Indoor Air Quality Monitoring and Characterization of Airborne Workstations Pollutants within Detergent Production Plant
Abstract
:1. Introduction
2. Materials and Methods
Study Design and Measurements
3. Statistical Analysis
4. Results
4.1. Visual Assessment of Workstation
4.2. PM2.5, PM10, Formaldehyde and Temperature—The FFA
4.3. Volatile Organic Compounds (VOCs)
4.4. Carbon Dioxide (CO2) and Relative Humidity (RH)
4.5. Particulate Matter Characterization
4.6. Sodium Oxide (Na2O)
4.7. Aluminum Oxide (Al2O3)
4.8. Silicon Dioxide (SiO2)
4.9. Sulfur Trioxide (SO3)
4.10. Calcium Oxide (CaO)
4.11. Iron (iii) Oxide (Fe2O3)
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UCSF Institute for Health & Aging; UC Berkeley Center for Environmental Research and Children’s Health; Informed Green Solutions; California Department of Pesticide Regulation. Green Cleaning, Sanitizing, and Disinfecting: A Toolkit for Early Care and Education; University of California, San Francisco School of Nursing: San Francisco, CA, USA, 2013; Available online: https://cerch.berkeley.edu/sites/default/files/green_cleaning_toolkit.pdf (accessed on 14 June 2021).
- EPA402-08/008; Care for Your Air: A Guide to Indoor Air Quality. United Stated Environmental Protection Agency [USEPA] : Washington, DC, USA, 2008. Available online: https://www.epa.gov/report-environment/indoor-air-quality (accessed on 20 June 2022).
- Basketter, D.; Berg, N.; Kruszewski, F.H.; Sarlo, K.; Concoby, B. Relevance of sensitization to occupational allergy and asthma in the detergent industry. J. Immunotoxicol. 2012, 9, 314–319. [Google Scholar] [CrossRef]
- Flindt, M.L. Biological miracles and misadventures: Identification of sensitization and asthma in enzyme detergent workers. Am. J. Ind. Med. 1996, 29, 99–110. [Google Scholar] [CrossRef]
- Zachariae, H.; Høech-Thomsen, J.; Witmeur, O.; Wide, L. Detergent enzymes and occupational safety: Observations on sensitization during Esperase production. Allergy 1981, 36, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Massachusetts Department of Environmental Protection [MDEP]. Health and Environmental Effects of Air Pollution; Massachusetts Department of Environmental Protection [MDEP]: Boston, MA, USA, 2013. Available online: https://www.mass.gov/doc/2013-annual-air-quality-report/download (accessed on 12 May 2022).
- Phillips, T.J. Protecting Commercial Buildings from Outdoor Pollutants: School Office and Multifamily Buildings; ROCIS Initiative: Pittsburgh, PA, USA, 2014. [Google Scholar]
- Agency for Toxic Substances and Disease Registry [ATSDR]. Case Study in Environmental Medicine: Taking an Exposure History; Agency for Toxic Substances and Disease Registry [ATSDR]: Atlanta, GA, USA, 2015. [Google Scholar]
- Omrane, F.; Gargouri, J.; Khadhraoui, M.; Elleuch, B.; Navier, D.Z. Risk assessment of occupational exposure to heavy metal mixtures: A study protocol. J. Bio. Med. Cent. Public Health 2018, 18, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United State Environmental Protection Agency (USEPA). Biological Pollutants’ Impact on Indoor Air Quality; United State Environmental Protection Agency (USEPA): Washington, DC, USA, 2017. Available online: https://www.epa.gov/indoor-air-quality-iaq/biological-pollutants-impact-indoor-air-quality (accessed on 12 December 2021).
