The Role of Ferroptosis in the Damage of Human Proximal Tubule Epithelial Cells Caused by Perfluorooctane Sulfonate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Determination of PFOS Concentration
2.3. Determination of Fer-1 Concentration
2.4. Cell Treatment
2.5. Measurement of ROS Production by Flowcytometry
2.6. Determination of ROS Production by Immunofluorescence
2.7. Cell Apoptosis Determined by Immunofluorescence
2.8. Expression Analysis of Ferroptosis, Endoplasmic Reticulum Stress, Oxidative Stress and Apoptosis Related Protein by Western Blot Analysis
2.9. Statistical Analysis
3. Results
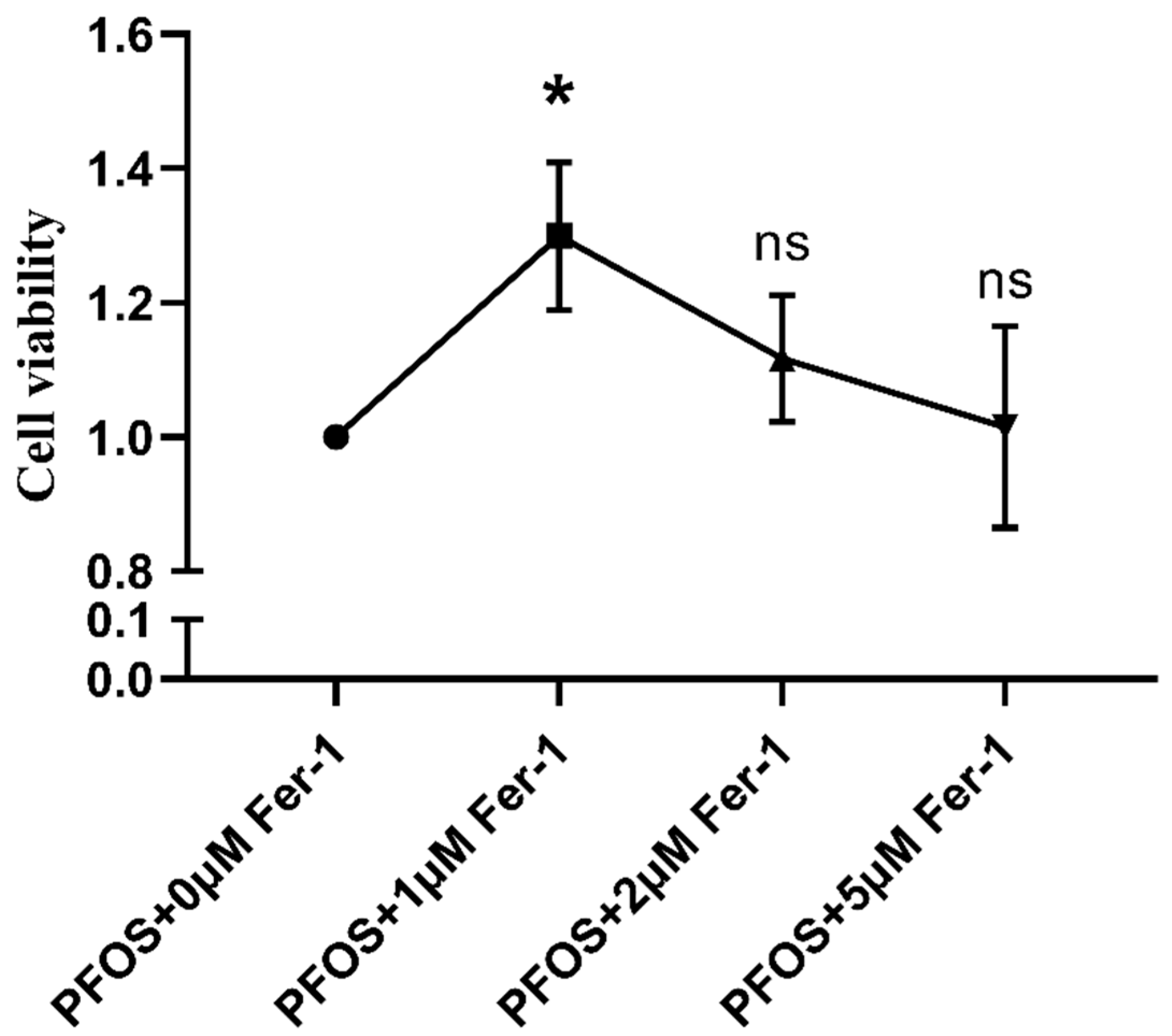
3.1. Determination of PFOS and Fer-1 Concentration
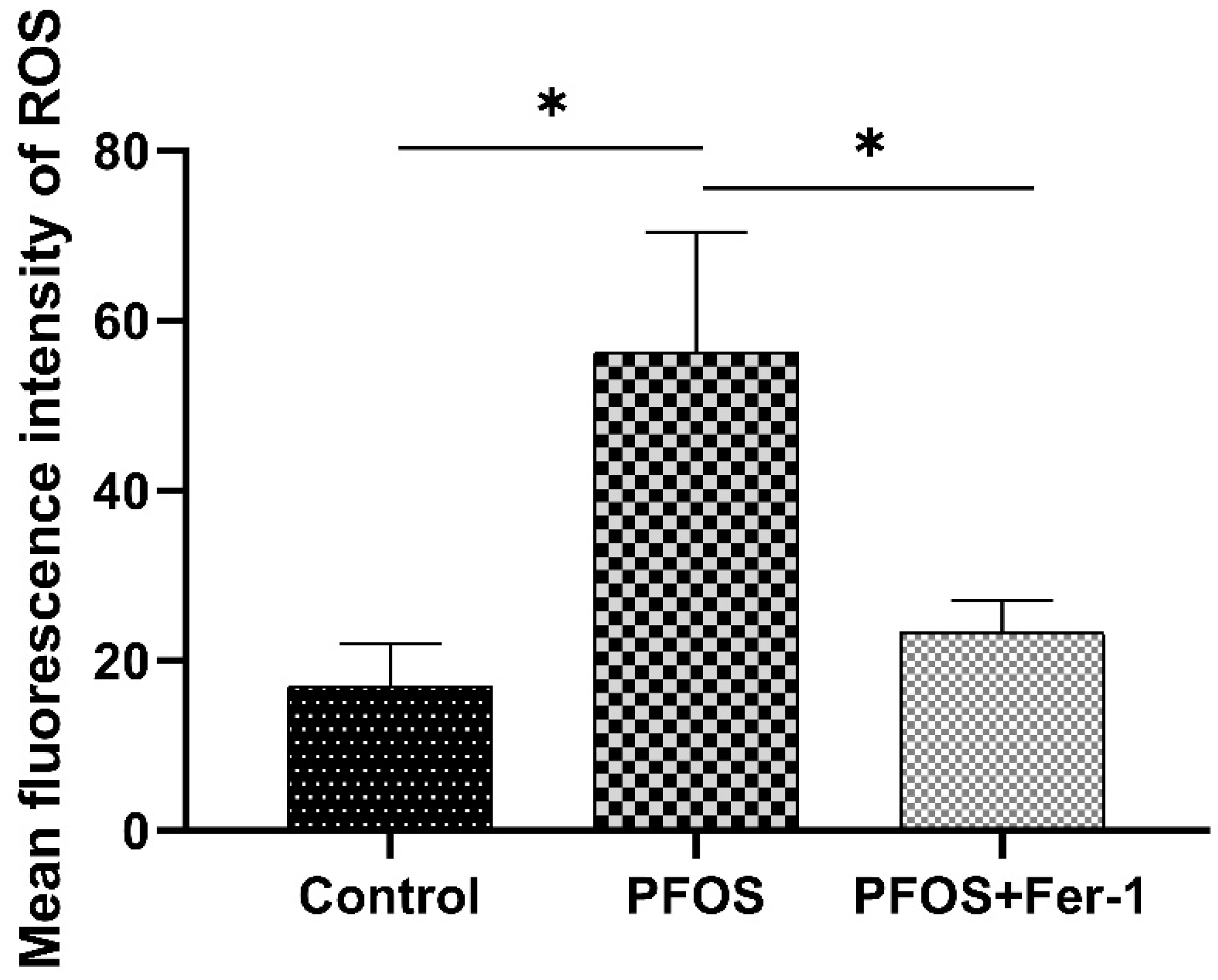
3.2. ROS Production in HK-2 Cells by Exposure to PFOS
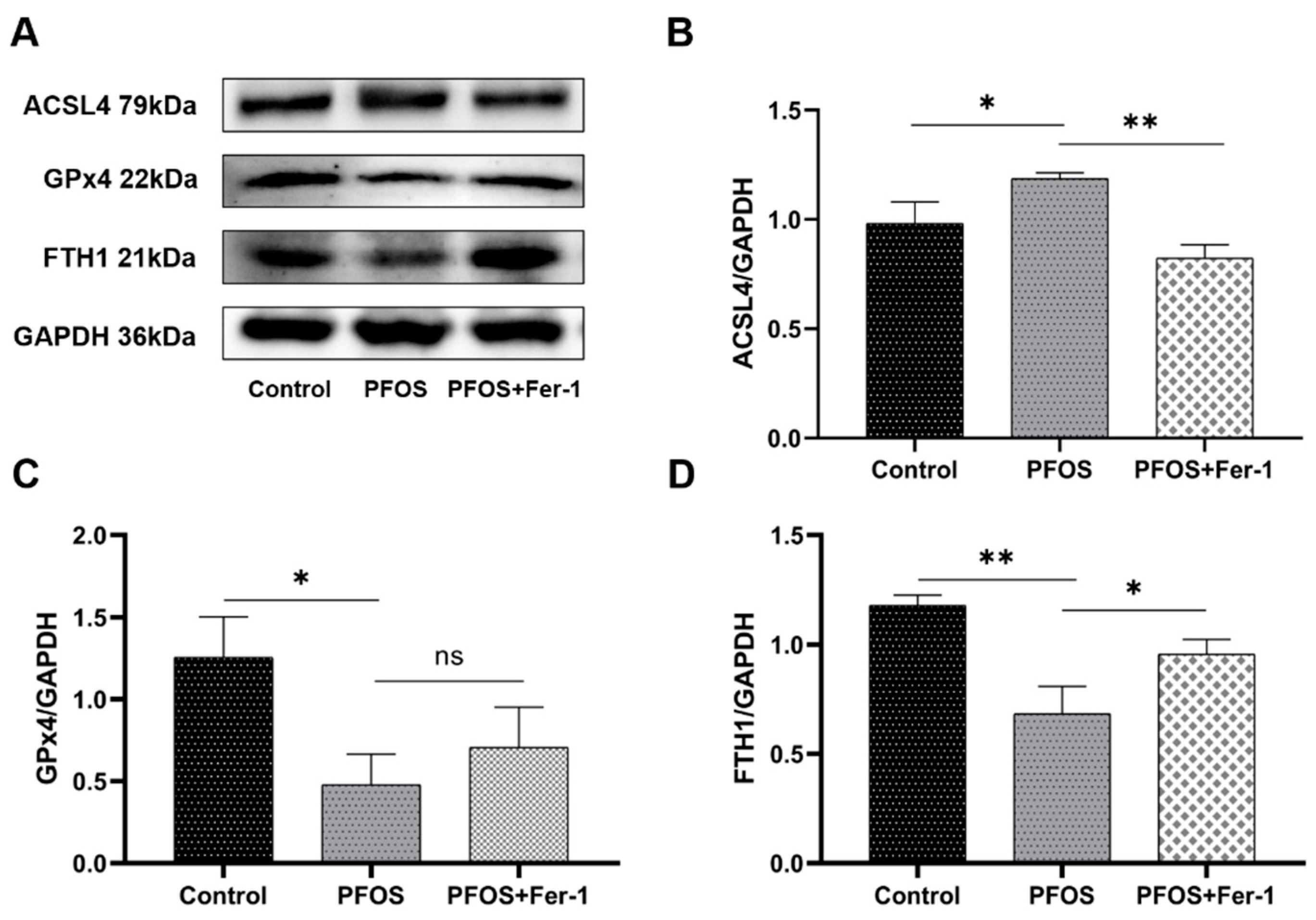
3.3. Ferroptosis in HK-2 Cells Induced by PFOS
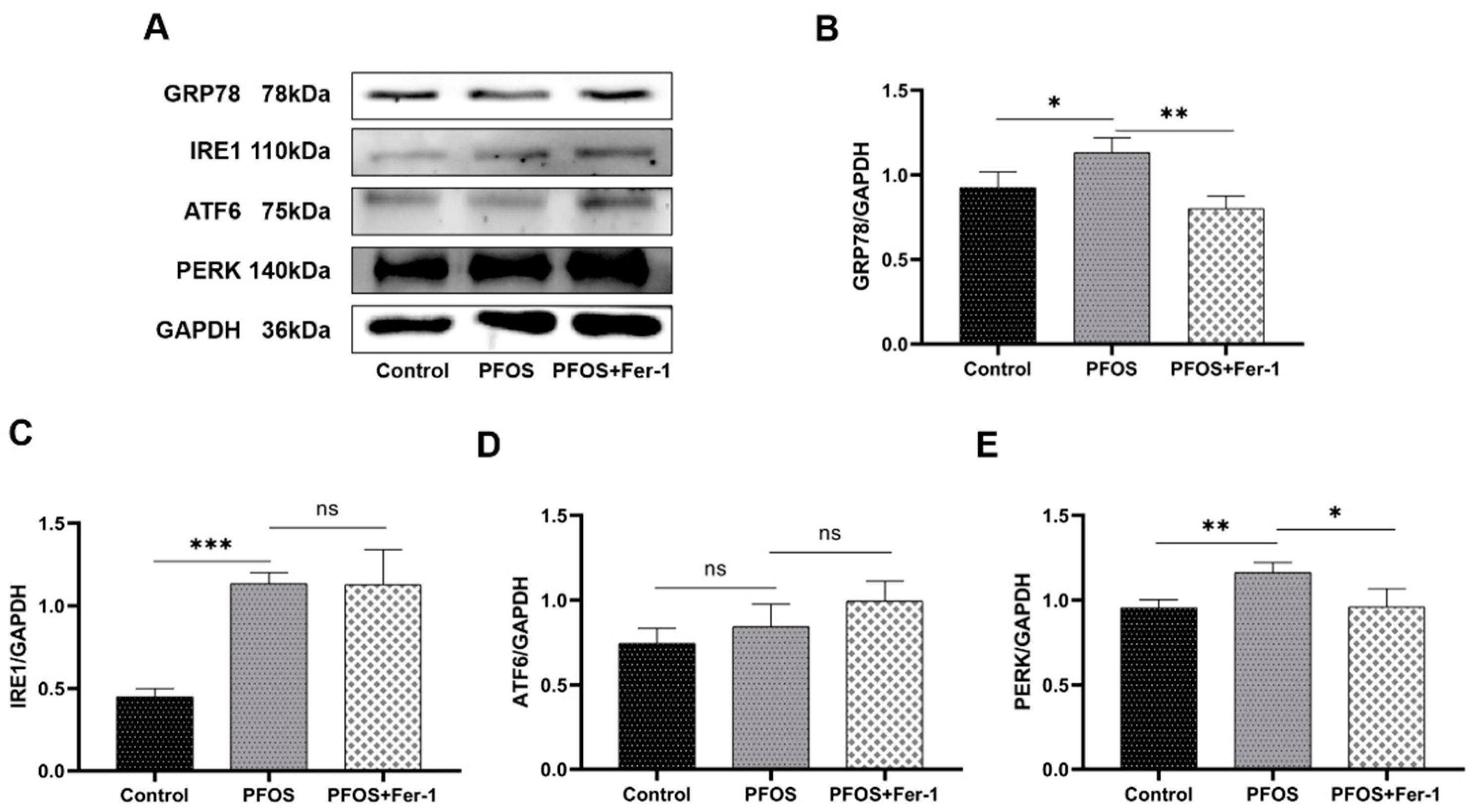
3.4. Endoplasmic Reticulum Stress in HK-2 Cells Caused by PFOS
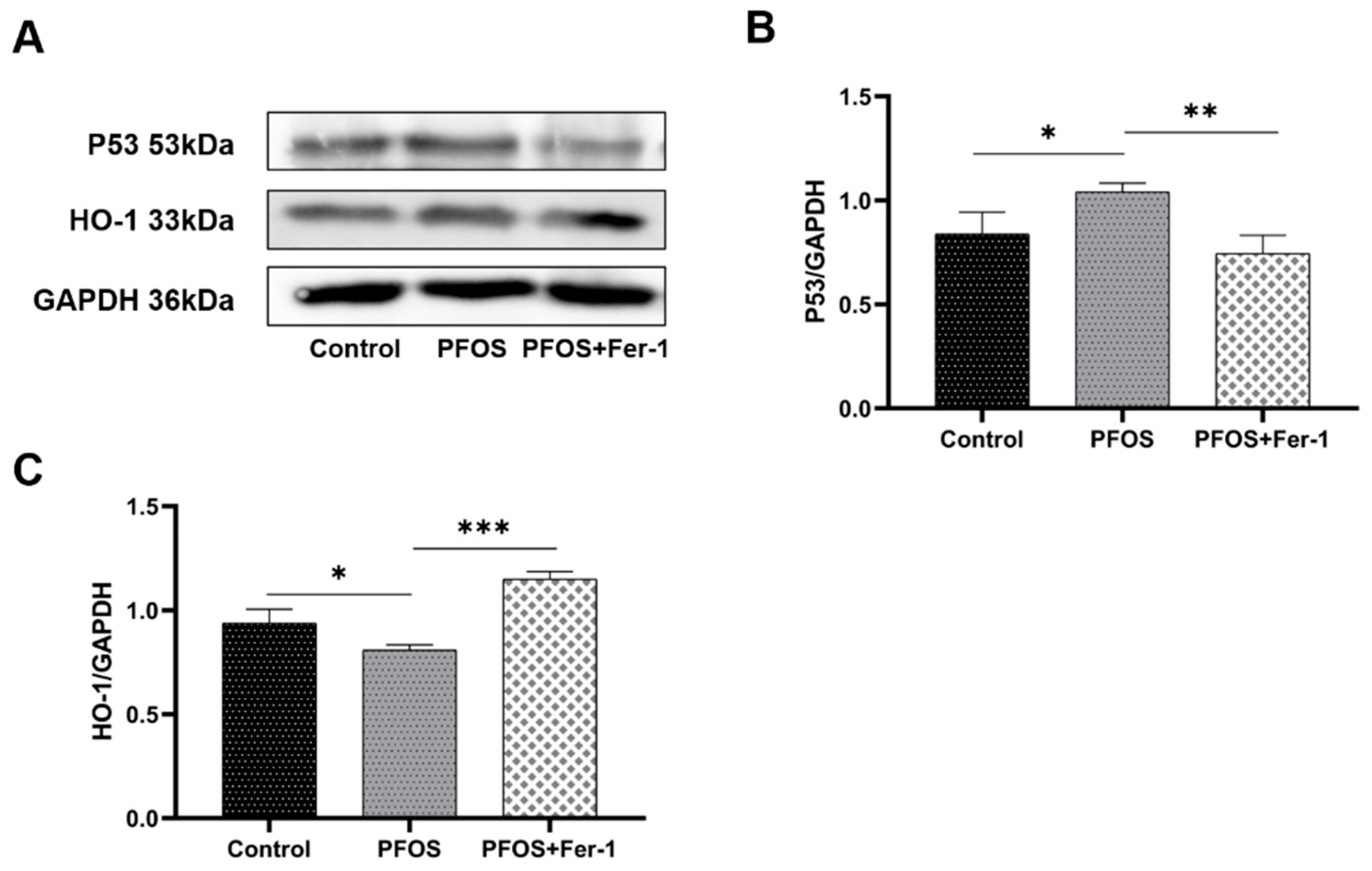
3.5. Antioxidation Suppression by PFOS Exposure
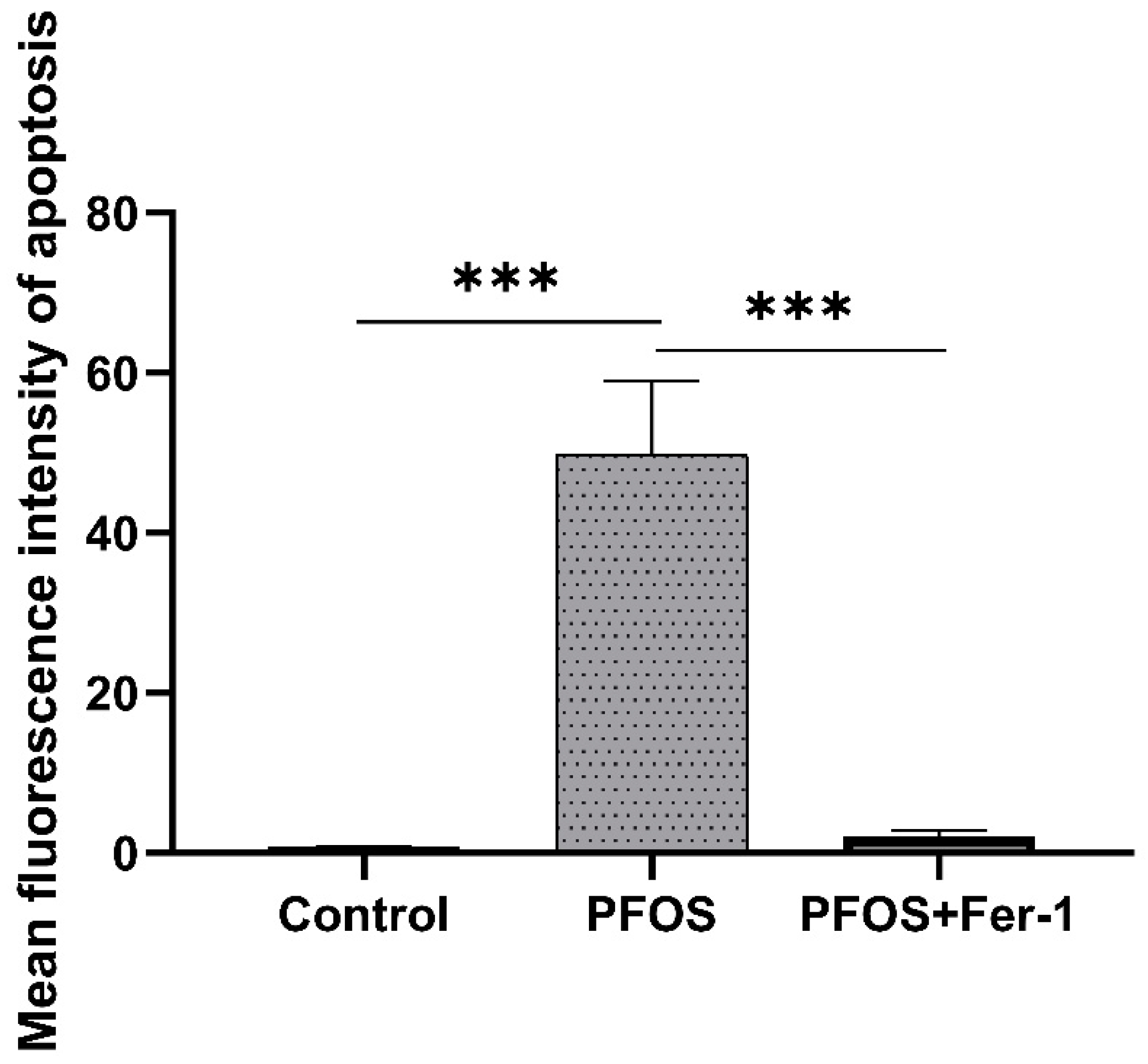
3.6. Apoptosis of HK-2 Cells Caused by PFOS
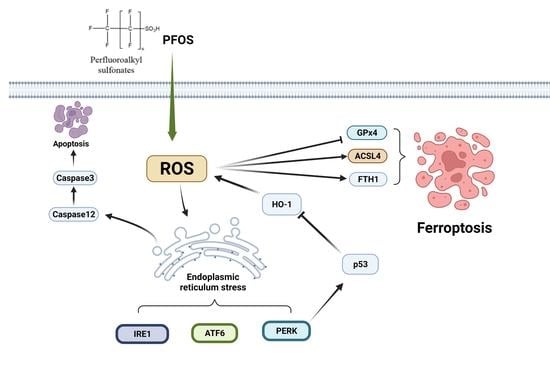
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, S.; Shukla, P.; Chen, D.; Eftekhari, E.; An, H.; Zare, F.; Ghasemi, N.; Zhang, D.; Nguyen, N.T.; Li, Q. Emerging technologies for PFOS/PFOA degradation and removal: A review. Sci. Total Environ. 2022, 827, 153669. [Google Scholar] [CrossRef] [PubMed]
