Effects of Amendments and Indigenous Microorganisms on the Growth and Cd and Pb Uptake of Coriander (Coriandrum sativum L.) in Heavy Metal-Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Amendments
2.2. Pot Experiment
2.3. Harvest and Sample Analysis
2.3.1. Plant Analysis
2.3.2. Soil Analysis
2.4. Statistical Analysis
3. Results
3.1. Plant Growth Responses
3.2. Cd and Pb Uptake by Coriander Tissues
3.3. Soil Analysis
3.3.1. Soil pH
3.3.2. Available Cd and Pb Concentrations
3.4. Correlation Analysis of Dry Biomass, Soil pH, Cd and Pb Concentrations, and Available Cd and Pb Concentrations
4. Discussion
4.1. Effect of Pb and Cd Toxicity on Plant Growth
4.2. Effect of n-HAP with and without IMOs
4.3. Effect of γ-PGA with and without IMOs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, Q.; Tang, Y.; Li, W.; Xu, J.; Wu, J.; Wang, F.; Brookes, P.C. Human health risk assessment of heavy metals in soil-vegetable system: A multi-medium analysis. Sci. Total Environ. 2013, 463–464, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Doabi, S.A.; Karami, M.; Afyuni, M.; Yeganeh, M. Pollution and health risk assessment of heavy metals in agricultural soil, atmospheric dust and major food crops in Kermanshah province, Iran. Ecotoxicol. Environ. Saf. 2018, 163, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, Y.; Weng, L.; Ma, J.; Ma, Y.; Li, Y.; Islam, M.S. Comparisons of heavy metal input inventory in agricultural soils in North and South China: A review. Sci. Total Environ. 2019, 660, 776–786. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Shi, C.; Cui, Y.; Lu, J.; Zhang, B. Sulfur-based autotrophic biosystem for efficient vanadium (V) and chromium (VI) reductions in groundwater. Chem. Eng. J. 2020, 395, 124972. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, Y.; Xie, H.; Wei, J.; Zhang, X.; Huang, X.; Wang, J.; Lou, X. Effect of cornstalk biochar on phytoremediation of Cd-contaminated soil by Beta vulgaris var. cicla L. Ecotoxicol. Environ. Saf. 2020, 205, 111144. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, X.; Xu, H.; Xia, P.; Wang, H.; Jing, H.; Li, J.; Zhao, J. High zinc removal from water and soil using struvite-supported diatomite obtained by nitrogen and phosphate recovery from wastewater. Environ. Chem. Lett. 2017, 16, 569–573. [Google Scholar] [CrossRef]
- Seshadri, B.; Bolan, N.S.; Choppala, G.; Kunhikrishnan, A.; Sanderson, P.; Wang, H.; Currie, L.D.; Tsang, D.C.W.; Ok, Y.S.; Kim, G. Potential value of phosphate compounds in enhancing immobilization and reducing bioavailability of mixed heavy metal contaminants in shooting range soil. Chemosphere 2017, 184, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, M.; Chen, W.; Zhu, S.; Liu, N.; Zhu, L. Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ. Pollut. 2010, 158, 514–519. [Google Scholar] [CrossRef] [PubMed]