- Mukurarinda, J. Characterization of Industrial Volatile Organic Compounds Emission in Rwanda and Bio Filtration of Acetone, Dimethyl Sulphide and Hexane. Master’s Thesis, Ghent University, Ghent, Belgium, 2016. [Google Scholar]
- Diodu, R.; Bucur, E.; Galaon, T.; Pascu, L.F. Indoor air exposure to aldehydes and ketones in rooms with new and old furniture of a new office building. J. Environ. Prot. Ecol. 2015, 16, 832–838. [Google Scholar]
- Szczurek, A.; Maciejewska, A.; Poloczanski, R.; Teuerle, M.; Wylomanska, A. Dynamics of carbon dioxide concentration in indoor air. J. Stoch. Environ. Res. Risk Manag. 2014, 29, 2193. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Laurent, O.; Li, L.; Hu, J.; Kleeman, M. Adverse Reproductive Health Outcomes and Exposure to Gaseous and Particulate-Matter Air Pollution in Pregnant Women. Res. Rep. Health Eff. Inst. 2016, 2016, 1–58. [Google Scholar]
- Zhang, N.; Lu, H.; Zhang, R.; Sun, X. Protective effects of methane-rich saline on mice with allergic asthma by inhibiting inflammatory response, oxidative stress and apoptosis. J. Zhejiang Univ.-Sci. B 2019, 20, 828–837. [Google Scholar] [CrossRef]
- Agida, G.O.; Forcados, G.E.; Usman, Y.; Muraina, I.; Ottor, M.; Samuel, A.L.; Usman, A.S.; Makoshi, M.S.; Atiku, A.; Gotep, J.G.; et al. Proximate, Antinutritional And Mineral Composition of Some Pasture Plants Growing In Vom, Nigeria. Res. J. Agric. 2017, 4, 1–8. Available online: https://researchjournali.com/view.php? id=3600 (accessed on 19 March 2020).
- Google Earth. Satellite Map Showing NHPL Jos Plateau State, Nigeria. 2019. Available online: https://www.nasco.net/companies/nasco-household/ (accessed on 22 June 2022).
- NASCO Group Report. (n.d.). History and Heritage; NASCO Press: Jos, Nigeria; Available online: https://www.nasco.net/about-us/history-heritage/ (accessed on 20 June 2022).
- NASCO Brochure. NASCO Press: Jos, Nigeria, 2022. Available online: https://www.nasco.net/news/downloads/ (accessed on 20 June 2022).
- Occupational Safety and Health Administration [OSHA]. Indoor air quality investigation. In OSHA Technical Manual (OTM); 2018; Section iii, Chapter 2. Available online: https://www.osha.gov/otm/section-3-health-hazards/chapter-2 (accessed on 13 May 2021).
- National Environmental Standards and Regulations Enforcement Agency [NESREA]. Draft of the National Environmental (Air Quality Control) Regulations; NESREA Press: Abuja, Nigeria, 2014; pp. 16–17. Available online: https://lawnigeria.com/2020/08/national-environmental-air-quality-control-regulations-2014/ (accessed on 15 June 2022).
- Chen, Q.F.; Milburn, R.K. Real time monitoring of hazardous airborne chemicals: A styrene investigation. J. Hazard. Mater. 2006, 132, 261–268. [Google Scholar] [CrossRef]
- Whalley, J.; Zandi, S. Particulate matter sampling technique and data modelling methods. Open Access 2016, 2, 29–54. [Google Scholar]
- Napoli, C.; Marcotrigiano, V.; Montagna, M.T. Air sampling procedures to evaluate microbial contamination: A comparison between active and passive methods in operating theatres. BMC Public Health 2012, 12, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specac. Analyzing the Composition of Cement—XRF Sample Prep. Azo Mater. 2018. Available online: https://www.environmental-expert.com/articles/xrf-sample-preparation-techniques-pellets-versus-loose-powder-spectroscopy-guides-778859 (accessed on 17 July 2021).
- Yamada, Y. Sample Preparation for X-Ray Florescence Analysis. Rigaku J. 2014, 30, 29. Available online: https://www.rigaku.com/newsletters/mabu/may2016/RigakuJournal_XRF.pdf (accessed on 23 January 2021).
