.A.; Pedersen, J.E.; Løhr, M.; Kermanizadeh, A.; Loft, S.; Ebbehøj, N.; Hansen, Å.M.; Pedersen, P.B.; et al. Association between polycyclic aromatic hydrocarbon exposure and peripheral blood mononuclear cell DNA damage in human volunteers during fire extinction exercises. Mutagenesis 2018, 33, 105–115. [Google Scholar] [CrossRef]
- Wingfors, H.; Nyholm, J.R.; Magnusson, R.; Wijkmark, C.H. Impact of Fire Suit Ensembles on Firefighter PAH Exposures as Assessed by Skin Deposition and Urinary Biomarkers. Ann. Work. Expo. Health 2018, 62, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Rossbach, B.; Wollschläger, D.; Letzel, S.; Gottschalk, W.; Muttray, A. Internal exposure of firefighting instructors to polycyclic aromatic hydrocarbons (PAH) during live fire training. Toxicol. Lett. 2020, 331, 102–111. [Google Scholar] [CrossRef]
- Sancini, A.; Caciari, T.; Sinibaldi, F. Blood pressure changes and polycyclic aromatic hydrocarbons in outdoor workers. Clin. Ter. 2014, 165, e295–e303. [Google Scholar] [CrossRef]
- Rossner, P.; Svecova, V.; Milcova, A.; Lnenickova, Z.; Solansky, I.; Sram, R.J. Seasonal variability of oxidative stress markers in city bus drivers: Part I. Oxidative damage to DNA. Mutat. Res. Mol. Mech. Mutagen. 2008, 642, 14–20. [Google Scholar] [CrossRef]
- Andersen, M.H.G.; Saber, A.T.; Frederiksen, M.; Clausen, P.A.; Sejbaek, C.S.; Hemmingsen, C.H.; Ebbehøj, N.E.; Catalán, J.; Aimonen, K.; Koivisto, J.; et al. Occupational exposure and markers of genetic damage, systemic inflammation and lung function: A Danish cross-sectional study among air force personnel. Sci. Rep. 2021, 11, 17998. [Google Scholar] [CrossRef]
- Guilbert, A.; De Cremer, K.; Heene, B.; Demoury, C.; Aerts, R.; Declerck, P.; Brasseur, O.; Van Nieuwenhuyse, A. Personal exposure to traffic-related air pollutants and relationships with respiratory symptoms and oxidative stress: A pilot cross-sectional study among urban green space workers. Sci. Total Environ. 2019, 649, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, P.T.J.; Van Houtum, J.; Anzion, R.B.; Champmartin, C.; Hertsenberg, S.; Bos, R.P.; Van Der Valk, P. The occupational exposure of dermatology nurses to polycyclic aromatic hydrocarbons—Evaluating the effectiveness of better skin protection. Scand. J. Work. Environ. Health 2009, 35, 212–221. [Google Scholar] [CrossRef]
- Van Gestel, E.A.; Linssen, E.S.; Creta, M.; Poels, K.; Godderis, L.; Weyler, J.J.; De Schryver, A.; Vanoirbeek, J.A. Assessment of the absorbed dose after exposure to surgical smoke in an operating room. Toxicol. Lett. 2020, 328, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.A.; Torres, V.M.; Louro, H.; Gomes, F.; Lopes, C.; Marçal, N.S.; Fragoso, E.; Martins, C.; Oliveira, C.L.; Hagenfeldt, M.; et al. Effects of Occupational Exposure to Tobacco Smoke: Is There a Link Between Environmental Exposure and Disease? J. Toxicol. Environ. Health Part A 2013, 76, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Capelas, S.; Delerue-Matos, C.; Morais, S. Grill Workers Exposure to Polycyclic Aromatic Hydrocarbons: Levels and Excretion Profiles of the Urinary Biomarkers. Int. J. Environ. Res. Public Health 2020, 18, 230. [Google Scholar] [CrossRef]
- Hansen, A.M.; Mathiesen, L.; Pedersen, M.; Knudsen, L.E. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies—A review. Int. J. Hyg. Environ. Health 2008, 211, 471–503. [Google Scholar] [CrossRef]
- Vital, N.; Antunes, S.; Louro, H.; Vaz, F.; Simões, T.; Penque, D.; Silva, M.J. Environmental Tobacco Smoke in Occupational Settings: Effect and Susceptibility Biomarkers in Workers from Lisbon Restaurants and Bars. Front. Public Health 2021, 9, 674142. [Google Scholar] [CrossRef]
- Petit, P.; Bicout, D.J.; Persoons, R.; Bonneterre, V.; Barbeau, D.; Maître, A. Constructing a Database of Similar Exposure Groups: The Application of the Exporisq-HAP Database from 1995 to 2015. Ann. Work Expo. Health 2017, 61, 440–456. [Google Scholar] [CrossRef]
- Unwin, J.; Cocker, J.; Scobbie, E.; Chambers, H. An Assessment of Occupational Exposure to Polycyclic Aromatic Hydrocarbons in the UK. Ann. Occup. Hyg. 2006, 50, 395–403. [Google Scholar] [CrossRef]
- Alhamdow, A.; Tinnerberg, H.; Lindh, C.; Albin, M.; Broberg, K. Cancer-related proteins in serum are altered in workers occupationally exposed to polycyclic aromatic hydrocarbons: A cross-sectional study. Carcinogenesis 2019, 40, 771–781. [Google Scholar] [CrossRef]
- Valière, M.; Petit, P.; Persoons, R.; Demeilliers, C.; Maître, A. Consistency between air and biological monitoring for assessing polycyclic aromatic hydrocarbon exposure and cancer risk of workers. Environ. Res. 2022, 207, 112268. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Bisceglia, L.; Cavone, D.; Caputi, A.; De Maria, L.; Delfino, M.C.; Corrado, V.; Ferri, G.M. Environmental Monitoring of PAHs Exposure, Biomarkers and Vital Status in Coke Oven Workers. Int. J. Environ. Res. Public Health 2020, 17, 2199. [Google Scholar] [CrossRef] [PubMed]