- Savvaides, T.; Koelmel, J.P.; Zhou, Y.; Lin, E.Z.; Stelben, P.; Aristizabal-Henao, J.J.; Bowden, J.A.; Godri Pollitt, K.J. Prevalence and Implications of Per- and Polyfluoroalkyl Substances (PFAS) in Settled Dust. Curr. Environ. Health Rep. 2021, 8, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Baabish, A.; Sobhanei, S.; Fiedler, H. Priority perfluoroalkyl substances in surface waters—A snapshot survey from 22 developing countries. Chemosphere 2021, 273, 129612. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Mokra, K. Endocrine disruptor potential of short- and long-chain perfluoroalkyl substances (PFASs)—A synthesis of current knowledge with proposal of molecular mechanism. Int. J. Mol. Sci. 2021, 22, 2148. [Google Scholar] [CrossRef]
- Yang, C.; Lee, H.K.; Zhang, Y.; Jiang, L.L.; Chen, Z.F.; Chung, A.; Cai, Z. In Situ detection and imaging of PFOS in mouse kidney by matrix-assisted laser desorption/ionization imaging mass spectrometry. Anal. Chem. 2019, 91, 8783–8788. [Google Scholar] [CrossRef]
- Midgett, K.; Peden-Adams, M.M.; Gilkeson, G.S.; Kamen, D.L. In vitro evaluation of the effects of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on IL-2 production in human T-cells. J. Appl. Toxicol. 2015, 35, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Duan, X.; Sun, W.; Sun, H. Perfluorooctane sulfonate acute exposure stimulates insulin secretion via GPR40 pathway. Sci. Total Environ. 2020, 726, 138498. [Google Scholar] [CrossRef]
- Chen, J.; Miao, Y.; Gao, Q.; Cui, Z.; Xiong, B. Exposure to perfluorooctane sulfonate in vitro perturbs the quality of porcine oocytes via induction of apoptosis. Environ. Pollut. 2021, 284, 117508. [Google Scholar] [CrossRef] [PubMed]
- Umar Ijaz, M.; Rauf, A.; Mustafa, S.; Ahmed, H.; Ashraf, A.; Al-Ghanim, K.; Swamy Mruthinti, S.; Mahboob, S. Pachypodol attenuates Perfluorooctane sulphonate-induced testicular damage by reducing oxidative stress. Saudi J. Biol. Sci. 2022, 29, 1380–1385. [Google Scholar] [CrossRef]
