Diclofenac Concentrations in Post-Mortem Specimens—Distribution, Case Reports, and Validated Method (UHPLC-QqQ-MS/MS) for Its Determination
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Blank Samples
2.4. Case Reports
2.5. Working Solutions, Calibration Curve, and Quality Control Samples
2.6. Sample Preparation
2.7. Method Validation
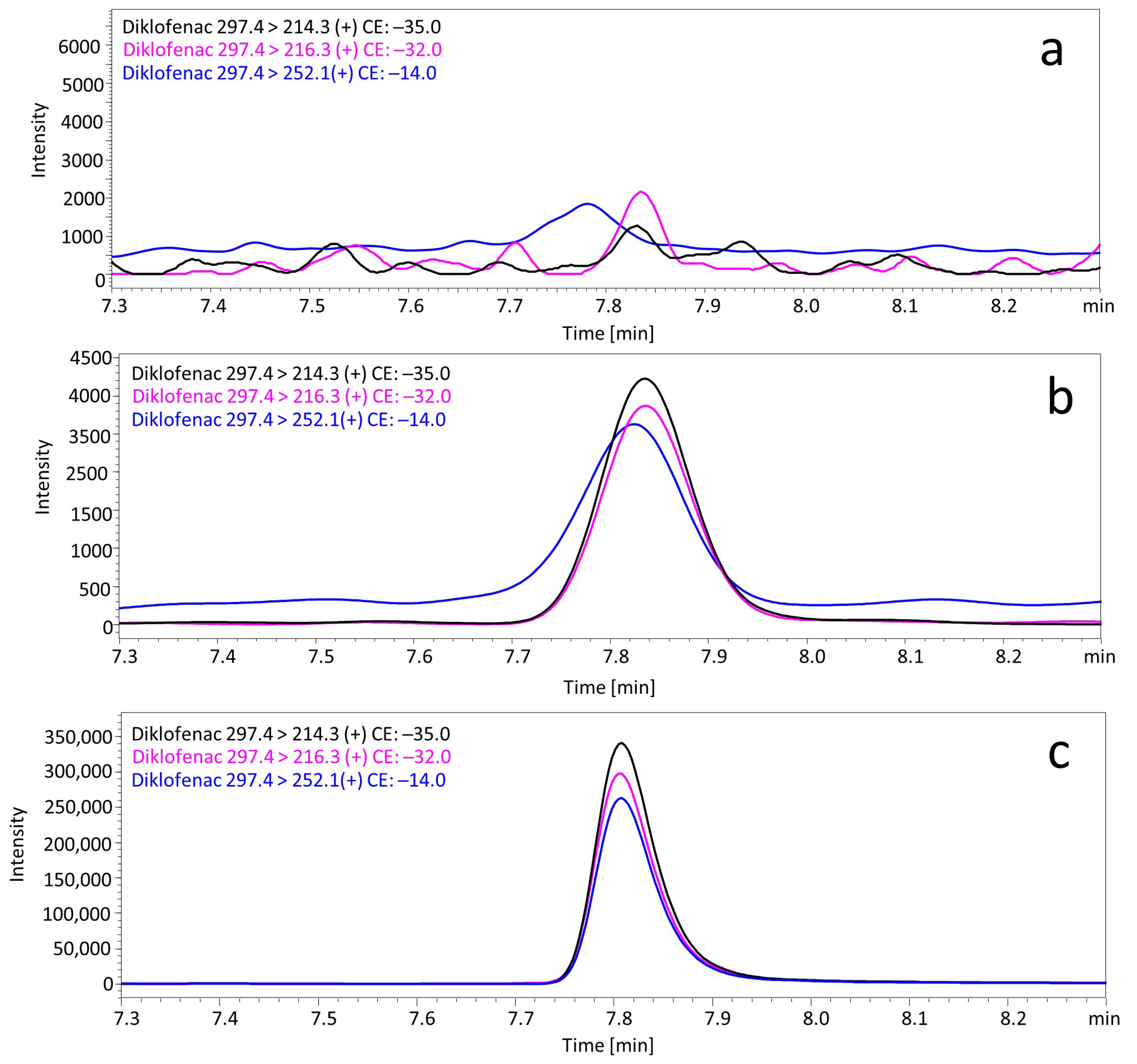
2.7.1. Selectivity
2.7.2. Linearity
2.7.3. Precision and Accuracy
2.7.4. Lower Limits of Quantification (LLOQ)
2.7.5. Recovery and Matrix Effect
3. Results
3.1. Method Development
3.2. Diclofenac Concentrations in Biological Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- O’Brien, W.M. Adverse reactions to nonsteroidal anti-inflammatory drugs diclofenac compared with other nonsteroidal anti-inflammatory drugs. Am. J. Med. 1986, 80, 70–80. [Google Scholar] [CrossRef]
- Netter, P.; Lambert, H.; Larcan, A.; Godbillon, J.; Gosset, G. Diclofenac sodium-chlormezanone poisoning. Eur. J. Clin. Pharmacol. 1984, 26, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Moneret-Vautrin, D.A.; Morisset, M.; Flabbee, J.; Beaudouin, E.; Kanny, G. Epidemiology of life-threatening and lethal anaphylaxis: A review. Allergy 2005, 60, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Van Der Klauw, M.M.; Wilson, J.H.P.; Stricker, B.H.C.H. Drug-associated anaphylaxis: 20 years of reporting in the Netherlands (1974–1994) and review of the literature. Clin. Exp. Allergy 1996, 26, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Van der Klauw, M.M.; Stricker, B.H.C.H.; Herings, R.M.C.; Cost, W.S.; Valkenburg, H.A.; Wilson, J.H. A population based case-cohort study of drug-induced anaphylaxis. Br. J. Clin. Pharmacol. 1993, 35, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.; Conforti, A.; Venegoni, M.; Motola, D.; Moretti, U.; Meneghelli, I.; Cocci, A.; Cellini, G.S.; Scotto, S.; Montanaro, N.; et al. Drug-induced anaphylaxis: Case/non-case study based on an italian pharmacovigilance database. Drug Saf. 2005, 28, 547–556. [Google Scholar] [CrossRef]
- Van Puijenbroek, E.P.; Egberts, A.C.G.; Meyboom, R.H.B.; Leufkens, H.G.M. Different risks for NSAID induced anaphylaxis. Ann. Pharmacother. 2002, 36, 24–29. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Nguyen, H.A.; Vu, D.H.; Le, T.T.; Nguyen, H.A., Jr.; Dang, B.V.; Nguyen, T.N.; Nguyen, D.H.; Nguyen, T.B.; Montastruc, J.L.; et al. Drug-Induced Anaphylaxis in a Vietnamese Pharmacovigilance Database: Trends and Specific Signals from a Disproportionality Analysis. Drug Saf. 2019, 42, 671–682. [Google Scholar] [CrossRef]
- Sen, I.; Mitra, S.; Gombar, K.K. Fatal anaphylactic reaction to oral diclofenac sodium. Can. J. Anesth. 2001, 48, 421. [Google Scholar] [CrossRef][Green Version]
- Milman, U.; Hermoni, D. Anaphylactic reaction to oral diclofenac sodium sustained-release tablet. Isr. J. Med. Sci. 1994, 30, 909–910. [Google Scholar]