- Yin, H.; Liu, C.; Zhang, L.; Li, A.; Ma, Z. Measurement and evaluation of indoor air quality in naturally ventilated residential buildings. Indoor Built Environ. J. 2019, 28, 1307–1323. [Google Scholar] [CrossRef]
- Occupational Safety and Health Administration (OSHA). Record Retention Requirements for Indoor Air Quality Documents and Reports; 2002. Available online: https://www.osha.gov/laws-regs/standardinterpretations/2002-08-0 (accessed on 16 February 2021).
- Ling, Z.H.; Zhao, J.; Fan, S.J.; Wang, X.M. Sources of formaldehyde and their contributions to photochemical O3 formation at an urban site in the Pearl River Delta, southern China. Chemosphere 2017, 168, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- OSHA. Formaldehyde Standard; 29FCR 1910.48; 2002. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1048 (accessed on 13 February 2021).
- Ozdamar, M.; Umarogullari, F. Thermal comfort and indoor air quality. Int. J. Sci. Res. Innov. Technol. 2018, 5, 2313–3759. [Google Scholar]
- Abbasi, A.M.; Motamedzade, M.; Aliabadi, M.; Golmohammadi, R.; Tapak, L. The impact of indoor air temperature on the executive functions of human brain and the physiological response of body. Health Promot. Perspect. 2019, 9, 55–64. [Google Scholar] [CrossRef] [Green Version]
- OSHA. Office Temperature, Humidity and Environmental Tobacco Smoke; Washington, DC, USA, 2003. Available online: https://www.osha.gov/laws-regs/standardinterpretations/2003-02-28 (accessed on 11 April 2022).
- Aziah, D.; Abdin, E.; Azwan, A.; Lin, N.; Rusli, N. Assessment of Indoor Air Quality and Heat Stress Exposure in an Automotive Assembly Plant. Air Quality 2010, 343–365. Available online: https://www.intechopen.com/chapters/11393 (accessed on 13 September 2021).
- Rumchev, K.; Brown, H.; Spickett, J. Volatile organic compounds: Do they present a risk to our health? Rev. Environ. Health 2007, 22, 39–55. [Google Scholar] [CrossRef]
- Arif, A.A.; Shah, S.M. Association between personal exposure to volatile organic compounds and asthma among US adult population. Int. Arch. Occup. Environ. Health 2007, 80, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Bu, Z.; Liu, W.; Kan, H.; Zhao, Z.; Deng, F.; Huang, C.; Zhao, B.; Zeng, X.; Sun, Y.; et al. Health effects of exposure to indoor volatile organic compounds from 1980 to 2017: A systematic review and meta-analysis. Indoor Air 2022, 32, e13038. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.H.; Spengler, J.D.; Neas, L.M.; Samet, J.M.; Wagner, G.R.; Coultas, D.; Ozkaynak, H.; Schwab, M. Respiratory and irritant health effects of ambient volatile organic compounds. The Kanawha County Health Study. Am. J. Epidemiol. 1993, 137, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Volatile Organic Compounds’ Impact on Indoor Air Quality; 2022. Available online: https://www.epa.gov/indoor-air-quality-iaq/volatile-organic-compounds-impact-indoor-air-quality# (accessed on 15 June 2020).
- Li, Y.; Yuan, Y.; Li, C.; Han, X.; Zhang, X. Human responses to high temperature, relative humidity and carbon dioxide concentration in underground refuge chamber. Build. Environ. J. 2018, 131, 53–62. [Google Scholar] [CrossRef]
- Bonino, S. Carbon Dioxide Detection and Indoor Air Quality Control. Occup Health Saf. 2016, 85, 46–48. Available online: https://pubmed.ncbi.nlm.nih.gov/27183813/ (accessed on 12 December 2021).
- OSHA Fact Sheet. Carbon dioxide Poisoning. 2012. Available online: https://www.osha.gov/sites/default/files/publications/carbonmonoxide-factsheet.pdf (accessed on 19 December 2020).
- Wikipedia. Geography of Nigeria and Climate Types Found in Nigeria. 2018. Available online: https://en.wikipedia.org/wiki/Geography_of_Nigeria (accessed on 12 November 2020).