- Sams, C. Urinary Naphthol as a Biomarker of Exposure: Results from an Oral Exposure to Carbaryl and Workers Occupationally Exposed to Naphthalene. Toxics 2017, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Sram, R.J.; Svecova, V.; Rossnerova, A. Systematic review of the use of the lymphocyte cytokinesis-block micronucleus assay to measure DNA damage induced by exposure to polycyclic aromatic hydrocarbons. Mutat. Res. Mutat. Res. 2016, 770, 162–169. [Google Scholar] [CrossRef]
- Alhamdow, A.; Lindh, C.; Albin, M.; Gustavsson, P.; Tinnerberg, H.; Broberg, K. Cardiovascular disease-related serum proteins in workers occupationally exposed to polycyclic aromatic hydrocarbons. Toxicol. Sci. 2019, 171, 235–246. [Google Scholar] [CrossRef]
- Alhamdow, A.; Zettergren, A.; Kull, I.; Hallberg, J.; Andersson, N.; Ekström, S.; Berglund, M.; Wheelock, C.E.; Essig, Y.J.; Krais, A.M.; et al. Low-level exposure to polycyclic aromatic hydrocarbons is associated with reduced lung function among Swedish young adults. Environ. Res. 2021, 197, 111169. [Google Scholar] [CrossRef]
- Gao, P.; da Silva, E.; Hou, L.; Denslow, N.D.; Xiang, P.; Ma, L.Q. Human exposure to polycyclic aromatic hydrocarbons: Metabolomics perspective. Environ. Int. 2018, 119, 466–477. [Google Scholar] [CrossRef]
- Keir, J.L.A.; Akhtar, U.S.; Matschke, D.M.J.; Kirkham, T.; Chan, L.; Ayotte, P.; White, P.A.; Blais, J.M. Elevated Exposures to Polycyclic Aromatic Hydrocarbons and Other Organic Mutagens in Ottawa Firefighters Participating in Emergency, On-Shift Fire Suppression. Environ. Sci. Technol. 2017, 51, 12745–12755. [Google Scholar] [CrossRef]
- Thein, N.; Møller, P.; Amtoft, H.; Vogel, U.; Korsholm, B.; Autrup, H.; Wallin, H. A strong genotoxic effect in mouse skin of a single painting of coal tar in hairless mice and in MutaMouse. Mutat. Res. Mol. Mech. Mutagen. 2000, 468, 117–124. [Google Scholar] [CrossRef]
- Hadrup, N.; Zhernovkov, V.; Jacobsen, N.R.; Voss, C.; Strunz, M.; Ansari, M.; Schiller, H.B.; Halappanavar, S.; Poulsen, S.S.; Kholodenko, B.; et al. Acute Phase Response as a Biological Mechanism-of-Action of (Nano)particle-Induced Cardiovascular Disease. Small 2020, 16, e1907476. [Google Scholar] [CrossRef]
- Saber, A.T.; Jacobsen, N.R.; Jackson, P.; Poulsen, S.S.; Kyjovska, Z.O.; Halappanavar, S.; Yauk, C.L.; Wallin, H.; Vogel, U. Particle-induced pulmonary acute phase response may be the causal link between particle inhalation and cardiovascular disease. WIREs Nanomed. Nanobiotechnology 2014, 6, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.; Miller, M.R.; Clift, M.; Elder, A.; Mills, N.L.; Møller, P.; Schins, R.P.; Vogel, U.; Kreyling, W.; Jensen, K.A.; et al. Nanomaterials Versus Ambient Ultrafine Particles: An Opportunity to Exchange Toxicology Knowledge. Environ. Health Perspect. 2017, 125, 106002. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; Wilson, P.G.; Shridas, P.; Ji, A.; de Beer, M.; de Beer, F.C.; Webb, N.R.; Tannock, L.R. Serum amyloid A3 is pro-atherogenic. Atherosclerosis 2018, 268, 32–35. [Google Scholar] [CrossRef] [PubMed]
- RAC. Note on Reference Dose-Response Relationship for the Carcinogenicity of Pitch, Coal Tar, High Temperature and on PBT and vPvB Properties. 2018. Available online: https://echa.europa.eu/documents/10162/17229/ctpht_rac_note_en.pdf/a184ee42-0642-7454-2d18-63324688e13d?t=1544526560573 (accessed on 12 April 2022).
- Lafontaine, M.; Gendre, C.; Morele, Y.; Laffitte-Rigaud, G. Excretion of Urinary 1-Hydroxypyrene in Relation to the Penetration Routes of Polycyclic Aromatic Hydrocarbons. Polycycl. Aromat. Compd. 2002, 22, 579–588. [Google Scholar] [CrossRef]
- Cherry, N.; Aklilu, Y.-A.; Beach, J.; Britz-McKibbin, P.; Elbourne, R.; Galarneau, J.-M.; Gill, B.; Kinniburgh, D.; Zhang, X. Urinary 1-hydroxypyrene and Skin Contamination in Firefighters Deployed to the Fort McMurray Fire. Ann. Work Expo. Health 2019, 63, 448–458. [Google Scholar] [CrossRef]
- Cequier, E.; Pérez Luzardo, O.; Kasper-Sonnenberg, M.; Koch, H.; Haug, L.S.; Bury, D.; Vorkamp, K.; Knudsen, B.E.; Hajeb, P.; Pardo, O.; et al. Prioritised List of Biomarkers, Matrices and Analytical Methods for the 1st Prioritisation Round of Substances. Deliverable Report D9.WP9—Laboratory Analysis and Quality Assurance. 2017. Available online: https://www.hbm4eu.eu/work-packages/deliverable-9-2-prioritised-list-of-biomarkers-matrices-and-analytical-methods-for-the-1st-prioritisation-round-of-substances/ (accessed on 18 July 2022).