- Mao, B.; Mruk, D.; Lian, Q.; Ge, R.; Li, C.; Silvestrini, B.; Cheng, C.Y. Mechanistic Insights into PFOS-mediated sertoli cell injury. Trends Mol. Med. 2018, 24, 781–793. [Google Scholar] [CrossRef]
- Coperchini, F.; Awwad, O.; Rotondi, M.; Santini, F.; Imbriani, M.; Chiovato, L. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J. Endocrinol. Investig. 2017, 40, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.D.; Cardenas, A.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.F.; Calafat, A.M.; Webster, T.F.; Horton, E.S.; Oken, E. Per- and polyfluoroalkyl substances and kidney function: Follow-up results from the diabetes prevention program trial. Environ. Int. 2021, 148, 106375. [Google Scholar] [CrossRef]
- Poothong, S.; Papadopoulou, E.; Padilla-Sánchez, J.A.; Thomsen, C.; Haug, L.S. Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environ. Int. 2020, 134, 105244. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Hliwa, P.; Sznajder-Katarzyńska, K.; Wiczkowski, W.; Topolska, J.; Zieliński, H. Perfluoroalkyl Substance contamination levels of pike (Esox lucius L.) and roach (Rutilus rutilus L.) from selected Masurian lakes in Eastern Europe. Environ. Toxicol. Chem. 2021, 40, 3317–3327. [Google Scholar] [CrossRef] [PubMed]
- Bursian, S.J.; Link, J.E.; McCarty, M.; Harr, K.; Roberts, J.; Simcik, M.F. Dietary Exposure of Japanese Quail (Coturnix japonica) to Perfluorooctane Sulfonate (PFOS) and a Legacy Aqueous Film-Forming Foam (AFFF) Containing PFOS: Effects on Reproduction and Chick Survivability and Growth. Environ. Toxicol. Chem. 2021, 40, 2521–2537. [Google Scholar] [CrossRef]