- El-Nagar, D.A.; Massoud, S.A.; Ismail, S.H. Removal of some heavy metals and fungicides from aqueous solutions using nano-hydroxyapatite, nano-bentonite and nanocomposite. Arab. J. Chem. 2020, 13, 7695–7706. [Google Scholar] [CrossRef]
- Yan, Y.; Qi, F.; Zhao, S.; Luo, Y.; Gu, S.; Li, Q.; Zhang, L.; Zhou, S.; Bolan, N. A new low-cost hydroxyapatite for efficient immobilization of lead. J. Colloid Interface Sci. 2019, 553, 798–804. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, J.; Liu, W.; Yan, Y.; Wang, Y. Hydroxyapatite as a passivator for safe wheat production and its impacts on soil microbial communities in a Cd-contaminated alkaline soil. J. Hazard. Mater. 2021, 404, 124005. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J. Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol. Engin. 2015, 74, 319–326. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Yin, J.; Qi, L.P.; Feng, Y. Combined amendments of nano-hydroxyapatite immobilized cadmium in contaminated soil-potato (Solanum tuberosum L.) system. Bull. Environ. Contam. Toxicol. 2018, 100, 581–587. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, N.; Zhu, C.; Gan, L. Exogenously applied poly-gamma-glutamic acid alleviates salt stress in wheat seedlings by modulating ion balance and the antioxidant system. Environ. Sci. Pollut. Res. 2017, 24, 6592–6598. [Google Scholar] [CrossRef]
- Yang, Z.H.; Dong, C.D.; Chen, C.W.; Sheu, Y.T.; Kao, C.M. Using poly-glutamic acid as soil-washing agent to remediate heavy metal-contaminated soils. Environ. Sci. Pollut. Res. 2018, 25, 5231–5242. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Feng, X.; Li, S.; Xu, H. Analysis of the metabolic pathways affected by poly (gamma-glutamic acid) in Arabidopsis thaliana based on genechip microarray. J. Agric. Food Chem. 2016, 64, 6257–6266. [Google Scholar] [CrossRef]
- Sakamoto, S.; Kawase, Y. Adsorption capacities of poly-gamma-glutamic acid and its sodium salt for cesium removal from radioactive wastewaters. J. Environ. Radioact. 2016, 165, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shi, W. Poly-γ-glutamic acid improves water-stable aggregates, nitrogen and phosphorus uptake efficiency, water-fertilizer productivity, and economic benefit in barren desertified soils of Northwest China. Agric. Water Manag. 2021, 245, 106551. [Google Scholar] [CrossRef]
- Bhat, A.R.; Irorere, V.U.; Bartlett, T.; Hill, D.; Kedia, G.; Morris, M.R.; Charalampopoulos, D.; Radecka, I. Bacillus subtilis natto: A non-toxic source of poly-γ-glutamic acid that could be used as a cryoprotectant for probiotic bacteria. AMB Express 2013, 3, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajdu, I.; Bodnár, M.; Csikós, Z.; Wei, S.; Daróczi, L.; Kovács, B.; Győri, Z.; Tamás, J.; Borbély, J. Combined nano-membrane technology for removal of lead ions. J. Memb. Sci. 2012, 409–410, 44–53. [Google Scholar] [CrossRef]