- Santonen, T.; Alimonti, A.; Bocca, B.; Duca, R.C.; Galea, K.S.; Godderis, L.; Göen, T.; Gomes, B.; Hanser, O.; Iavicoli, I.; et al. Setting up a collaborative European human biological monitoring study on occupational exposure to hexavalent chromium. Environ. Res. 2019, 177, 108583. [Google Scholar] [CrossRef]
- Scheepers, P.T.J.; Duca, R.C.; Galea, K.S.; Godderis, L.; Hardy, E.; Knudsen, L.E.; Leese, E.; Louro, H.; Mahiout, S.; Ndaw, S.; et al. HBM4EU Occupational Biomonitoring Study on e-Waste—Study Protocol. Int. J. Environ. Res. Public Health 2021, 18, 12987. [Google Scholar] [CrossRef]
- Jones, K.; Galea, K.S.; Scholten, B.; Loikala, M.; Porras, S.P.; Bousoumah, R.; Ndaw, S.; Leese, E.; Louro, H.; Silva, M.J.; et al. HBM4EU Diisocyanates Study—Research Protocol for a Collaborative European Human Biological Monitoring Study on Occupational Exposure. Int. J. Environ. Res. Public Health 2022, 19, 8811. [Google Scholar] [CrossRef]
| Source | Keywords | Filters |
|---|---|---|
| PubMed | PAHs; polycyclic aromatic hydrocarbons; environmental monitoring; biomonitoring; occupational exposure | Full text; January 2008 to March 2022; English; human |
| Web of Science | PAHs; polycyclic aromatic hydrocarbons; biomonitoring; occupational exposure | All databases; January 2008 to March 2022; English |
| Scopus | polycyclic aromatic hydrocarbons; biomonitoring; occupational exposure | All databases; January 2008 to March 2022; English |
| Criteria | |
|---|---|
| Inclusion criteria | Publication date between January 2008 and March 2022 * English language Report exposure from European workplaces Studies with quantitative data concerning occupational exposure to PAHs. This includes data obtained from HBM and from air monitoring in different exposure scenarios |
| Exclusion criteria | Published before 2008 Non-English language Studies reporting exposure outside of Europe Studies reporting exposure of general population Grey literature/not published in a peer reviewed journal Dissertations/theses Proceedings Only abstract available Reviews containing only published data |
| Assessment Component | TIER 1 | TIER 2 | TIER 3 |
|---|---|---|---|
| Study participants | >20 occupationally exposed individuals | 5–20 occupationally exposed individuals | Any other study (<5 occupationally exposed individuals) |
| Chemicals under investigation | 8 carcinogenic PAHs in entry 50 of Annex XVII to REACH (PAH8): BaP, benzo[e]pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, and dibenzo[a,h]anthracene. | Other than PAH8 or fewer than the 8 carcinogenic PAHs in entry 50 of Annex XVII to REACH | Other contaminants that result from the same process where PAHs can be produced. |
| Exposure biomarker and matrix | Biomarker(s) in a specified matrix has accurate and precise quantitative relationship with external exposure, internal dose, or target dose. E.g., BaP-specific metabolites, in particular 3-hydroxyBaP (3-OH-BaP). | Evidence exists for a relationship between biomarker in a specified matrix and external exposure, internal dose, or target dose but limited application. E.g., 1-OH-PYR, metabolite of pyrene-indirect marker of exposure to PAH mixtures that include BaP; glucuronide of 1-OH-PYR; OH-PHEs. | Biomarker in a specified matrix is a poor surrogate (low accuracy and precision) for exposure/dose. E.g., carboxyhemoglobin or volatile organic compounds (VOC) in exhaled air. |
| Biomarker specificity | Biomarker is at least one of the PAH8 or a specific metabolite. E.g., BaP and/or 3-OH-BaP | Biomarker is one PAH or a specific metabolite not included in PAH8. E.g., pyrene and/or 1-OH-PYR | Biomarker is derived from contaminants that result from the same process where PAHs can be produced. |
| Technique | Analytical methods that provide unambiguous identification and quantitation of the biomarker at the required sensitivity (e.g., HPLC-FD, GC-HRMS, LC-MS/MS). | Other analytical methods that provide quantitative but potentially ambiguous identification of the biomarker (e.g., HPLC). | Analytical methods that only allows for detection of the biomarkers but is not able to quantify. |
| Method characteristics | Acceptable LOD; Samples with a known history and documented stability data; samples are contamination-free | Stability not specifically assessed, but samples were stored appropriately and analyzed promptly. | LOD above current state-of–the-art; specific reason to query stability; known contamination issues |
| Quality assurance (QA) | Study has used external QA where appropriate | Some QA used (note details) | No QA |
| Sampling strategy and matrix adjustment | - | Study includes results for adjusted concentrations if adjustment is needed; |
| Occupational Sector | Study Design and Country | Biomarkers of Exposure and Effect | External Exposure Monitoring | Comments | Ref. |
|---|---|---|---|---|---|
| Metallurgic Industry | 129 male subjects (18–65 years old) working in anode, graphite cathode, and silicon production; smokers and non-smokers; urine samples collected at the beginning of the first shift of the working week, post-shift on the last-but-one workday, and the beginning of the last shift of the week; France | Exposure: urinary 1-OH-PYR and 3-OH-BaP | 3-OH-BaP is an essential biomarker of exposure to carcinogenic PAHs | [42] | |
| Seven male subjects (30–60 yearsold) working in a pre-baked electrode production plan; non-smokers; spot urine samples collected in pre- and post- shift every day of the working week; Germany | Exposure: urinary 1-OH-PYR and 3-OH-BaP | Personal air sampling | Urinary 3-OH-BaP is the most relevant biomarker for estimating carcinogenic risk in subjects exposed to complex mixtures of PAHs. It has the advantage of assessing exposure to BaP, the only PAH classified as carcinogenic to humans. | [43] | |