- Kataria, A.; Trachtman, H.; Malaga-Dieguez, L.; Trasande, L. Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ. Health Glob. Access Sci. Source 2015, 14, 89. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Q.; Liu, T.; Yang, S.; Sun, L.; Zhao, Z.Y.; Li, L.Y.; She, Y.C.; Zheng, Y.Y.; Ye, X.Y.; Bao, Q.; et al. Perfluoroalkyl substance pollutants activate the innate immune system through the AIM2 inflammasome. Nat. Commun. 2021, 12, 2915. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, L.; Zhang, R.; Shi, Z.; Xie, C.; Hong, Y.; Wang, J.; Cai, Z. Spatially revealed perfluorooctane sulfonate-induced nephrotoxicity in mouse kidney using atmospheric pressure MALDI mass spectrometry imaging. Sci. Total Environ. 2022, 838 Pt 3, 156380. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Yang, Z. Ferroptosis and its role in epilepsy. Front. Cell. Neurosci. 2021, 15, 696889. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Chen, C. Umbelliferone delays the progression of diabetic nephropathy by inhibiting ferroptosis through activation of the Nrf-2/HO-1 pathway. Food Chem. Toxicol. 2022, 163, 112892. [Google Scholar] [CrossRef]
- Dutta, R.K.; Kondeti, V.K.; Sharma, I.; Chandel, N.S.; Quaggin, S.E.; Kanwar, Y.S. Beneficial effects of myo-inositol oxygenase deficiency in cisplatin-induced AKI. J. Am. Soc. Nephrol. JASN 2017, 28, 1421–1436. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Xue, X.; Hou, Q.; Dai, C. Targeting ferroptosis attenuates interstitial inflammation and kidney fibrosis. Kidney Dis. 2021, 8, 57–71. [Google Scholar] [CrossRef]
- Yao, W.; Liao, H.; Pang, M.; Pan, L.; Guan, Y.; Huang, X.; Hei, Z.; Luo, C.; Ge, M. Inhibition of the NADPH oxidase pathway reduces ferroptosis during septic renal injury in diabetic mice. Oxidative Med. Cell. Longev. 2022, 2022, 1193734. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Jiang, J.; Han, J.; Li, W.; Li, X.; Yee Leung, K.M.; Snyder, S.A.; Alvarez, P. Which micropollutants in water environments deserve more attention globally? Environ. Sci. Technol. 2022, 56, 13–29. [Google Scholar] [CrossRef]