- Yin, A.; Jia, Y.; Qiu, T.; Gao, M.; Cheng, S.; Wang, X.; Sun, Y. Poly-γ-glutamic acid improves the drought resistance of maize seedlings by adjusting the soil moisture and microbial community structure. Appl. Soil Ecol. 2018, 129, 128–135. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Feng, X.; Xu, X.; Liang, J.; Chi, B.; Xu, H. Calcium involved in the poly (gamma-glutamic acid)-mediated promotion of Chinese cabbage nitrogen metabolism. Plant Physiol. Biochem. 2014, 80, 144–152. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-gamma-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels 2016, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Lei, P.; Xu, Z.; Liang, J.; Luo, X.; Zhang, Y.; Feng, X.; Xu, H. Poly (γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul. 2015, 78, 233–241. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Wang, J.S.; Lu, J.F.; Siao, F.Y.; Chen, B.H. Adsorption of toxic mercury(II) by an extracellular biopolymer poly (gamma-glutamic acid). Bioresour. Technol. 2009, 100, 200–207. [Google Scholar] [CrossRef]
- Pang, X.; Lei, P.; Feng, X.; Xu, Z.; Xu, H.; Liu, K. Poly-gamma-glutamic acid, a bio-chelator, alleviates the toxicity of Cd and Pb in the soil and promotes the establishment of healthy Cucumis sativus L. seedling. Environ. Sci. Pollut. Res. 2018, 25, 19975–19988. [Google Scholar] [CrossRef]
- Ye, S.; Zhao, Z. Seismic response of prestressed anchors with frame structure. Math. Probl. Eng. 2020, 2020, 9029045. [Google Scholar] [CrossRef]
- Park, J.H.; Bolan, N.; Megharaj, M.; Naidu, R. Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. J. Hazard. Mater. 2011, 185, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Oziegbe, O.; Oluduro, A.O.; Oziegbe, E.J.; Ahuekwe, E.F.; Olorunsola, S.J. Assessment of heavy metal bioremediation potential of bacterial isolates from landfill soils. Saudi J. Biol. Sci. 2021, 28, 3948–3956. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.L.; Yao, J.; Wang, F.; Min, N.; Gu, J.H.; Li, Z.F.; Sunahara, G.; Duran, R.; Solevic-Knudsen, T.; Hudson-Edwards, K.A.; et al. Bacterial diversity in typical abandoned multi-contaminated nonferrous metal(loid) tailings during natural attenuation. Environ. Pollut. 2019, 247, 98–107. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Azcon, R.; Diaz, G.; Roldan, A. The combination of compost addition and arbuscular mycorrhizal inoculation produced positive and synergistic effects on the phytomanagement of a semiarid mine tailing. Sci. Total Environ. 2015, 514, 42–48. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.W.; Yue, F.X.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 2018, 194, 495–503. [Google Scholar] [CrossRef]