| Seven non-smoking male subjects (30–60 years old) working in a prebaked electrode production plant; urine samples collected in pre-shift and post-shift every day of the working week; France | Exposure: urinary 1- and 2-OH-NAP, 2-, 3- and 9-OH-FLU, 1-, 2-, 3-, 4- and 9-OH-PHE | Personal air sampling | 1- and 2-OH-NAP, 1- and 3-OH-PHE concentrations profiles were weakly explained by occupational exposure, while 2- and 3-OH-FLU and 2-OH-PHE were strongly linked with atmospheric levels. 2-OH-FLU and 2-OH-PHE were the best biomarkers of gaseous PAH exposure | [44] | |
| 26 subjects working in production of graphite electrodes; smokers and non-smokers; analyses carried out in post-shift spot urine samples; Germany | Exposure: urinary 1-OH-PYR, OH-PHEs, and 3-OH-BaP | Personal air sampling | A strong correlation was found between 1-OH-PYR, OH-PHEs, and 3-OH-BaP concentrations (Pearson r = 0.618–0.867, p < 0.001). Thus 3-OH-BaP can be regarded as a reliable and sensitive biomarker for PAHs. Poor correlation was observed between BaP in air and 3-OH-BaP for workers in the production of graphite electrodes. Because of the carcinogenic potency of BaP, 3-OH-BaP is regarded as the most relevant biomarker for risk assessment rather than 1-OH-PYR and OH-PHEs. | [45] | |
| 19 subjects working in an aluminum production plant and 66 subjects working in a graphite electrode production plant; smokers and non-smokers; urine samples pre-shift, after the weekend and after three days of exposure (at the end of the third work day), or at the end of the work week; Slovakia | Exposure: urinary 1-OH-PYR | Stationary and personal air sampling | Strong correlation between the concentration of urinary 1-OH-PYR and concentration of pyrene or PAHs in air | [46] | |
| Six male subjects (30–60-years old) working in a pre-baked electrode production plan; non-smokers; urine samples collected at the beginning and at the post-shift on the last work day of the week; France | Exposure: 1-OH-PYR and 3-OH-BaP | The delay observed for maximum urinary excretion rates of 3-OH-BaP confirmed that sampling time should be performed the next morning after exposure | [41] | ||
| 26 male subjects working in production of graphite electrodes *; smokers and non-smokers; urine and blood samples collected in post-shift; Germany | Exposure: urinary 1-OH-PYR, and OH-PHEs Effect: 8-oxo-dGuo and DNA strand breaks (blood cells) | Personal air sampling | Urinary concentrations of 1-OH-PYR and OH-PHEs in relation to PAH exposures suggested additional routes of exposure at various workplaces rather than inhalation only. Significantly increased levels of 8-oxo-dGuo and DNA strand breaks when comparing all PAH exposed workers as one group to controls. The levels of DNA damage differed between the industries. The highest levels of DNA damage were found in the graphite electrode workers (more than 2-fold compared to the other groups of PAH-exposed workers). A regression model showed a weak association between air concentration of PAH and DNA strand breaks in blood cells, while no association was found between 8-oxo-Guo and PAH air exposure. | [47] | |
| 24 male subjects working in production of graphite electrodes; smokers and non-smokers; urine samples collected in post-shift; Germany | Exposure: 1-OH-PYR, 1,6- and 1,8-OH-PYR, 1-, 2-, 3-, 4-, and 9-OH-PHE, 1,2-, 3,4-, and 9,10-OH-PHE | Personal air sampling | Association of PYR with 1-OH-PYR and 1,6- and 1,8-OH-PYR was weaker than for PHE with OH-PHEs and 1,2-OH-PHE. 1,2-OH-PHE is a new biomarker to assess occupational exposure to PHE | [48] | |
| Converter infeed | 26 workers exposed to binding pitch-containing refractories and stamping materials; smokers and non-smokers; analyses carried out in post-shift spot urine samples; Germany | Exposure: urinary 1-OH-PYR, OH-PHEs, and 3-OH-BaP | Personal air sampling | A strong correlation was found between 1-OH-PYR, OH-PHEs, and 3-OH-BaP concentrations. Thus 3-OH-BaP can be regarded as a reliable and sensitive biomarker for PAHs. No correlation was observed between BaP in air and 3-OH-BaP for workers in converter infeed. Because of the carcinogenic potency of BaP, 3-OH-BaP is regarded as the most relevant biomarker for risk assessment rather than 1-OH-PYR and OH-PHEs. | [45] |
| Six male subjects exposed to PAHs during feeding converters in steel production; smokers and non-smokers; urine samples collected in post-shift; Germany | Exposure: 1-OH-PYR, 1,6- and 1,8-OH-PYR, 1-, 2-, 3-, 4-, and 9-OH-PHE, 1,2-, 3,4-, and 9,10-OH-PHE | Personal air sampling | Association of PYR with 1-OH-PYR and 1,6- and 1,8-OH-PYR was weaker than for PHE with OH-PHEs and 1,2-OH-PHE. 1,2-OH-PHE was pointed as a new biomarker to assess occupational exposure to PHE | [48] | |
| Construction and maintenance of bituminous and asphalt roads | 73 mastic asphalt workers exposed to bitumen fumes and 49 not exposed construction workers; smokers and non-smokers; pre- and post-shift urinary samples collected; Germany | Exposure: 1-OH-PYR, 1-, 2- 3-, 4-, and 9-OH-PHE | Stationary sampling | Markedly higher urinary PAH concentrations were found in a subgroup of mastic asphalt workers. Further analysis at the working place demonstrated that the cause of significantly enhanced PAH exposure during handling with bitumen under high processing temperatures was not exclusively based on the fumes of bitumen alone, but also on the contamination with coal-tar traces in the underground material as a major source of PAH exposure | [49] |
| Six workers working with rolled asphalt followed by mastic asphalt one week late; smokers and non-smokers; spot urine, blood and induced sputum samples collected pre- and post-shift; Germany | Exposure: urinary 1-OH-PYR and OH-PHEs Effect: 8-Oxo-dGuo and (+)-anti-BPDE DNA adducts | Stationary and personal air sampling | Processing mastic asphalt was associated with a higher bitumen and PAH concentrations than in rolled asphalt application. However, the post-shift urinary PAH metabolite concentrations did not reflect these different exposure levels. No differences in the excretion of urinary PAH metabolites, lung function impairment or genotoxicity markers were detected comparing the two asphalt applications. Increase in 8-oxo-dGuo adducts were observed during shift while the DNA strand break levels decreased (independent of asphalt application) | [50] | |
