Abstract
Predictors of mortality in illicit drug users involving Novel Psychoactive Substances (NPS) and multiple substances have not been elucidated. We aimed to define predictors of mortality in the NPS endemic era’s illicit drug users to strengthen patient care in emergency treatment. This was a retrospective study. LC-MS/MS-confirmed positive illicit drug users who visited the emergency departments (ED) of six medical systems were enrolled. Demographic information, physical examinations, and laboratory data were abstracted for mortality analysis. There were 16 fatalities in 355 enrolled patients. The most frequently used illicit drugs were amphetamines, followed by opioids, cathinones, and ketamine. The most frequently detected cathinones among the 16 synthetic cathinones were eutylone, followed by mephedrone. The combined use of cathinones and ketamine was most commonly observed in our results. Univariate analysis revealed that the mortality patients were older, with deep coma, faster heart rate and respiratory rate, lower blood pressures and O2 room air saturation, more seizures, abnormal breath sounds, and had urine incontinence compared to the survivor patients. The mortality patients also had acute kidney injury, higher potassium, blood sugar, liver function test, and lactate level. The results of multiple logistic regression demonstrated that SBP < 90 mmHg, dyspnea, blood sugar > 140 mg/dl, and HCO3 < 20.6 mmHg were independent predictors of in-hospital mortality. Regardless of the pattern of the use of illicit drugs, the predictors allow for risk stratification and determining the optimal treatment.
1. Introduction
Over the past decade, there has been a worldwide increase in the opportunity to use and consume Novel Psychoactive Substances (NPS), which are synthetic alternatives to illicit drugs of abuse [1]. As defined by United Nations Office on Drugs and Crime (UNODC), NPS are substances not under international control but may pose a public health threat similar to substances under international control. NPS are easy to obtain, inexpensive, and not detected by standard toxicology screens [2]. The number of intoxicated people presenting with emergencies is increasing [3]. According to the 2019 World Drug Report, as of December 2020, UNODC identified a total of 1047 NPS [4]. In contrast to the effects of NPS at the population level, different NPS can be quite harmful at the individual level, with toxicology cases of single substances showing harmful effects, including death, because of their use [4].
There have been three major waves of drug epidemics in recorded history in Taiwan. Each wave involved different types of drugs tackled with different policies and measures. Since the early 2000s, club drugs, including methamphetamine, MDMA, ketamine, and flunitrazepam (also known as FM2 in Taiwan), have become popular in local rave parties and dance clubs. Some club drugs, such as ketamine, are now more familiar to the public as NPS [5]. Moreover, drug use is gradually shifting from traditional drugs to NPS in Taiwan. Ketamine remained the most popular NPS in Taiwan till 2016.
From 2017 onward, synthetic cathinones (such as mephedrone and MEAPP) have replaced ketamine as the most predominant NPS and deserve further attention in Taiwan for their abuse liability [6]. A recent clinical study reported six patients with “instant coffee sachets” which contained a mixture of illicit psychoactive substances, including amphetamines, ketamine, or cathinones [7]. The latest study in Taiwan associated with NPS was the “Taiwan Emergency Department Drug Abuse Surveillance (TEDAS)” project [8]. This observational study included collecting and analyzing urine samples and assessing the clinical presentation of patients from 79 emergency departments (EDs) across Taiwan. According to their result, the most frequently detected drug was methamphetamine/amphetamine, followed by synthetic cathinones, ketamine, and opioids. The main synthetic cathinones were mephedrone, ephylone, eutylone, and dibutylone. In addition to that, TEDAS also reported that nearly half of their enrolled patients used two or more two kinds of illicit drugs. The most common drug combination was cathinones plus ketamine and its analogs. In addition to that, TEDAS also reported that nearly half of their enrolled patients used two or more two kinds of illicit drugs. The most common drug combination was cathinones plus ketamine and its analogs.
Given the heterogeneous mix of components, abuse of NPS is expected to cause variable adverse health effects [9]. Synthetic stimulants may cause paranoia, hallucinations, and even seizure. Synthetic cannabinoids are associated with a wide range of side effects, including cardiovascular and respiratory complications, hemodynamic instability, renal injury, and cerebrovascular accidents. Synthetic hallucinogens could cause tachycardia, hypertension, hyperthermia, agitation, hallucinations, drowsiness, and even confusion. In Taiwan, according to the Institute of Forensic Medicine, Ministry of Justice, 250 cases of drug use-related death collected from 1361 medico-legal autopsy cases in 2018 shows that ketamine ranked fifth (n = 32, 12.8%) on the drug use-related death list. The other top four death-related drugs were: methamphetamine (n = 82, 32.8%), heroin (n = 65, 26.0%), flunitrazepam (FM2) (n = 35, 14.0%), and estazolam (n = 33, 13.2%) [6]. When facing the emergence of NPS and multiple substances use, we need to be familiar with clinical manifestations, management of mixed drug intoxications, prognosis, and clinical outcomes. Although a few reports have described the clinical course and outcomes following different kinds of NPS ingestion, predictors of serious complications and even mortality have not been elucidated in today’s complex situation [10,11,12]. This study aimed to define predictors of mortality in NPS endemic era’s illicit drug users to strengthen the accuracy and timeliness of the emergency treatment.
2. Materials and Methods
2.1. Study Design, Setting, and Selection of Participants
This was a retrospective study. From February 2019 to November 2019, liquid chromatography/mass spectrometer (LC-LM/MS) confirmed positive illicit drug users who visited the emergency departments (ED) of the following medical centers or hospitals were enrolled: Chang Gung Memorial Hospitals (including Linkou, Keelung and Kaohsiung CGMH), MacKay Memorial Hospital, National Taiwan University Hospital, Taipei Veterans General Hospital, Hualien Tzu Chi Hospital, and China Medical University. Enrolled patients were part of the recruit patients of the 2019 “Taiwan Emergency Department Drug Abuse Surveillance (TEDAS)” project. TEDAS project provided LC-LM/MS qualitative analysis of 110 kinds of illicit drugs (Supplementary Table S1), including non-NPS illicit drugs and NPS for the 79 EDs participating in the project across Taiwan. When ED patients were suspected of illicit drugs being used, urine samples were collected and sent to either of the two toxicological laboratories, the Forensic and clinical toxicology center of National Taiwan University and Taipei Veterans General Hospital Department of Clinical Toxicology and Occupational Medicine.
This study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [13].
2.2. Measurements
We designed a standardized abstraction form to collect all variables from electronic medical records retrospectively. The following variables were collected: patient’s demographics including age, gender, and date of admission, triage vital signs, Glasgow Coma Scale (GCS), and positive physical examination of any neurological, psychiatric, cardiovascular, respiratory, or other symptoms upon ED admission, laboratory variables, and outcomes. Laboratory variables include white blood count (WBC), hemoglobin (Hb), platelet count, biochemical markers such as creatinine, sodium (Na), potassium (K), aspartate transaminase (AST), alanine aminotransferase (ALT), creatine-phospho-kinase (CPK) and venous/arterial blood gas were all recorded. Lab results of the urine drug test from TEDAS were abstracted. The primary outcome was in-hospital mortality.
2.3. Statistics
All statistical analysis was performed using SAS statistical software version 7.1 (SAS Institute, Cary, NC, USA). For univariate analysis between groups, continuous variables were expressed as the Mann-Whitney U test or Student t-test. The categorical variables were indicated as the Chi-Square test or Fisher’s exact test. The univariate and multivariate logistic regression analyses were applied to analyze predictors influencing mortality in NPS intoxication. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics
A total of 355 patients were included in this study, 233 (62.9%) of whom were male, and 122 (37.1%) were female. The most frequently used illicit drugs were amphetamines, followed by opioids, cathinones, and ketamine. The most frequently detected cathinones among the 16 synthetic cathinones were eutylone, followed by mephedrone. The combined use of cathinones and ketamine was most commonly observed in our results. Supplementary Tables S2 and S3 described the details of the used drugs individually.
Table 1 describes the clinical manifestations and laboratory findings of the main drug classes in our study. The cathinones users were younger than amphetamines, opioids, and ketamine users. The cathinones users also had higher body temperature than amphetamines, opioids, and ketamine users. Regarding patients’ conscious levels, opioids, cathinones, and ketamine users had more comatose (GCS ≤ 8) patients. Regarding symptoms and signs, cathinones users experienced more palpitation, hallucination, agitation, and delirium than the other two kinds of drug users. Not surprisingly, opioid users had the smallest pupils. Regarding laboratory findings, cathinones, amphetamines, and ketamine users had higher hematocrit than opioid users. The higher hematocrit may reflect these patients were hemoconcentration or dehydrated. The cathinones users also had higher creatinine levels. The opioid users were more respiratory acidic because of the respiratory depression effects of opioids.