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 2015, 96, 114–121. [Google Scholar] [CrossRef]
- Hammer, E.C.; Balogh-Brunstad, Z.; Jakobsen, I.; Olsson, P.A.; Stipp, S.L.S.; Rillig, M.C. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem. 2014, 77, 252–260. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, Q.; Zhao, C.; Wang, S.; Shi, Y.; Liang, S.; Zhao, C.; Shen, S. Effects of Bacillus subtilis and nanohydroxyapatite on the metal accumulation and microbial diversity of rapeseed (Brassica campestris L.) for the remediation of cadmium-contaminated soil. Environ. Sci. Pollut. Res. 2018, 25, 25217–25226. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Wu, X.W.; Li, Y.L.; Zhao, H.W.; Fang, Y.Y.; Wang, M.; Wang, S.T. Nanohydroxyapatite combined with white rot fungus can effectively reduce cadmium activity and change the soil microbial populations structure. Fresenius Environ. Bull. 2020, 29, 9644–9653. [Google Scholar]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.Y.; Xue, D.W.; Zhao, L.M.; Zhang, X.Y. Concentration of heavy metals in vegetables and potential health risk assessment in China. Environ. Geochem. Health 2018, 40, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Swathi, A.; Rajendra, H. Evaluation of antiarthritic activity of Coriander seed essential oil in Wistar albino rats. Res. J. Pharm. Technol. 2020, 13, 761–766. [Google Scholar] [CrossRef]
- Zhu, F.; He, S.Y.; Shang, Z.F. Effect of vegetables and nano-particle hydroxyapatite on the remediation of cadmium and phosphatase activity in rhizosphere soil through immobilization. Int. J. Phytorem. 2019, 21, 610–616. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Carvalho, M.E.A.; Piotto, F.A.; Gaziola, S.A.; Jacomino, A.P.; Jozefczak, M.; Cuypers, A.; Azevedo, R.A. New insights about cadmium impacts on tomato: Plant acclimation, nutritional changes, fruit quality and yield. Food Energy Secur. 2018, 7, e00131. [Google Scholar] [CrossRef] [Green Version]
- Poschenrieder, C.; Cabot, C.; Martos, S.; Gallego, B.; Barcelo, J. Do toxic ions induce hormesis in plants? Plant Sci. 2013, 212, 15–25. [Google Scholar] [CrossRef]
- Vivas, A.; Vörös, A.; Biró, B.; Barea, J.M.; Ruiz-Lozano, J.M.; Azcón, R. Beneficial effects of indigenous Cd-tolerant and Cd-sensitive Glomus mosseae associated with a Cd-adapted strain of Brevibacillus sp. in improving plant tolerance to Cd contamination. Appl. Soil Ecol. 2003, 24, 177–186. [Google Scholar] [CrossRef]
- Metwally, A.; Safronova, V.I.; Belimov, A.A.; Dietz, K.J. Genotypic variation of the response to cadmium toxicity in Pisum sativum. J. Exp. Bot. 2005, 56, 167–178. [Google Scholar] [CrossRef]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.Y.; Ahammed, G.J.; Qi, Z.Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [Green Version]