- Sanuki, T.; Sugioka, S.; Kotani, J. Anaphylactic Reaction Induced by Diclofenac: A Case Report. Oral. Sci. Int. 2010, 7, 34–36. [Google Scholar] [CrossRef]
- Dux, S.; Groslop, I.; Garty, M.; Rosenfeld, J.B. Anaphylactic shock induced by diclofenac. Br. Med. J. 1983, 286, 1861. [Google Scholar] [CrossRef] [PubMed]
- Badyal, D.K.; Gulrez, G.; Mahindru, D. Anaphylactic reaction induced by intravenous diclofenac: A case report. Indian J. Pharmacol. 2019, 51, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Alkhawajah, A.M.; Eifawal, M.; Mahmoud, S.F. Fatal anaphylactic reaction to diclofenac. Forensic Sci. Int. 1993, 60, 107–110. [Google Scholar] [CrossRef]
- Colak, S.; Gunes, H.; Afacan, M.A.; Kandis, H.; Erdogan, M.O.; Ayranci, M.; Saritas, A. Anaphylaxis after intramuscular injection of diclofenac sodium. Am. J. Emerg. Med. 2014, 32, 815-e1. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Tomar, G.S.; Ganguly, S.; Kapoor, M.C. Kounis syndrome resulting from anaphylaxis to diclofenac. Indian J. Anaesth. 2013, 57, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Hadar, A.; Holcberg, G.; Mazor, M. Anaphylactic shock after diclofenac sodium (Voltaren). Harefuah 2000, 138, 211–212. (In Hebrew) [Google Scholar] [PubMed]
- Jonker, M.J.; Bruynzeel, D.P. Anaphylactic reaction elicited by patch testing with diclofenac. Contact Dermat. 2003, 49, 114–115. [Google Scholar] [CrossRef]
- Picaud, J.; Beaudouin, E.; Renaudin, J.M.; Pirson, F.; Metz-Favre, C.; Dron-Gonzalvez, M.; Moneret-Vautrin, D.A. Anaphylaxis to diclofenac: Nine cases reported to the Allergy Vigilance Network in France. Allergy 2014, 69, 1420–1423. [Google Scholar] [CrossRef]
- Breen, E.G.; McNicholl, J.; Cosgrove, E.; McCabe, J.; Stevens, F.M. Fatal hepatitis associated with diclofenac. Gut 1986, 27, 1390–1393. [Google Scholar] [CrossRef]
- Arslan, M.N.; Melez, D.O.; Akcay, A.; Gur, A.; Sam, B.; Guven Apaydın, S. Coincidence of nicolau syndrome and rhabdomyolysis: Report of a forensic autopsy case and review of the literature. J. Forensic Sci. 2016, 61, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Jürgensen, J.S.; Seltsam, A.; Jörres, A. Fatal acute diclofenac-induced immune hemolytic anemia. Ann. Hematol. 2001, 80, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Verfaillie, G.; Knape, S.; Corne, L. A case of fatal necrotizing fasciitis after intramuscular administration of diclofenac. Eur. J. Emerg. Med. 2002, 9, 270–273. [Google Scholar] [CrossRef]
- Bakkali, H.; Belyamani, L.; Massou, S.; Elwartiti, L.; Aboulaala, K.; Balkhi, H.; Haimeur, C. Rhabdomyolysis Associated to Glossopharyngeal Edema: A Rare Side Effect of Diclofenac. Am. J. Clin. Exp. Med. 2014, 6, 161–164. [Google Scholar] [CrossRef]