- Wolkoff, P. Indoor air humidity, air quality and health—An overview. Int. J. Hyg. Environ. Health 2018, 221, 376–390. [Google Scholar] [CrossRef]
- Moda, H.M.; King, D. Assessment of Occupational Safety and Hygiene Perception among Afro-Caribbean Hair Salon Operators in Manchester, United Kingdom. Int. J. Environ. Res. Public Health 2019, 16, 3284. [Google Scholar] [CrossRef] [Green Version]
- Alomirah, H.F.; Moda, H.M. Assessment of indoor air quality and users perception of a renovated office building. Int. J. Environ. Res. Public Health 2020, 17, 1972. [Google Scholar] [CrossRef] [Green Version]
- Boguski, T.K. Understanding units of measurements. In Environmental Science and Technology Briefs for Citizens; Centre for Hazardous Substance Research (CHSR), Kansas State University: Manhattan, KS, USA, 2008; Available online: https://engg.k-state.edu/CHSR/outreach/resources/docs/2UnitsofMeasure022508.pdf (accessed on 18 August 2021).
- Ausetute. Parts Per Million (ppm) Concentration. Key Concepts. 2020. Available online: https://www.ausetute.com.au/partspm.html (accessed on 13 February 2022).
- Teesing. Parts Per Million to Milligram Per meter Cube Converter. 2020. Available online: https://www.justintools.com/unit-conversion/density.php?k1=parts-per-million&k2=milligrams-per-cubic-meter (accessed on 11 February 2022).
- OSHA. OSHA Permissible Exposure Limits. OSHA Annotated Table Z-1 & Z-3 (Mineral Dusts); 29. CFR 1910.1000; 2018. Available online: https://www.osha.gov/annotated-pels/table-z-1 (accessed on 11 June 2022).
- INCHEM. Sodium Oxide. 2006. Available online: https://inchem.org/documents/icsc/icsc/eics1653.htm (accessed on 15 June 2022).
- Treesubsuntorn, C.; Setiawan, G.D.; Permana, B.H.; Citra, Y.; Krobthong, S.; Yingchutrakul, Y.; Siswanto, D.; Thiravetyan, P. Particulate matter and volatile organic compound phytoremediation by perennial plants: Affecting factors and plant stress response. Sci. Total Environ. 2021, 794, 148–779. [Google Scholar] [CrossRef]
- International Labour Organization/World Health Organization [ILO/WHO]. Aluminium Oxide. 2017. Available online: https://www.ilo.org/wcmsp5/groups/public/@ed_dialogue/@lab_admin/documents/publication/wcms_819788.pdf (accessed on 11 June 2022).
- Xiao, Y.; Reuter, M.A.; Boin, U. Aluminium recycling and environmental issues of salt slag treatment. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2005, 40, 1861–1875. [Google Scholar] [CrossRef]
- OSHA. Crystalline Silica Exposure Health Hazard Information for Industry Employees. US Department of Labour. 2018. Available online: https://www.osha.gov/sites/default/files/2018-12/fy16_sh-29661-sh6_SilicaTrainingReferences.pdf (accessed on 11 June 2022).
- Heaviside, C.; Witham, C.; Vardoulakis, S. Potential health impacts from sulphur dioxide and sulphate exposure in the UK resulting from an Icelandic effusive volcanic eruption. Sci. Total Environ. 2021, 774, 145549. [Google Scholar] [CrossRef] [PubMed]
- Cain, W.S.; Jalowayski, A.A.; Kleinman, M.; Lee, N.-S.; Lee, B.-R.; Ahn, B.-H.; Magruder, K.; Schmidt, R.; Hillen, B.K.; Warren, C.B.; et al. Sensory and irritating reactions to mineral dusts: Sodium borate, calcium oxide, and calcium sulfate. J. Occup. Environ. Hyg. 2004, 1, 222–236. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Occupational Safety and Health [NIOSH]. Iron Oxide Fumes. In In Centre for Disease Control; 2011. Available online: https://www.cdc.gov/niosh/npg/npgd0344.html (accessed on 19 June 2022).