- Shekari, L.; Aroiee, H.; Mirshekari, A.; Nemati, H. Protective role of selenium on cucumber (Cucumis sativus L.) exposed to cadmium and lead stress during reproductive stage role of selenium on heavy metals stress. J. Plant Nutr. 2019, 42, 529–542. [Google Scholar] [CrossRef]
- Hoque, M.N.; Tahjib-Ul-Arif, M.; Hannan, A.; Sultana, N.; Akhter, S.; Hasanuzzaman, M.; Akter, F.; Hossain, M.S.; Abu Sayed, M.; Hasan, M.T.; et al. Melatonin modulates plant tolerance to heavy metal stress: Morphological responses to molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 11445. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Saeed, R.; Wu, L.H. Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef]
- Zhang, H.H.; Xu, Z.S.; Guo, K.W.; Huo, Y.Z.; He, G.Q.; Sun, H.W.; Guan, Y.P.; Xu, N.; Yang, W.; Sun, G.Y. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, N.; Yoshikawa, S.; Ikeda, K. Influences of heavy application of nitrogen on soil acidification and root growth in tea fields. Jpn. J. Crop. Sci. 1995, 64, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.J.; Chen, J.H.; Fan, T.T.; Zhou, D.M.; Wang, Y.J. Effect of nanoparticle hydroxyapatite on the immobilization of Cu and Zn in polluted soil. Environ. Sci. Pollut. Res. 2018, 25, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Zhang, S.Q.; Cheng, P.; Zhang, S.W.; Sun, Y.H. Effects of Soil amendments on heavy metal immobilization and accumulation by maize grown in a multiple-metal-contaminated soil and their potential for safe crop production. Toxics 2020, 8, 102. [Google Scholar] [CrossRef]
- Lee, H.H.; Owens, V.N.; Park, S.; Kim, J.; Hong, C.O. Adsorption and precipitation of cadmium affected by chemical form and addition rate of phosphate in soils having different levels of cadmium. Chemosphere 2018, 206, 369–375. [Google Scholar] [CrossRef]
- Zeng, G.M.; Wan, J.; Huang, D.L.; Hu, L.; Huang, C.; Cheng, M.; Xue, W.J.; Gong, X.M.; Wang, R.Z.; Jiang, D.N. Precipitation, adsorption and rhizosphere effect: The mechanisms for phosphate-induced Pb immobilization in soils—A review. J. Hazard. Mater. 2017, 339, 354–367. [Google Scholar] [CrossRef]
- Cao, X.D.; Wahbi, A.; Ma, L.N.; Li, B.; Yang, Y.L. Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 2009, 164, 555–564. [Google Scholar] [CrossRef]