- Delrio, F.G.; Park, Y.; Herzlich, B.; Grob, D. Case report: Diclofenac-induced rhabdomyolysis. Am. J. Med. Sci. 1996, 312, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, K.; Rossner, D.; Gössling, T.; Lichtenberg, A.; Richter, M.; Krettek, C. Rhabdomyolysis after administration of diclofenac. Unfallchirurg 2005, 108, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Güzel, A.; Biner, B.O.; Karasalihoğlu, S.; Aylanç, H. Fatal acute diclofenac-induced rhabdomyolysis in a pediatric patient. Balk. Med. J. 2009, 28, 102–103. [Google Scholar] [CrossRef]
- Watzer, B.; Lusthof, K.J.; Schweer, H. Abortion after deliberate Arthrotec® addition to food. Mass spectrometric detection of diclofenac, misoprostol acid, and their urinary metabolites. Int. J. Legal Med. 2015, 129, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Swan, G.E.; Cuthbert, R.; Quevedo, M.; Green, R.E.; Pain, D.J.; Bartels, P.; Cunningham, A.A.; Duncan, N.; Meharg, A.A.; Oaks, J.L.; et al. Toxicity of diclofenac to Gyps vultures. Biol. Lett. 2006, 22, 279–822. [Google Scholar] [CrossRef]
- Green, R.E.; Newton, I.; Shultz, S.; Cunningham, A.A.; Gilbert, M.; Pain, D.J.; Prakash, V. Diclofenac poisoning as a cause of vulture population declines across the Indian subcontinent. J. Appl. Ecol. 2004, 41, 793–800. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Chaudhry, M.J.; Arshad, M.; et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 6975, 630–633. [Google Scholar] [CrossRef]
- Shultz, S.; Baral, H.S.; Charman, S.; Cunningham, A.A.; Das, D.; Ghalsasi, G.R.; Goudar, M.S.; Green, R.E.; Jones, A.; Nighot, P.; et al. Diclofenac poisoning is widespread in declining vulture populations across the Indian subcontinent. Proc. Biol. Sci. 2004, 271 (Suppl. S6), S458–S460. [Google Scholar] [CrossRef]
- El-Yazbi, F.A.; Amin, O.A.; El-Kimary, E.I.; Khamis, E.F.; Younis, S.E. High-performance thin-layer chromatographic methods for the determination of febuxostat and febuxostat/diclofenac combination in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1086, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lala, L.G.; D’Mello, P.M.; Naik, S.R. HPTLC determination of diclofenac sodium from serum. J. Pharm. Biomed. Anal. 2002, 29, 539–544. [Google Scholar] [CrossRef]
- Moncrieff, J. Extractionless determination of diclofenac sodium in serum using reversed-phase high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. 1992, 577, 185–189. [Google Scholar] [CrossRef]
- Chmielewska, A.; Konieczna, L.; Plenis, A.; Bieniecki, M.; Lamparczyk, H. Determination of diclofenac in plasma by high-performance liquid chromatography with electrochemical detection. Biomed. Chromatogr. 2006, 20, 119–124. [Google Scholar] [CrossRef]
- Kuhlmann, O.; Stoldt, G.; Struck, H.G.; Krauss, G.J. Simultaneous determination of diclofenac and oxybuprocaine in human aqueous humor with HPLC and electrochemical detection. J. Pharm. Biomed. Anal. 1998, 17, 1351–1356. [Google Scholar] [CrossRef]
- Kole, P.L.; Millership, J.; McElnay, J.C. Determination of diclofenac from paediatric urine samples by stir bar sorptive extraction (SBSE)-HPLC-UV technique. Talanta 2011, 85, 1948–1958. [Google Scholar] [CrossRef]