| 218 workers exposed to vapors and aerosols of bitumen and 96 roadside construction workers not working with asphalt (not exposed); urine and blood samples collected in pre- and post-shift; Germany | Exposure: urinary 1-, 2 + 9-, 3-, and 4-OH-PHE and 1-OH-PYR Susceptibility: The influence of 18 single-nucleotide polymorphisms (SNP) in genes coding for enzymes involved in PAH and amine metabolism regarding their impact on urinary markers (1-OH-PYR) and the sum of 1-, 2 + 9-, 3-, 4- (OH-PHE). | Personal air sampling | Significant modulation of the levels of OH-PHEs but not of 1-OH-PYR by two (GSTM1 * 1 and NAT2 * 803GG) out of 18 sequence variants of metabolizing enzymes. | [51] | |
| 320 male workers exposed to bitumen fumes and 118 road-side construction workers (not exposed); smokers and non-smokers; spot urine and blood samples collected in pre- and post-shift; Germany | Exposure: urinary 1-OH-PYR, the sum of 1-, 2-, 3-,4-, and 9-OH-PHEs, and 1- and 2-OH-NAP Effect: (+)-anti-BPDE, 8-oxo-dGuo and DNA strand breaks (blood cells) | Personal air sampling | Increased levels of 8-oxo-dGuo adducts and DNA strand breaks in exposed workers (both pre- and post shift) compared to controls. The level of 8-oxo-dGuo was increased post- compared to pre-shift, whereas the level of DNA strand breaks was decreased post- compared to pre-shift. No difference in the level of (+)-anti-BPDE-DNA adducts between exposed and controls No association between air exposure to bitumen and biomarkers of exposure and biomarkers of effect were found. Similar no associations between PAH metabolites and DNA damage except for a weak association between 1-OH-PYR and DNA strand breaks (rs = 0.18, p = 0.0012) were found. | [52] | |
| 317 male bitumen-exposed workers and 117 roadside construction workers as not exposed; smokers and non-smokers; Germany | Exposure: urinary 1- and 2-OH-NAP, 1-, 2,9-, 3-, and 4-OH-PHE, 1-OH-PYR | Personal air sampling | Exposure to asphalt resulted in an additional but marginal internal PAH exposure when assessed with 1-OH-PYR and OH-PHEs in post-shift urines, whereas OH-NAP concentrations were dominated by smoking. The dose–response relation between airborne bitumen concentrations and PAH metabolites was weak | [53] | |
| Seven subjects working in bitumen production and asphalting plant; smokers and non-smokers; urine samples collected before work, after the weekend and after three days of exposure (at the end of the third work day), or at the end of the work week; Slovakia | Exposure: urinary 1-OH-PYR | Stationary and personal air sampling | Strong correlation between the concentration of urinary 1-OH-PYR and concentration of pyrene or PAHs in air | [46] | |
| 91 mastic asphalt workers (bitumen-exposed group) and 42 workers from outdoor construction sites (not bitumen-exposed group); smokers and non-smokers; urine samples collected in pre- and post-shift; Germany | Exposure: urinary 1-OH-PYR, 1,6- and 1,8-OH-PYR, 1-, 2-, 3-, 4-, and 9-OH-PHE, and 1,2- and 9,10-OH-PHE | Personal air sampling | None of the PAH metabolites can be considered as a specific biomarker for bitumen exposure | [54] | |
| 144 male workers (22–62 years) working on the paving of a new highway; smokers and non-smokers; urine samples collected in post-shift after at least three work-shifts; Italy | Exposure: urinary 2-OH-NAP and 1-OH-PYR | Stationary and personal air sampling | Higher relevance of 1-OH-PYR as compared to 2-OH-NAP for the risk assessment of hot mix asphalt exposed workers | [55] | |
| Roofing | 73 roofers and 57 subjects not occupationally exposed to PAHs; three exposure groups including soft-applied roofing using polymer-modified bitumen, hot-applied roofing using oxidized bitumen and the tearing off of old roof coatings containing coal tar; smokers and non-smokers; urine samples collected at the beginning of the week at the beginning of a shift and at the end of the week (following 3–5 days of roofing activities) either in pre- or post-shift, or 16 h after the end of a shift France | Exposure: urinary 1-OH-PYR, 3-OH-BaP, tetraolBaP, 1- and 2-OH-NAP, 1-, 2-, 3-and 9-OH-FLU and 1-, 2-,3-, 4 and 9-OH-PHE | Personal air sampling | Urinary 1-OH-PYR, 3-OH-FLU and 2-OH-PHE appeared to be the most relevant biomarkers for assessing PAH exposure in roofers due to their correlation with airborne levels of parent PAHs. They were hardly influenced by confounding factors (smoking and other environmental sources). Conversely, 1- and 2-OH-NAP should not be used for occupational exposure as they originate from many environmental sources (vehicle exhaust and tobacco) | [56] |
| Coke production | 87 coke-oven workers; smokers and non-smokers; analyses carried out in post-shift spot urine samples; Germany | Exposure: urinary 1-OH-PYR, OH-PHEs, and 3-OH-BaP | Personal air sampling | A good correlation was found between 1-OH-PYR, OH-PHE, and 3-OH-BaP concentrations. Thus 3-OH-BaP can be regarded as a reliable and sensitive biomarker for PAHs. Statistically significant correlations are observed for workers in coking plants. Because of the carcinogenic potency of BaP, 3-OH-BaP is regarded as the more relevant biomarker for risk assessment rather than 1-OH-PYR r and OH-PHEs | [45] |
| 27 male coke-oven workers; smokers and non-smokers; urine samples collected post-shift; Germany | Exposure: 1-OH-PYR, 1,6- and 1,8-OH-PYR, 1-, 2-, 3-, 4-, and 9-OH-PHE, 1,2-, 3,4-, and 9,10-OH-PHE | Personal air sampling | Association of PYR with 1-OH-PYR and 1,6- and 1,8-OH-PYR was weaker than for PHE with OH-PHEs and 1,2-OH-PHE. 1,2-OH-PHE was resulted to be a new biomarker to assess occupational exposure to PHE | [48] | |