- Xu, Y.P.; Schwartz, F.W.; Traina, S.J. Sorption of Zn2+ and Cd2+ on hydroxyapatite surface. Environ. Sci. Technol. 1994, 28, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.A.; Zhang, P.C.; Hesterberg, D.; Chou, J.; Sayers, D.E. Formation of chloropyromorphite in a lead-contaminated soil amended with hydroxyapatite. Environ. Sci. Technol. 2001, 35, 3798–3803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, F.R.; Ali, S.; Zhang, H.T.; Ouyang, Y.B.; Qiu, B.Y.; Wu, F.B.; Zhang, G.P. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Collins, C. Uptake of organic pollutants and potentially toxic elements (PTEs) by crops. In Persistent Organic Pollutants and Toxic Metals in Foods; Rose, M., Fernandes, A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 129–144. [Google Scholar]
- Lim, J.E.; Ahmad, M.; Lee, S.S.; Shope, C.L.; Hashimoto, Y.; Kim, K.R.; Usman, A.R.A.; Yang, J.E.; Ok, Y.S. Effects of lime-based waste materials on immobilization and phytoavailability of cadmium and lead in contaminated soil. Clean-Soil Air Water 2013, 41, 1235–1241. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Cui, H.B.; Shi, Y.; Zhou, J.; Chu, H.Y.; Cang, L.; Zhou, D.M. Effect of different grain sizes of hydroxyapatite on soil heavy metal bioavailability and microbial community composition. Agric. Ecosys. Environ. 2018, 267, 165–173. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.M.; Luo, L.; Liu, Y.; Wei, J.H.; Zhang, J.C.; Yang, Y.; Chen, A.W.; Mao, Q.M.; Zhou, Y.Y. Response of soil microbial communities to red mud-based stabilizer remediation of cadmium-contaminated farmland. Environ. Sci. Pollut. Res. 2018, 25, 11661–11669. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, F.F.; Xie, M.D.; Jiang, Q.Q.; Chen, W.Q.; Ao, T.Q. The impact of stabilizing amendments on the microbial community and metabolism in cadmium-contaminated paddy soils. Chem. Eng. J. 2020, 395, 125132. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Sierra, C.; Martinez-Blanco, D.; Blanco, J.A.; Gallego, J.R. Optimisation of magnetic separation: A case study for soil washing at a heavy metals polluted site. Chemosphere 2014, 107, 290–296. [Google Scholar] [CrossRef]
- Panczuk-Figura, I.; Kolodynska, D. Biodegradable chelating agent for heavy metal ions removal. Separat. Sci. Technol. 2016, 51, 2576–2585. [Google Scholar] [CrossRef]
- Knight, B.P.; McGrath, S.P.; Chaudri, A.M. Biomass carbon measurements and substrate utilization patterns of microbial populations from soils amended with cadmium, copper, or zinc. Appl. Environ. Microbiol. 1997, 63, 39–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]



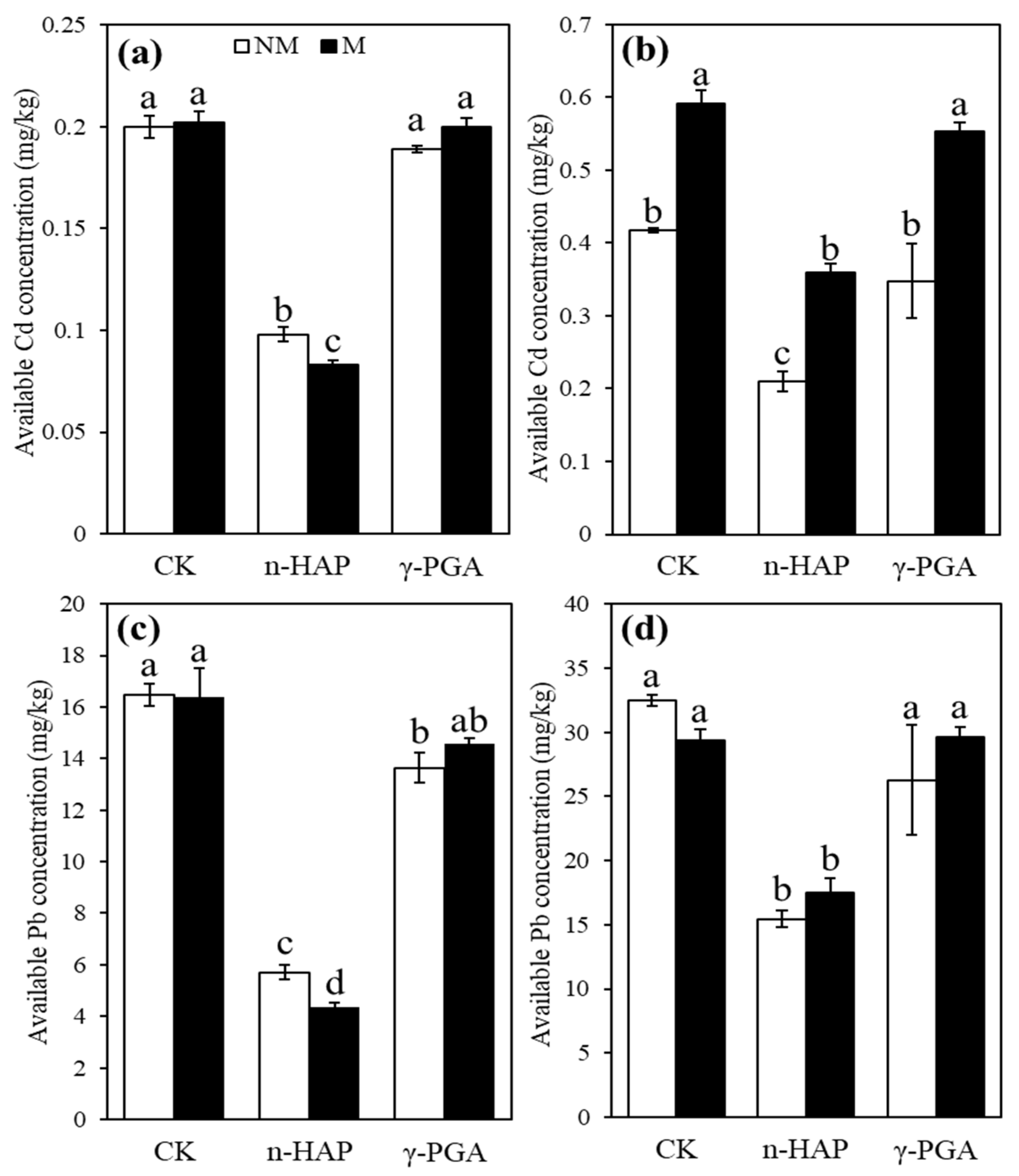
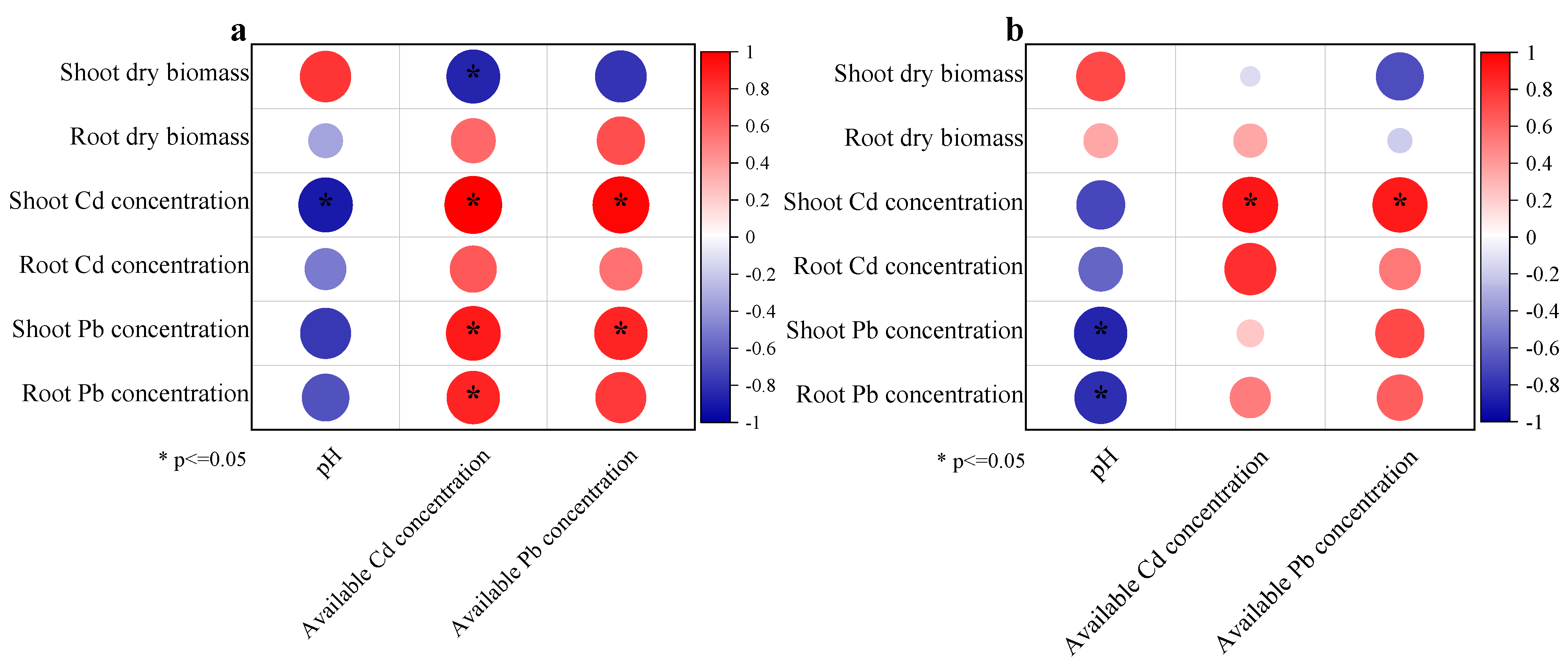
| Soils | pH | EC | TP | Olsen-P | SOC | Available Cd | Total Cd | Available Pb | Total Pb |
|---|---|---|---|---|---|---|---|---|---|
| (ds/cm) | (g/kg) | (mg/kg) | (g/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | ||
| Slightly contaminated | 4.56 ± 0.25 | 376 ± 7 | 0.50 ± 0.10 | 17.9 ± 0.5 | 26.5 ± 1.1 | 0.21 ± 0.01 | 0.29 ± 0.01 (0.30) 1 | 13.8 ± 0.5 | 50.9 ± 3.0 (70.0) 1 |