- Yilmaz, B.; Asci, A.; Palabiyik, S.S. HPLC method for determination of diclofenac in human plasma and its application to a pharmacokinetic study in Turkey. J. Chromatogr. Sci. 2011, 49, 422–427. [Google Scholar] [CrossRef]
- Kaphalia, L.; Kaphalia, B.S.; Kumar, S.; Kanz, M.F.; Treinen-Moslen, M. Efficient high performance liquid chromatograph/ultraviolet method for determination of diclofenac and 4’-hydroxydiclofenac in rat serum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 830, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, A.; Karidas, T.; Malamataris, S. Extractionless high-performance liquid chromatographic method for the determination of diclofenac in human plasma and urine. J. Chromatogr. 1993, 619, 324–329. [Google Scholar] [CrossRef]
- Arcelloni, C.; Lanzi, R.; Pedercini, S.; Molteni, G.; Fermo, I.; Pontiroli, A.; Paroni, R. High-performance liquid chromatographic determination of diclofenac in human plasma after solid-phase extraction. J. Chromatogr. B Biomed. Sci. Appl. 2001, 763, 195–200. [Google Scholar] [CrossRef]
- Roskar, R.; Kmetec, V. Liquid chromatographic determination of diclofenac in human synovial fluid. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 788, 57–64. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, C.K.; Choi, S.J.; Kim, S.B.; Lee, M.H.; Ko, G.I.; Sohn, D.H. Simultaneous determination of aceclofenac and diclofenac in human plasma by narrowbore HPLC using column-switching. J. Pharm. Biomed. Anal. 2000, 23, 775–781. [Google Scholar] [CrossRef]
- Dahivelkar, P.P.; Bhoir, S.I.; Bari, S.B.; Surana, S.J.; Bhagwat, A.M. Simultaneous determination of diclofenac potassium and drotaverine hydrochloride in human plasma using reversed-phase high-performance liquid chromatography. J. Chromatogr. Sci. 2012, 50, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Cuklev, F.; Kristiansson, E.; Fick, J.; Asker, N.; Forlin, L.; Larsson, D.G.J. Diclofenac in fish: Blood plasma levels similar to human therapeutic levels affect global hepatic gene expression. Environ. Toxicol Chem. 2011, 30, 2126–2134. [Google Scholar] [CrossRef]
- Shah, I.; Barker, J.; Naughton, D.P.; Barton, S.J.; Ashraf, S.S. Determination of diclofenac concentrations in human plasma using a sensitive gas chromatography mass spectrometry method. Chem. Cent. J. 2016, 10, 52. [Google Scholar] [CrossRef]
- Del Puppo, M.; Cighetti, G.; Kienle, M.G.; Paroni, R.; Borghi, C. Determination of diclofenac in human plasma by selected ion monitoring. Biol. Mass Spectrom. 1991, 20, 426–430. [Google Scholar] [CrossRef]
- Sioufi, A.; Pommier, F.; Godbillon, J. Determination of diclofenac in plasma and urine by capillary gas chromatography-mass spectrometry with possible simultaneous determination of deuterium-labelled diclofenac. J. Chromatogr. 1991, 571, 87–100. [Google Scholar] [CrossRef]