| 37 male coke-oven workers *; smokers and non-smokers; urine and blood samples collected in post-shift; Germany | Exposure: urinary 1-OH-PYR, and OH-PHEs Effect: 8-oxo-dGuo and DNA strand breaks (blood cells) | Personal air sampling | Urinary concentrations of 1-OH-PYR and OH-PHEs in relation to PAH exposures suggested additional routes of exposure at various workplaces rather than inhalation only. Significantly increased levels of 8-oxo-dGuo and DNA strand breaks when comparing all four groups of PAH exposed workers to controls. The levels of DNA damage differed between the industries. The highest levels of DNA damage were found in the graphite electrode workers (more than 2-fold compared to the other groups of PAH exposed workers). A regression model showed a weak association between air concentration of PAH and DNA strand breaks in blood cells, while no association was found between 8-oxo-dGuo and PAH air exposure. | [47] | |
| 104 male coke-oven workers and 49 male subjects from the general population living in the same area (controls); smokers and non-smokers; urine samples collected at the end of an 8-h work shift, after three consecutive days of work; Poland | Exposure: urinary un-metabolized PAHs (PHE, ANT, FLT, PYR, CHR, BaA, BkF, BbF, BaP, DahA, BghiP, In[c,d]P) | BaP and other carcinogenic PAHs were quantified for the first time in urine samples from both occupationally and environmentally exposed subjects, assessing exposure to specific compounds | [57] | ||
| 619 coke-oven workers (average age, 50 years old); smokers and non-smokers; urine samples collected after two non-working days before work and after the fourth day of work; Poland | Exposure: urinary 1-OH-PYR | Stationary and personal air sampling | 1-OH-PYR resulted to be an effective biomarker for exposure to PAHs, due to positive correlation with air monitoring | [58] | |
| 647 coke plant workers (18−63 years old) and 206 non-exposed subjects (18−73 years old) living in the vicinity of the coke plant but not employed therein; smokers and non-smokers; worker urine samples collected before the work shift after two non-working days and at the end of the last day of the working week; Poland | Exposure: urinary 1-OH-PYR | Urine samples of coke plant workers collected before and after the working week had higher 1-OH-PYR concentrations compared to non-occupationally exposed subjects, thus indicating that samples collected at the beginning of the working week are not suitable for assessment of the worker background exposure. Despite the fact that occupational exposure induces a much greater influence on urinary 1-OH-PYR concentrations than cigarettes smoking, the latter’s effect was still significant | [59] | ||
| Wood preservation | 144 male workers (22–62 years old) handling with creosote; smokers and non-smokers; urine samples in pre- and post-shift; Germany | Exposure: urinary 1,2-OH-NAP, 1- and 2-OH-NAP, 1-NMA and 2-NMA | Stationary and personal air sampling | No significant correlations were observed between the naphthalene concentration in the air and the naphthalene metabolites, probably due to an additional uptake via the skin that may be a relevant absorption pathway in these workers. 1,2-OH-NAP and 1-NMA resulted to be specific biomonitoring parameter of naphthalene exposure | [60] |
| Workers exposed to creosote and its constituents; urine samples collected before the first shift at the start of a working week that was preceded by a work-free weekend (pre-shift samples), and after three successive work days at the end of the shift (post-shift samples); Germany | Exposure: urinary 1-OH-PYR | Personal air sampling and dermal PAH exposure measurement (body Tyvek™ coveralls and split leather gloves as dermal samplers) | The results of the study confirmed the eminent extent of dermal exposure at these workplaces | [61] | |
| Oil spills and cleanup operations | 22 subjects participating in an ‘oil-on-water’ field trial in the North Sea; smokers and non-smokers; urine samples collected in pre- and post-shift; Norway | Exposure: urinary 1-OH-PYR | Personal air sampling | Urinary levels of 1-OH-PYR were within the reference range of what is considered as background level | [62] |
| Waste incinerator | 29 workers including plant workers (incinerator operators, boiler maintenance, furnace maintenance, control panel, and waste-gas-washing operators), laboratory workers, and administration workers; Spain | Exposure: urinary 1-OH-PYR (analysis of pooled samples) | No evidence of occupational exposure to PAHs was observed | [63] | |
| 29 workers including plant workers (incinerator operators, boiler maintenance, furnace maintenance, control panel, and waste-gas-washing operators), laboratory workers, and administration workers; Spain | Exposure: urinary 1-OH-PYR (analysis of pooled samples) | No evidence of occupational exposure to PAHs was observed | [64] | ||
| Firefighters | Four smoke diving trainers participating in three fire house simulator tests; urine samples collected before exposure, immediately after the work shift, 6 h and the next morning after exposure; Finland | Exposure: urinary 1-OH-PYR, 1-OH-NAP, and muconic acid | Stationary sampling and dermal exposure measurements | Glueless wood or gas were resulted as the safest burning material, and sinol as the safest firing liquid. Moreover, it is absolutely imperative that polystyrene foam no longer be burned | [40] |
| 13 male smoke diving trainers in different smoke diving simulators; urinary samples collected before exposure, immediately after the work shift, 6 h and the next morning after exposure; Finland | Exposure: urinary 1-OH-PYR and 1-OH-NAP | Stationary sampling | Dermal exposure plays a role in PAH exposure during smoke diving. In order to measure dermal exposure, a second urine sample should be taken 6 h after the exposure has ended | [65] | |
| 153 workers including non- exposed firefighters (subjects not involved in fire combat activities within 48 h prior the urine collection) and exposed firefighters (subjects actively involved in fires combat and extinction); non-smokers; urine samples collected in post-shift; Portugal | Exposure: urinary 1-OH-NAP, 1-OH-ACE, 2-OH-FLU, 1-OH-PHE, 1-OH-PYR, and 3-OH-BaP | Personal air sampling | 1-OH-NAP and 1-OH-ACE were the most abundant urinary metabolites in non-exposed and exposed firefighters. 1-OH-PYR was the less abundant biomarkers. Inclusion of other metabolites was suggested, in addition to 1-OH-PYR to better estimate occupational exposure to PAHs | [66] | |