- Shi, B.; Wang, T.; Yang, H.; Zhou, Y.; Bi, R.; Yang, L.; Yoon, S.J.; Kim, T.; Khim, J.S. Perfluoroalkyl acids in rapidly developing coastal areas of China and South Korea: Spatiotemporal variation and source apportionment. Sci. Total Environ. 2021, 761, 143297. [Google Scholar] [CrossRef] [PubMed]
- Greaves, A.K.; Letcher, R.J.; Sonne, C.; Dietz, R.; Born, E.W. Tissue-specific concentrations and patterns of perfluoroalkyl carboxylates and sulfonates in East Greenland polar bears. Environ. Sci. Technol. 2012, 46, 11575–11583. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, N.M.; Minucci, J.M.; Mullikin, A.; Slover, R.; Cohen Hubal, E.A. Human exposure pathways to poly- and perfluoroalkyl substances (PFAS) from indoor media: A systematic review. Environ. Int. 2022, 162, 107149. [Google Scholar] [CrossRef] [PubMed]
- Poothong, S.; Padilla-Sánchez, J.A.; Papadopoulou, E.; Giovanoulis, G.; Thomsen, C.; Haug, L.S. Hand wipes: A useful tool for assessing human exposure to poly- and perfluoroalkyl substances (PFASs) through hand-to-mouth and dermal contacts. Environ. Sci. Technol. 2019, 53, 1985–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Hammarstrand, S.; Midberg, B.; Xu, Y.; Li, Y.; Olsson, D.S.; Fletcher, T.; Jakobsson, K.; Andersson, E.M. Cancer incidence in a Swedish cohort with high exposure to perfluoroalkyl substances in drinking water. Environ. Res. 2022, 204 Pt C, 112217. [Google Scholar] [CrossRef]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; ADQI XIII Work Group. Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. J. Am. Soc. Nephrol. JASN 2016, 27, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Ren, X.; Zhao, L.; Guo, L. Exposure to perfluorooctane sulfonate reduced cell viability and insulin release capacity of β cells. J. Environ. Sci. 2022, 115, 162–172. [Google Scholar] [CrossRef]
- Bi, C.; Junaid, M.; Liu, Y.; Guo, W.; Jiang, X.; Pan, B.; Li, Z.; Xu, N. Graphene oxide chronic exposure enhanced perfluorooctane sulfonate mediated toxicity through oxidative stress generation in freshwater clam Corbicula fluminea. Chemosphere 2022, 297, 134242. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, M.; Kagan, V.E.; Bayir, H.; Pagnussat, G.C.; Head, B.; Traber, M.G.; Stockwell, B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018, 32, 602–619. [Google Scholar] [CrossRef] [Green Version]
- Kinowaki, Y.; Kurata, M.; Ishibashi, S.; Ikeda, M.; Tatsuzawa, A.; Yamamoto, M.; Miura, O.; Kitagawa, M.; Yamamoto, K. Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab. Investig. 2018, 98, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, X.; Tan, Q.; Zhou, H.; Xu, J.; Gu, Q. Inhibiting Ferroptosis through Disrupting the NCOA4-FTH1 Interaction: A New Mechanism of Action. ACS Cent. Sci. 2021, 7, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Zheng, J.; Xu, X.N.; Gu, C.; Li, W. Uranium induces kidney cells apoptosis via reactive oxygen species generation, endoplasmic reticulum stress and inhibition of PI3K/AKT/mTOR signaling in culture. Environ. Toxicol. 2022, 37, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, B.; Liu, X.; Li, P.; Zhang, P.; Ye, F.; Zhao, T.; Kuang, Y.; Chen, W.; Jin, X.; et al. PERK Regulates the sensitivity of hepatocellular carcinoma cells to High-LET carbon ions via either apoptosis or ferroptosis. J. Cancer 2022, 13, 669–680. [Google Scholar] [CrossRef]