- Dowling, G.; Gallo, P.; Fabbrocino, S.; Serpe, L.; Regan, L. Determination of ibuprofen, ketoprofen, diclofenac and phenylbutazone in bovine milk by gas chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1497–1508. [Google Scholar] [CrossRef][Green Version]
- Yilmaz, B. GC–MS determination of diclofenac in human plasma. Chroma 2010, 71, 549–551. [Google Scholar] [CrossRef]
- Krokos, A.; Tsakelidou, E.; Michopoulou, E.; Raikos, N.; Theodoridis, G.; Gika, H. NSAIDs determination in human serum by GC-MS. Separations 2018, 5, 37. [Google Scholar] [CrossRef]
- Choi, M.H.; Choi, Y.K.; Chung, B.C. Rapid and Sensitive Analysis of Diclofenac in Human Plasma by GC/SIM/MS. Anal. Lett. 1999, 32, 2245–2253. [Google Scholar] [CrossRef]
- Borenstein, M.R.; Xue, Y.; Cooper, S.; Tzeng, T.B. Sensitive capillary gas chromatographic-mass spectrometric-selected-ion monitoring method for the determination of diclofenac concentrations in human plasma. J. Chromatogr. B Biomed. Appl. 1996, 685, 59–66. [Google Scholar] [CrossRef]
- de Jong, E.G.; Kiffers, J.; Maes, R.A. The determination of non-steroidal anti-inflammatory drugs by GC-MS-MS in equine urine. J. Pharm. Biomed. Anal. 1989, 12, 1617–1622. [Google Scholar] [CrossRef]
- Szpot, P.; Wachełko, O.; Zawadzki, M. Application of ultra-sensitive GC-QqQ-MS/MS (MRM) method for the determination of diclofenac in whole blood samples without derivatization. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1179, 122860. [Google Scholar] [CrossRef] [PubMed]
- Saraji, M.; Maleki, B.; Khayamian, T.; Mehrafza, N. Electrospray ionization-ion mobility spectrometry in the negative mode combined with hollow fiber liquid–liquid–liquid microextraction for the determination of diclofenac in urine and plasma samples. Chromatographia 2017, 80, 951–959. [Google Scholar] [CrossRef]
- Nazario, C.E.D.; Lancas, F.M. Determination of Diclofenac in Bovine Milk at Low Levels Using Ultra High Performance Liquid Chromatography–Tandem Mass Spectrometry. Food Anal. Methods 2017, 10, 2490–2496. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, X.W.; Kong, X.J.; Qin, Z.; Li, S.H.; Jiao, Z.H.; Li, J.Y. An LC-MS/MS method for the quantification of diclofenac sodium in dairy cow plasma and its application in pharmacokinetics studies. Biomed. Chromatogr. 2019, 33, e4520. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. High-throughput ultra-performance LC-MS-MS method for analysis of diclofenac sodium in rabbit plasma. J. Chromatogr. Sci. 2015, 53, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Sparidans, R.W.; Lagas, J.S.; Schinkel, A.H.; Schellens, J.H.; Beijnen, J.H. Liquid chromatography-tandem mass spectrometric assay for diclofenac and three primary metabolites in mouse plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 872, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.; Wagrowski-Diehl, D.M.; Lu, Z.; Mazzeo, J.R. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J. Chromatogr. B 2007, 852, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.X.; Namiranian, K.; Dehghanyar, P.; Stroh, R.; Mascher, H.; Müller, M. Comparison of UV and tandem mass spectrometric detection for the high-performance liquid chromatographic determination of diclofenac in microdialysis samples. J. Pharm. Biomed. Anal. 2003, 33, 745–754. [Google Scholar] [CrossRef]