| 108 healthy firefighters serving at three different fire stations, smokers and non-smokers; urine samples collected post-shift between Tuesday and Thursday; Portugal | Exposure: urinary 1-OH-NAP, 1-OH-ACE, 2-OH-FLU, 1-OH-PHE, 1-OH-PYR, and 3-OH-BaP | 2-OH-FLU was the most affected compound by firefighting activities; 1-OH-NAP and 1-OH-ACE presented the highest increments due to tobacco consumption | [67] | ||
| 48 firefighter workers, non-smokers; urine samples collected post-shift; Portugal | Exposure: urinary 1-OH-NAP, 1-OH-ACE, 2-OH-FLU, 1-OH-PHE, 1-OH-PYR, and 3-OH-BaP | Personal air sampling | Moderate to strong correlations were observed between PAHs and urinary OH-PAHs. In accordance with the airborne PAHs profile, urinary 1-OH-NAP and 1-OH-ACE were the predominant metabolites. Thus, total body burden of PAHs should not be based exclusively on 1-OH-PYR biomonitoring | [68] | |
| 43 young subjects training to become firefighters **; biological measurements and samples collected 14 days before the smoke-diving course, immediately after the 3-day course exercises and 14 days subsequent to the end of the training session, Denmark Denmark | Exposure: urinary 1-OH-PYR Effect: Cardiovascular effects (microvascular function, heart rate variability) | Stationary and personal air sampling | Fire extinction exercises were associated with increased urinary 1-OH-PYR levels, decreased microvascular function and altered heart rate variability. However, the association between urinary 1-OH-PYR excretion and cardiovascular effects was not statistically significant in models that included smoke exposure as categorical variable | [69] | |
| 53 young subjects training to become firefighters; biological samples collected 14 days before the smoke-diving course, immediately after the 3-day course exercises and 14 days subsequent to the end of the training session; Denmark | Exposure: urinary 1-OH-PYR Effect: Inflammatory markers in plasma (ICAM-1, VCAM-1, SAA, CRP, IL-6, IL-8. DNA damage in peripheral blood mononuclear cells (DNA strand breaks and Fpg-sensitive sites). Lung function | Dermal wiping of the neck | Increased urinary excretion of 1-OH-PYR after the firefighting exercise compared with the two control measurements performed 2 weeks before and 2 weeks after the firefighting course, respectively. The level of DNA strand breaks was strongly associated with total PAH and pyrene levels on skin and urinary excretion of 1-OH-PYR. | [70] | |
| 22 male subjects from 3 consecutive 24 h work shift days (39–59 years old); urine samples collected pre- and post-shift and 24 h after the shift; Denmark | Exposure: urinary 1-OH-PYR Effect: Inflammatory markers in plasma (ICAM-1, VCAM-1, SAA, CRP, IL-6, IL-8. DNA damage in PBMC (DNA strand breaks, Fpg-sensitive sites), lung function; | Dermal wiping of the neck | No increase in the PAH levels on the skin (back of the neck) nor 1-OH-PYR concentrations in urine was observed. The work shift was not associated with increased levels of genotoxicity or decreased lung function, while increased levels of VCAM-1 was observed. | [6] | |
| 20 students (19–29 years old) at the Swedish Civil Contingencies Agency for prospective firefighters; non-smokers; urine samples collected pre-shift and at 6 and 20 h after exposure; Sweden | Exposure: urinary 1- and 2-OH-NAP, 1-OH-ACE, 2- and 9-OH-FLU, 9-OH-PHE, 1-OH-PYR, 3-OH-BaP | Stationary sampling inside the smoke container, air sampling inside the instructor’s Jacket, skin wipe sampling | Median urinary 1-OH-PYR levels were significantly increased after 6 h and 20 h as compared to baseline levels. The other OH-PAHs showed significantly increased levels after 6 h but not after 20 h, indicating a faster overall biotransformation. 1-OH-PYR showed the strongest correlation to skin-deposited PAHs and therefore it is suggested that this is a better indicator of exposure during smoke diving exercises when wood is used as fuel | [71] | |
| Six firefighting instructors (20–41 years old) exposed to combustion products in a compartment fire behavior training unit; non-smoker; repeated collection of urine samples before and immediately after, as well as 1, 3, 6, 9, 11, and 18 h after each training session (five training sessions, the time interval between two consecutive training sessions of the same test person being at least six days); Germany | Exposure: urinary 1- and 2-OH-NAP, 2-, 3-and 9-OH-FLU, 1-, 2-,3- and 4-OH-PHE, and 1-OH-PYR | A significant effect of the training sessions on the time course of internal exposure was found. OH-PAHs concentration increased at the latest 3 h after end of training. Due to the use of self-containing breathing apparatuses, dermal absorption is assumed as major exposure route | [72] | ||
| Policemen | 374 workers; smokers and non-smokers; urine samples collected after 5 working days, post-shift; Italy | Exposure: urinary 1-OH-PYR Effect: blood pressure | Occupational exposure to PAHs may be able to significantly influence the blood pressure probably acting on the autonomic nervous system. Inverse correlation between 1-OH-PYR and blood pressure | [73] | |
| Bus drivers | 50 bus drivers and 50 controls spending >90% of daily time indoors, non-smokers; spot urine samples and blood collected post-shift; Czech Republic | Effect: 8-oxo-dG (urine) | Personal air sampling (once in each season) | Bus drivers were exposed to significantly higher levels of PAHs in winter, while in the other two seasons the exposure of controls was unexpectedly higher than that of bus drivers. 8-oxo-dG levels were higher in bus drivers than in controls in all seasons | [74] |