- Kalo, E.; Kogan-Sakin, I.; Solomon, H.; Bar-Nathan, E.; Shay, M.; Shetzer, Y.; Dekel, E.; Goldfinger, N.; Buganim, Y.; Stambolsky, P.; et al. Mutant p53R273H attenuates the expression of phase 2 detoxifying enzymes and promotes the survival of cells with high levels of reactive oxygen species. J. Cell Sci. 2012, 125 Pt 22, 5578–5586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Wang, S.; Jiang, L.; Sun, X.; Li, J.; Liu, X.; Yao, X.; Zhang, C.; Wang, N.; Deng, H.; et al. 2-undecanone protects against fine particles-induced heart inflammation via modulating Nrf2/HO-1 and NF-κB pathways. Environ. Toxicol. 2022, 37, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, B.; Nemeth, Z.; Correa-Costa, M.; Bulmer, A.C.; Otterbein, L.E. Heme oxygenase-1: A metabolic nike. Antioxid. Redox Signal. 2014, 20, 1709–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Dilxat, T.; Shi, Q.; Qiu, T.; Lin, J. The combination of nicotinamide mononucleotide and lycopene prevents cognitive impairment and attenuates oxidative damage in D-galactose induced aging models via Keap1-Nrf2 signaling. Gene 2022, 822, 146348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, M.; Zhu, P.; Huang, H.; Zhuang, Q.; Shen, J.; Cai, Y.; Zhao, M.; He, Q. New Insights into the Nrf-2/HO-1 Signaling Axis and Its Application in Pediatric Respiratory Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3214196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; An, Y.; Jiao, W.; Zhang, Z.; Han, H.; Gu, X.; Teng, X. Selenium Protects against Lead-induced Apoptosis via Endoplasmic Reticulum Stress in Chicken Kidneys. Biol. Trace Elem. Res. 2018, 182, 354–363. [Google Scholar] [CrossRef]