- Nirogi, R.; Padala, N.S.; Boggavarapu, R.K.; Kalaikadhiban, I.; Ajjala, D.R.; Bhyrapuneni, G.; Muddana, N.R. Skin sample preparation by collagenase digestion for diclofenac quantification using LC-MS/MS after topical application. Bioanalysis 2016, 12, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ma, H.; Cen, N.; Zhou, A.; Tao, H. A pharmacokinetic study of diclofenac sodium in rats. Biomed. Rep. 2017, 7, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Dehghanyar, P.; Seigfried, B.; Martin, W.; Menke, G.; Müller, M. Favourable dermal penetration of diclofenac after administration to the skin using a novel spray gel formulation. Br. J. Clin. Pharmacol. 2005, 60, 573–577. [Google Scholar] [CrossRef]
- Suenami, K.; Lim, L.W.; Takeuchi, T.; Sasajima, Y.; Sato, K.; Takekoshi, Y.; Kanno, S. Rapid and simultaneous determination of nonsteroidal anti-inflammatory drugs in human plasma by LC-MS with solid-phase extraction. Anal. Bioanal. Chem. 2006, 384, 1501–1505. [Google Scholar] [CrossRef]
- Di Rago, M.; Saar, E.; Rodda, L.N.; Turfus, S.; Kotsos, A.; Gerostamoulos, D.; Drummer, O.H. Fast targeted analysis of 132 acidic and neutral drugs and poisons in whole blood using LC-MS/MS. Forensic Sci. Int. 2014, 243, 35–43. [Google Scholar] [CrossRef]
- Sørensen, L.K. Determination of acidic and neutral therapeutic drugs in human blood by liquid chromatography-electrospray tandem mass spectrometry. Forensic Sci. Int. 2011, 206, 119–126. [Google Scholar] [CrossRef]
- Di Rago, M.; Pantatan, S.; Hargreaves, M.; Wong, K.; Mantinieks, D.; Kotsos, A.; Glowacki, L.; Drummer, O.H.; Gerostamoulos, D. High throughput detection of 327 drugs in blood by LC-MS-MS with automated data processing. J. Anal. Toxicol. 2021, 45, 154–183. [Google Scholar] [CrossRef]
- Al-Asmari, A.I. Method for the identification and quantification of sixty drugs and their metabolites in postmortem whole blood using liquid chromatography tandem mass spectrometry. Forensic Sci. Int. 2020, 309, 110193. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.E.; Fletcher, A.K. Fatal nefopam overdose. Emerg. Med. J. 2010, 27, 407–408. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, I.M.; Mallett, P.; Stolberg, S.; Haas, E.A.; Mena, O. Striking increases in postmortem compared to antemortem drug concentrations in a suicidal overdose: A case report. Aust. J. Forensic Sci. 2015, 48, 37–41. [Google Scholar] [CrossRef]