- Hartwig, A.; Commission, M.A.K. Iron oxides (inhalable fraction. MAK-Collect. Occup Health Saf. Annu. Threshold. Cl. Workplace 2016, 1, 1804–1869. [Google Scholar] [CrossRef]

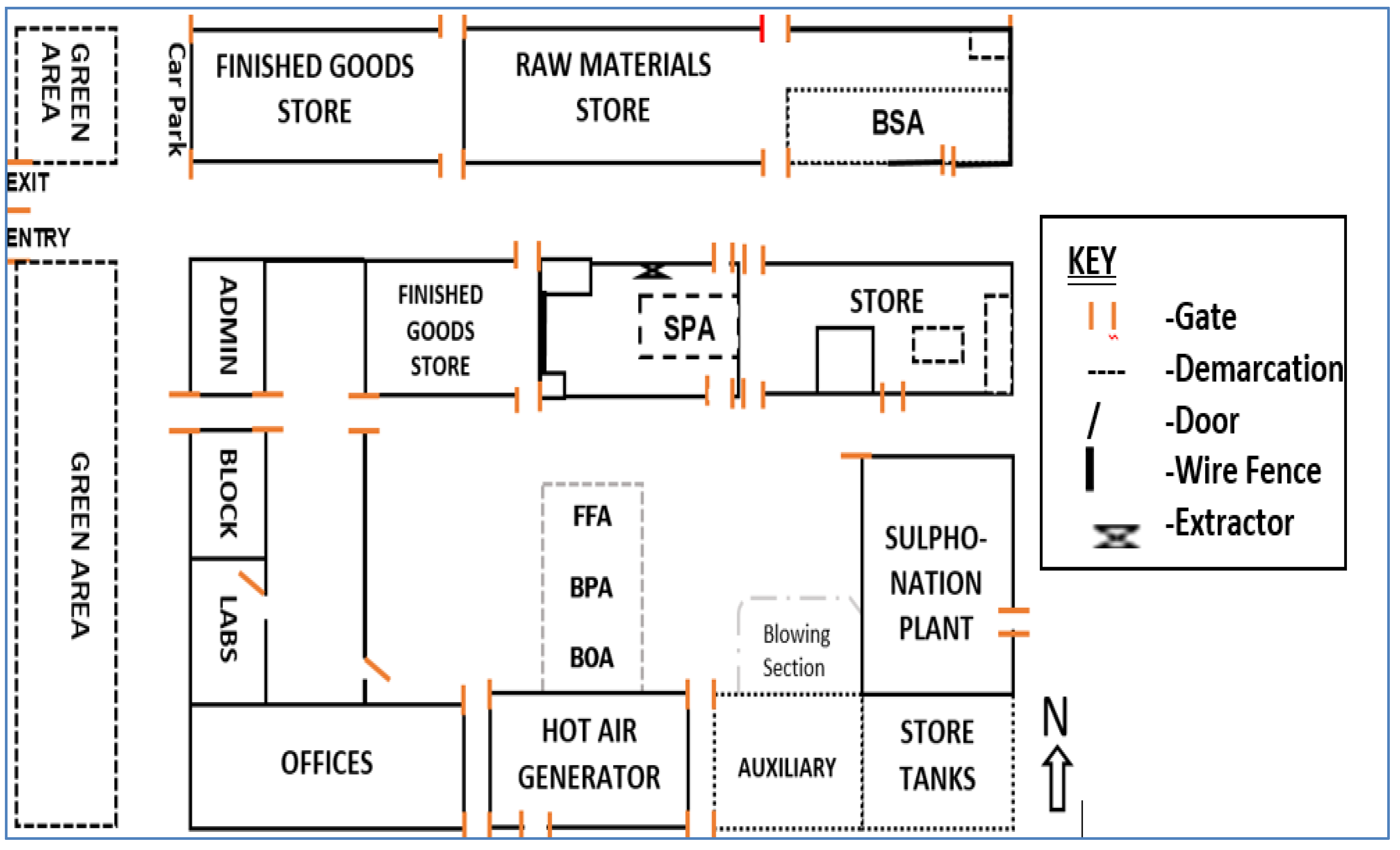


| No. | Workstation | Floor | Floor Area (m2) | Minimum No. of Workforce per Shift | Maximum No of Workers per Shift |
|---|---|---|---|---|---|
| 1 | BPA | Ground floor | 50 | 9 | 32 |
| 2 | BOA | Ground floor | 50 | 3 | 8 |
| 3 | FFA | First floor | 200 | 7 | 17 |
| 4 | SPA | Ground floor | 400 | 34 | 71 |
| 5 | BSP | Ground floor | 200 | 6 | 8 |
| Workstation | Reported Exposure Health Complaints | ||
|---|---|---|---|
| Respiratory Related | Eyes Related | Other Surface Injuries | |
| BPA (32) | 7 | 10 | 0 |
| BOA (8) | 2 | 2 | 0 |
| FFA (17) | 4 | 3 | 1 |
| SPA (71) | 17 | 9 | 6 |
| BSP (8) | 2 | 3 | 0 |
| Total | 32 | 26 | 7 |
| Sampling Points | ||||||
|---|---|---|---|---|---|---|
| Parameters | (BPA) | (BOA) | (FFA) | (SPA) | (BSP) | p Value |
| PM2.5 (µg/m3) | 44 ± 5.66 | 24.5 ± 2.38 | 48.5 ± 6.25 | 28.75 ± 1.89 | 38.25 ± 4.11 | S |
| PM10 (µg/m3) | 54 ± 8.17 | 26.75 ± 2.75 | 61.75 ± 9.53 | 33.75 ± 3.95 | 45.25 ± 6.23 | S |
| HCHO (mg/m3) | 0.01 ± 0.02 | 0.0025 ± 0.00 | 0.0125 ± 0.00 | 0.00 ± 0.00 | 0.0025 ± 0.00 | NS |
| VOCs (mg/m3) | 1.35 ± 0.28 | 0.98 ± 0.40 | 0.93 ± 0.06 | 0.27 ± 0.04 | 0.09 ± 0.00 | S |
| CO2 (mg/m3) | 1227.75 ± 55.22 | 1265 ± 55.82 | 1190.25 ± 4.5 | 1155.25 ± 2.36 | 1137 ± 2.00 | S |
| T (°C) | 25.65 ± 0.42 | 27.6 ± 0.34 | 28.15 ± 0.10 | 26.95 ± 0.12 | 27.55 ± 0.12 | S |
| RH (%) | 20.13 ± 0.22 | 22.55 ± 0.20 | 22.7 ± 0.62 | 22.75 ± 0.17 | 23.80 ± 0.52 | S |