| Moderately contaminated | 3.85 ± 0.13 | 271 ± 4 | 0.82 ± 0.03 | 60.0 ± 2.9 | 17.0 ± 0.3 | 0.66 ± 0.06 | 1.08± 0.05 (1.50) 2 | 29.4 ± 1.0 | 100.0 ± 1.8 (400.0) 2 |
| Parameter | M | A | CL | M × A | M × CL | A × CL | M × A × CL |
|---|---|---|---|---|---|---|---|
| Shoot biomass | 13.3 *** | 56.8 *** | 5.9 * | 9.1 *** | 37.1 *** | 11.1 *** | 6.0 ** |
| Shoot Cd concentration | 7.0 * | 54.1 *** | 63.1 *** | 4.2 * | 0.9 | 1.0 | 0.7 |
| Shoot Pb concentration | 2.4 | 12.5 *** | 22.6 *** | 0.1 | 6.1 * | 2.9 | 0.5 |
| Root biomass | 6.1 * | 0.4 | 20.0 *** | 1.4 | 5.5* | 1.7 | 1.7 |
| Root Cd concentration | 4.0 | 4.1 * | 30.8 *** | 2.5 | 2.1 | 1.6 | 2.1 |
| Root Pb concentration | 0.6 | 20.6 *** | 0.8 | 2.7 | 1.3 | 6.0 ** | 1.2 |
| pH values | 1.0 | 5.0 * | 0.7 | 0.1 | 0.0 | 0.0 | 0.5 |
| Available Cd concentration | 98.2 *** | 128.0 *** | 804.0 *** | 1.8 | 99.7 *** | 12.6 *** | 0.3 |
| Available Pb concentration | 0.1 | 107.0 *** | 310.0 *** | 2.2 | 0.4 | 1.5 | 1.8 |
| Treatments | Dry Biomass (g/pot) | Cd Concentration (mg/kg) | Pb Concentration (mg/kg) | |||
|---|---|---|---|---|---|---|
| NM | M | NM | M | NM | M | |
| Slightly contaminated | ||||||
| CK | 0.064 a | 0.082 a | 3.18 cd | 4.69 bc | 23.8 b | 38.9 a |
| n-HAP | 0.040 a | 0.042 a | 2.47 d | 3.25 cd | 7.4 c | 9.51 c |
| γ-PGA | 0.050 a | 0.042 a | 6.04 ab | 6.73 a | 47.1 a | 49.8 a |
| Moderately contaminated | ||||||
| CK | 0.065 b | 0.190 ab | 9.62 b | 86.0 a | 34.5 ab | 40.7 a |
| n-HAP | 0.200 ab | 0.225 ab | 10.2 b | 7.6 b | 33.4 ab | 15.0 b |
| γ-PGA | 0.078 b | 0.353 a | 11.0 ab | 18.7 ab | 31.7 ab | 40.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, N.; Hao, W.; Zhou, Z.; Li, L.; Wang, F.; Gai, J. Effects of Amendments and Indigenous Microorganisms on the Growth and Cd and Pb Uptake of Coriander (Coriandrum sativum L.) in Heavy Metal-Contaminated Soils. Toxics 2022, 10, 408. https://doi.org/10.3390/toxics10080408
Mi N, Hao W, Zhou Z, Li L, Wang F, Gai J. Effects of Amendments and Indigenous Microorganisms on the Growth and Cd and Pb Uptake of Coriander (Coriandrum sativum L.) in Heavy Metal-Contaminated Soils. Toxics. 2022; 10(8):408. https://doi.org/10.3390/toxics10080408
Chicago/Turabian StyleMi, Nana, Wenying Hao, Zixin Zhou, Longcheng Li, Fayuan Wang, and Jingping Gai. 2022. "Effects of Amendments and Indigenous Microorganisms on the Growth and Cd and Pb Uptake of Coriander (Coriandrum sativum L.) in Heavy Metal-Contaminated Soils" Toxics 10, no. 8: 408. https://doi.org/10.3390/toxics10080408
APA StyleMi, N., Hao, W., Zhou, Z., Li, L., Wang, F., & Gai, J. (2022). Effects of Amendments and Indigenous Microorganisms on the Growth and Cd and Pb Uptake of Coriander (Coriandrum sativum L.) in Heavy Metal-Contaminated Soils. Toxics, 10(8), 408. https://doi.org/10.3390/toxics10080408