- Fels, H.; Lottner-Nau, S.; Sax, T.; Roider, G.; Graw, M.; Auwärter, V.; Musshoff, F. Postmortem concentrations of the synthetic opioid U-47700 in 26 fatalities associated with the drug. Forensic Sci. Int. 2019, 301, e20–e28. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Retention Time (min.) | Precursor Ions (m/z) | Product Ions (m/z) | Dwell Time (msec) | Q1 Pre Bias (V) | CE (V) | Q3 Pre Bias (V) |
|---|---|---|---|---|---|---|---|
| Diclofenac | 7.807 | 297.3 | 214.3 216.3 * 252.1 | 17 | −11 −15 −15 | −35 −32 −14 | −22 −23 −16 |
| Diclofenac-d4 | 7.794 | 301.5 | 220.2 218.3 * | 17 | −17 −18 | −37 −34 | −14 −14 |
| Biological Matrix | Validation Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| The Linear Concentration Range [ng/mL] or [ng/g] | The Coefficient of Determination (R2) | LLOQ [ng/mL] or [ng/g] | Concentration Level [ng/mL] or [ng/g] | Intraday | Interday | Recovery [%] * | Matrix Effect [%] * | |||
| Precision [%] * | Accuracy [%] * | Precision [%] * | Accuracy [%] * | |||||||
| Bile | 0.5–500 | >0.998 | 0.5 | 1 50 500 | 2.0 3.5 1.2 | −4.3 13.8 5.7 | 10.1 0.9 1.8 | −0.2 7.7 9.1 | 73.5 94.1 90.7 | −26.5 −5.9 −9.3 |
| Blood | 0.5–500 | >0.998 | 0.5 | 1 50 500 | 10.7 1.6 2.7 | −12.7 7.9 −4.0 | 1.0 2.8 0.3 | −5.0 7.7 −0.7 | 102.2 92.6 93.7 | 2.2 −7.4 −6.3 |
| Placenta | 0.5–500 | >0.997 | 0.5 | 1 50 500 | 2.8 7.6 8.7 | 8.3 14.0 11.2 | 11.6 7.9 11.1 | 3.3 11.6 9.4 | 79.5 95.9 93.1 | −20.5 −4.2 −6.9 |
| Urine | 0.5–500 | >0.997 | 0.5 | 1 50 500 | 0.8 10.8 8.1 | −9.5 2.2 −3.4 | 2.4 9.8 0.8 | 1.0 −3.9 −6.9 | 72.0 84.2 80.1 | −28.0 −15.8 −19.9 |
| Kidney | 0.5–500 | >0.999 | 0.5 | 1 50 500 | 2.2 4.2 11.9 | 10.7 2.7 2.9 | 14.6 2.4 5.0 | 14.9 −2.3 1.0 | 84.8 85.1 86.1 | −15.2 −14.9 −13.9 |
| Liver | 0.5–500 | >0.999 | 0.5 | 1 50 500 | 1.2 3.6 4.3 | −3.8 2.8 12.0 | 2.2 4.5 5.5 | 2.0 8.3 2.0 | 74.5 89.7 90.3 | −25.5 −10.3 −9.7 |
| Stomach content | 0.5–500 | >0.997 | 0.5 | 1 50 500 | 13.1 3.9 5.0 | 12.0 2.3 1.2 | 0.7 0.5 6.0 | 14.5 −9.4 2.1 | 85.2 82.0 85.8 | −14.8 −18.0 −14.2 |
| Concentrations of Diclofenac [ng/mL a or ng/g b] | |||||||
|---|---|---|---|---|---|---|---|
| Case 1 (Female Fetus) | Case 2 (Male) | Case 3 (Male) | Case 4 (Female Fetus) | Case 5 (Female Fetus) | Case 6 (Female) | ||
| Biological Fluids a | Blood | 429.5 | 108.2 | 121.7 | ─ | ─ | 207.2 |
| Vitreous humor | ─ | 10.7 | 37.8 | ─ | ─ | 15.1 | |
| Urine | ─ | 82.4 | 12 631.3 | ─ | ─ | ─ | |
| Bile | ─ | 14 931.1 | ─ | ─ | ─ | ─ | |
| Stomach content | ─ | 229.1 | ─ | ─ | ─ | ─ | |
| Solid Tissues b | Liver | ─ | 50.5 | ─ | ─ | 6938.0 | ─ |
| Kidney | ─ | 153.8 | ─ | ─ | ─ | ─ | |
| Heart | ─ | ─ | ─ | ─ | 6585.0 | ─ | |
| Bones | ─ | ─ | ─ | 50.0 | ─ | ─ | |
| Placenta | 1036.7 | ─ | ─ | ||||
| Biological Sample (Volume) | Sample Preparation | Instruments (Mode) | Recovery [%] /Internal Standard | LOQ [ng/mL] (Injection Volume) | References |
|---|---|---|---|---|---|
| Fish plasma (500 µL) | SPE | ESI-HPLC-QqQ-MS/MS (SRM) | 76.0 13CD3-labeled naproxen | ─ (10 µL) | [46] |