| Sample Values | Regulatory Limits | |||
|---|---|---|---|---|
| Parameters | Minimum | Maximum | OSHA | NESREA |
| PM2.5(µg/m3) | 24.50 | 48.50 | 50.00 | - |
| PM10(µg/m3) | 26.75 | 61.75 | 150.00 | 180.00 |
| HCHO (mg/m3) | 0.00 | 0.01 | 0.75 | 0.10 |
| VOCs (mg/m3) | 0.09 | 1.35 | 0.75 | 0.60 |
| CO2 (mg/m3) | 1137 | 1265.00 | 5000.00 | 10,000.00 |
| T (°C) | 25.65 | 28.15 | 24.44 | 25.50 |
| RH (%) | 20.13 | 23.80 | 60.00 | 70.00 |
| Parameter (wt. %) | Sampling Location | ||||
|---|---|---|---|---|---|
| BPA | BOA | FFA | SPA | BSP | |
| Na2O | 26.580 | 29.096 | 29.945 | 27.955 | 25.490 |
| MgO | 1.101 | 1.356 | 0.307 | 0.378 | 6.348 |
| Al2O3 | 3.183 | 4.178 | 2.407 | 2.904 | 16.498 |
| SiO2 | 41.722 | 40.711 | 26.193 | 25.279 | 25.788 |
| P2O5 | 0.446 | 0.000 | 0.133 | 0.139 | 0.014 |
| SO3 | 18.014 | 14.213 | 38.086 | 31.767 | 9.600 |
| Cl− | 0.544 | 0.567 | 0.674 | 0.341 | 0.834 |
| K2O | 0.075 | 0.017 | 0.111 | 0.113 | 0.150 |
| CaO | 6.406 | 9.287 | 1.657 | 2.025 | 14.311 |
| TiO2 | 0.250 | 0.272 | 0.091 | 0.145 | 0.528 |
| Cr2O3 | 0.008 | 0.002 | 0.002 | 0.015 | 0.003 |
| Mn2O3 | 0.017 | 0.006 | 0.006 | 0.071 | 0.010 |
| Fe2O3 | 1.629 | 0.279 | 0.376 | 8.834 | 0.402 |
| ZnO | 0.023 | 0.000 | 0.009 | 0.031 | 0.005 |
| SrO | 0.001 | 0.017 | 0.003 | 0.002 | 0.019 |
| Parameter (mg/m3) | Sampling Location | |||||
|---|---|---|---|---|---|---|
| BPA | BOA | FFA | SPA | BSP | OSHA PEL | |
| Na2O | 22.46 | 24.59 | 25.30 | 23.62 | 21.54 | 15 |
| MgO | 0.61 | 0.75 | 0.17 | 0.21 | 3.49 | 15 |
| Al2O3 | 4.42 | 5.81 | 3.34 | 4.04 | 22.93 | 15 |
| SiO2 | 34.17 | 33.35 | 21.45 | 20.70 | 21.12 | 21.06 |