| Air force personnel | 79 employees at an air force military base grouped by exposure based on job-function; non-smokers; first morning spot urine samples collected; Denmark | Exposure: urinary 1- and 2-OH-NAP, 2-OH-FLU, 1-, 2 + 3-, and 4-OH-PHE, and 1-OH-PYR Effect: Plasma level of acute phase response (SAA and CRP); genotoxicity (DNA strand breaks in PBMCs and micronuclei frequency in blood reticulocytes); lung function | PAH exposure assessed by silicon bands used as samplers; dermal exposure assessed by neck and hand palm wiping | No difference in exposure levels of total PAHs and OH-PAHs between exposure groups/job functions. No effects for biomarkers of systemic inflammation, genotoxicity; or lung function | [75] |
| Maintenance of parks, gardens, and reserves | 48 green space workers (20–55 years old) exposed to traffic-related air pollution; smokers or non-smoker; urine sample collected at the end of the fourth day follow-up; Belgium | Exposure: urinary 1- and 2-OH-NAP, 1-OH-PYR Effect: Airway inflammation (Exhaled NO); oxidative stress (8-OHdG in urine) | Personal air sampling (black carbon exposure) | With the exception of 2-OH-NAP, PAH biomarkers were measured below their detection limits in 70% (1-OH-PYR) and half of the samples (1-OH-NAP). Therefore, PAH exposure was not tested for potential correlations to health conditions. | [76] |
| Healthcare workers | 66 dermatology nurses involved in the topical application of coal tar ointment; smokers and non-smokers, The Netherlands | Exposure: urinary 1-OH-PYR | Personal air and dermal sampling | Use of gloves reduced the excretion of 1-OH-PYR by 51.5% | [77] |
| 15 surgeons, scrub assistants, and circulation nurses exposed to surgical smoke in an operating room; pre-, mid-, and end-shift urine samples collected (mid- and end-shift urinary sampling performed 30 min to 2 h after the cessation of smoke production); Belgium | Exposure: urinary 1-OH-PYR | Stationary and personal air sampling | 1-OH-PYR generally measured below its detection limit. No correlation was found between naphthalene in air and urinary 1-OH-NAP | [78] | |
| Workers in restaurants | 96 employees in restaurants, smokers and non-smokers; Portugal | Effect: Lung function, TAS (plasma), 8-OHdG (serum) and proteomics analysis of plasma | Stationary sampling | Increased levels of 8-OHdG in secondhand tobacco smoke exposed workers compared to non-exposed workers (regardless of smoking status). Proteomics analysis showed 9 differentially expressed proteins in plasma of second-hand tobacco smoke exposed non-smokers. Among these two acute phase proteins (ITIH4) and CP were expressed the most | [79] |
| 18 grill workers; non-smokers; urine samples collected at the end of the working day over a complete working week, including the resting days; Portugal | Exposure: urinary 1-OH-NAP + 1-OH-ACE, 2-OH-FLU, 1-OH-PHE, 1-OH-PYR, and 3-OH-BaP | OH-PAH concentrations were significantly increased during subjects’ working period compared with the following resting days, being 1-OH-NAP + 1-OH-ACE and 2-OH-FLU the compounds with the highest increments and 1-OH-PYR the lowest increments | [80] |
| Study Type | 8-Oxo-dGuo | 8-OHdG | 8-Oxo-dG | (+)-Anti-BPDE | DNA Strand Breaks | Fpg-Sensitive Sites | Micronuclei | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cross-sectional | ↑ (blood) | ↑ (blood) | [47] | |||||
| Cross-sectional/cross-shift | ↑ (blood) | = | ↑ (blood) | [52] | ||||
| Longitudinal study | ↑ during shift (blood) | = | [50] | |||||
| Cross-sectional | ↑ (urine) | [74] | ||||||
| Cross-shift (participants are their own controls) | =(PBMC) | ↓ (PBMC) | [6] | |||||
| Longitudinal (participants are their own controls) | = (PBMC) | ↑ (PBMC) | [70] | |||||
| Cross-sectional | = (PBMC) | = (PBR) | [75] | |||||
| Second-hand tobacco smoke exposed workers compared to non-exposed workers * | ↑ | [79] | ||||||
| Cross-sectional | ** | [76] |
| Study Type | Blood Pressure | Microvascular Function | Heart Rate Variability | SAA | CRP | IL-6 | IL-8 | I-CAM | V-CAM | ITIH4 and CP | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Second-hand tobacco smoke exposed workers compared to non-exposed workers | ↑ | [79] | |||||||||
| No control group: “when assessing the correlation between the dose of urinary 1-OH-PYR and BP in standing and supine positions, the “control group” was inherent in the sample” | Inverse correlation between 1-OH-PYR and blood pressure | [73] | |||||||||
| Human exposure study, where the participants were studied in three exposure scenarios, serving as their own controls. | ↓ | ↓ (SDNN, pNN50 and RMSSD, HF) ↑ (LF, LF/HF ratio) | [69] | ||||||||
| = | = | <LOD | <LOD | = | = | [70] | |||||
| Workshift, comparison of pre-shift with post-shift | = | = | <LOD | <LOD | = | ↑ | [6] | ||||
| Cross-sectional | = | = | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louro, H.; Gomes, B.C.; Saber, A.T.; Iamiceli, A.L.; Göen, T.; Jones, K.; Katsonouri, A.; Neophytou, C.M.; Vogel, U.; Ventura, C.; et al. The Use of Human Biomonitoring to Assess Occupational Exposure to PAHs in Europe: A Comprehensive Review. Toxics 2022, 10, 480. https://doi.org/10.3390/toxics10080480
Louro H, Gomes BC, Saber AT, Iamiceli AL, Göen T, Jones K, Katsonouri A, Neophytou CM, Vogel U, Ventura C, et al. The Use of Human Biomonitoring to Assess Occupational Exposure to PAHs in Europe: A Comprehensive Review. Toxics. 2022; 10(8):480. https://doi.org/10.3390/toxics10080480
Chicago/Turabian StyleLouro, Henriqueta, Bruno Costa Gomes, Anne Thoustrup Saber, Anna Laura Iamiceli, Thomas Göen, Kate Jones, Andromachi Katsonouri, Christiana M. Neophytou, Ulla Vogel, Célia Ventura, and et al. 2022. "The Use of Human Biomonitoring to Assess Occupational Exposure to PAHs in Europe: A Comprehensive Review" Toxics 10, no. 8: 480. https://doi.org/10.3390/toxics10080480
APA StyleLouro, H., Gomes, B. C., Saber, A. T., Iamiceli, A. L., Göen, T., Jones, K., Katsonouri, A., Neophytou, C. M., Vogel, U., Ventura, C., Oberemm, A., Duca, R. C., Fernandez, M. F., Olea, N., Santonen, T., Viegas, S., & Silva, M. J. (2022). The Use of Human Biomonitoring to Assess Occupational Exposure to PAHs in Europe: A Comprehensive Review. Toxics, 10(8), 480. https://doi.org/10.3390/toxics10080480