| Bovine milk (2000 µL) | Two steps LLE (ethyl acetate) | ESI-UHPLC-QqQ-MS/MS (MRM) | 85.5−89.1 diclofenac-d4 | 0.05 (30 μL) | [58] |
| Dairy cow plasma (200 μL) | Protein precipitation with ACN HCOOH | ESI-HPLC-QqQ-MS/MS (MRM) | 97.6−101.8 tolfenamic acid | 5 (5μL) | [59] |
| Rabbit plasma (100 µL) | Protein precipitation with ACN | ESI-UHPLC-QqQ-MS/MS (MRM) | 54.1−67.1 flufenamic acid | 80 (10 µL) | [60] |
| Mouse plasma (10 µL) | Protein precipitation with ACN | ESI-HPLC-QqQ-MS/MS (SRM) | 89.0−103.0 diclofenac-d4 | 20 (30 µL) | [61] |
| Ringer-microdialysis samples (25 µL) | Dissolution in methanol and formic acid | HPLC/-QqQ-MS/MS (SRM) | ─ indomethacine | 1 (20 µL) | [63] |
| Rat skin (50 μL of enzymatically treated and homogenized sample) | LLE (methyl tert-butyl ether) | ESI-HPLC-QqQ-MS/MS (MRM) | 64.5−68.4 diclofenac-d4 | 200 b (5 µL) | [64] |
| Rat plasma (50 µL) | Protein precipitation with MeOH | ESI-HPLC-QqQ-MS/MS (MRM) | ─ naproxen | ─ (7 µL) | [65] |
| Human plasma (1000 µL) | LLE (cyclohexane: tert. butylmethyl ether) | ESI-HPLC-QqQ-MS/MS (MRM) | ─ diclofenac-d6 | 0.15 (50 µL) | [66] |
| Human plasma (500 µL) | SPE | ESI-HPLC-MS (SIM) | 84.9 ─ | 100 a (5 µL) | [67] |
| Human whole blood (100 µL) | Protein precipitation with ACN | ESI-HPLC-QqQ-MS/MS (MRM) | 82.0−103.0 nimodipine-d7 | 500 a (100 µL) | [68] |
| Human whole blood (100 µL) | Protein precipitation with ACN | ESI-HPLC-QqQ-MS/MS (SRM) | 84.0−93.0 3-acetamidophenol | 60 (10 µL) | [69] |
| Human whole blood (100 µL) | LLE (pH 9.2; butyl chloride: isopropanol) | ESI-HPLC-QqQ-MS/MS (MRM) | −92.0 to −96.0 MDMA-d5 | 100 (20 µL) | [70] |
| Human whole blood (1000 µL) | SPE | UHPLC-QqQ-MS/MS (MRM) | 90.6−97.1 diazepam-d5 | 5 (1 µL) | [71] |
| Human whole blood (200 µL) | LLE (pH 9.0; ethyl acetate) | ESI-UHPLC-QqQ-MS/MS (MRM) | 92.6−102.2 diclofenac-d4 | 0.5 (2 µL) | Presented method |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpot, P.; Wachełko, O.; Zawadzki, M. Diclofenac Concentrations in Post-Mortem Specimens—Distribution, Case Reports, and Validated Method (UHPLC-QqQ-MS/MS) for Its Determination. Toxics 2022, 10, 421. https://doi.org/10.3390/toxics10080421
Szpot P, Wachełko O, Zawadzki M. Diclofenac Concentrations in Post-Mortem Specimens—Distribution, Case Reports, and Validated Method (UHPLC-QqQ-MS/MS) for Its Determination. Toxics. 2022; 10(8):421. https://doi.org/10.3390/toxics10080421
Chicago/Turabian StyleSzpot, Paweł, Olga Wachełko, and Marcin Zawadzki. 2022. "Diclofenac Concentrations in Post-Mortem Specimens—Distribution, Case Reports, and Validated Method (UHPLC-QqQ-MS/MS) for Its Determination" Toxics 10, no. 8: 421. https://doi.org/10.3390/toxics10080421
APA StyleSzpot, P., Wachełko, O., & Zawadzki, M. (2022). Diclofenac Concentrations in Post-Mortem Specimens—Distribution, Case Reports, and Validated Method (UHPLC-QqQ-MS/MS) for Its Determination. Toxics, 10(8), 421. https://doi.org/10.3390/toxics10080421