| P2O5 | 0.86 | 0.00 | 0.26 | 0.27 | 0.03 | 15(NE) |
| SO3 | 19.67 | 15.52 | 41.57 | 34.68 | 10.48 | 15 (NE) |
| Cl− | 0.26 | 0.27 | 0.33 | 0.17 | 0.40 | 15 |
| K2O | 0.10 | 0.02 | 0.14 | 0.15 | 0.19 | 15 (NE) |
| CaO | 4.90 | 7.10 | 1.27 | 1.55 | 10.94 | 5 |
| TiO2 | 0.27 | 0.30 | 0.10 | 0.16 | 0.57 | 15 |
| Cr2O3 | 0.02 | 0.01 | 0.01 | 0.03 | 0.01 | 0.5 |
| Mn2O3 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 5 (as Mn) |
| Fe2O3 | 3.55 | 0.61 | 0.82 | 19.23 | 0.88 | 10 (as fumes) |
| ZnO | 0.03 | 0.00 | 0.01 | 0.03 | 0.01 | 15 |
| SrO | 0.00 | 0.03 | 0.00 | 0..00 | 0.03 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gushit, J.S.; Mohammed, S.U.; Moda, H.M. Indoor Air Quality Monitoring and Characterization of Airborne Workstations Pollutants within Detergent Production Plant. Toxics 2022, 10, 419. https://doi.org/10.3390/toxics10080419
Gushit JS, Mohammed SU, Moda HM. Indoor Air Quality Monitoring and Characterization of Airborne Workstations Pollutants within Detergent Production Plant. Toxics. 2022; 10(8):419. https://doi.org/10.3390/toxics10080419
Chicago/Turabian StyleGushit, John Stephen, Salamatu Uba Mohammed, and Haruna Musa Moda. 2022. "Indoor Air Quality Monitoring and Characterization of Airborne Workstations Pollutants within Detergent Production Plant" Toxics 10, no. 8: 419. https://doi.org/10.3390/toxics10080419
APA StyleGushit, J. S., Mohammed, S. U., & Moda, H. M. (2022). Indoor Air Quality Monitoring and Characterization of Airborne Workstations Pollutants within Detergent Production Plant. Toxics, 10(8), 419. https://doi.org/10.3390/toxics10080419