- Guo, Y.; Hao, D.; Hu, H. High doses of dexamethasone induce endoplasmic reticulum stress-mediated apoptosis by promoting calcium ion influx-dependent CHOP expression in osteoblasts. Mol. Biol. Rep. 2021, 48, 7841–7851. [Google Scholar] [CrossRef]
- Baek, A.R.; Hong, J.; Song, K.S.; Jang, A.S.; Kim, D.J.; Chin, S.S.; Park, S.W. Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice. Exp. Mol. Med. 2020, 52, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Feng, X.; Li, L.; Luo, J.; Liu, X.; Zheng, J.; Fan, X.; Liu, Y.; Xu, X.; Zhou, G.; et al. Effects of quercetin on tenderness, apoptotic and autophagy signalling in chickens during post-mortem ageing. Food Chem. 2022, 383, 132409. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Liu, D.; Yan, S.; Liang, Y.; Cui, J.; Guo, L.; Ren, S.; Chen, P. The Role of Ferroptosis in the Damage of Human Proximal Tubule Epithelial Cells Caused by Perfluorooctane Sulfonate. Toxics 2022, 10, 436. https://doi.org/10.3390/toxics10080436
Wang P, Liu D, Yan S, Liang Y, Cui J, Guo L, Ren S, Chen P. The Role of Ferroptosis in the Damage of Human Proximal Tubule Epithelial Cells Caused by Perfluorooctane Sulfonate. Toxics. 2022; 10(8):436. https://doi.org/10.3390/toxics10080436
Chicago/Turabian StyleWang, Pingwei, Dongge Liu, Shuqi Yan, Yujun Liang, Jiajing Cui, Li Guo, Shuping Ren, and Peng Chen. 2022. "The Role of Ferroptosis in the Damage of Human Proximal Tubule Epithelial Cells Caused by Perfluorooctane Sulfonate" Toxics 10, no. 8: 436. https://doi.org/10.3390/toxics10080436
APA StyleWang, P., Liu, D., Yan, S., Liang, Y., Cui, J., Guo, L., Ren, S., & Chen, P. (2022). The Role of Ferroptosis in the Damage of Human Proximal Tubule Epithelial Cells Caused by Perfluorooctane Sulfonate. Toxics, 10(8), 436. https://doi.org/10.3390/toxics10080436