Table 1.
The clinical manifestations of the main drug classes.
There were 16 fatalities for a mortality rate of 4.5% (Table 2). The mean age of all patients was 39.32 ± 15.86. The mortality patients (49.94 ± 18.8 years old) were older than the survivors (38.81 ±15.6 years old, p = 0.01). Those who died also had higher heart rates (127.19 ± 28.33 vs. 104.61 ± 25.53, respectively, p = 0.00) and faster respiratory rates compared to the survivors (23.13 ± 5.75 vs. 19.65 ± 2.98, p = 0.01). Lower blood pressures were observed among the mortality patients when they admitted to EDs (systolic blood pressure 105.38 ± 28.91 mmHg vs. 127.01 ± 26.78 mmHg, p = 0.0; diastolic blood pressure 69.5 ± 30.32 mmHg vs. 78.78 ± 19.23 mmHg, p = 0.01; and mean arterial pressure 81.46 ± 29.04 mmHg vs. 94.86 ± 20.39 mmHg, p = 0.01). Also, we found those who died had lower O2 room air saturation than the survivors (89.17 ± 8.78% vs. 95.5 ± 6.23%, p = 0.0054). There were more patients with deep coma (GCS ≤ 8) in the mortality group (9/16 vs. 59/339, p = 0.0008).

Table 2.
Characteristics of the illicit drug users on arrival at the ED of survivors and mortality patients.
Regarding the clinical presentations, the most common symptoms/sings was agitation (n = 92, 26%). As well, 38 (11%) patients had dyspnea, 26 (7.3%) patients had hallucination, and 25 (7%) patients had sweating on the arrival at ED (Table 3). The mortality patients had more dyspnea (p < 0.00), seizure/status epilepticus (p = 0.00), abnormal breath sound (p = 0.00) and urine incontinence (p = 0.03) than the survivors.

Table 3.
Clinical presentations of the illicit drug users on arrival at the ED of survivors and mortality patients.
3.2. Laboratory Results
Univariate comparisons of laboratory data were presented in Table 4. When comparing to the survivors, the mortality patients had less platelet count (193.44 ± 128.2 vs. 273.98 ± 98.32 103/µL, p = 0.01), higher creatinine (2.06 ± 1.19 vs. 1.24 ± 1.46 mg/dL, p < 0.00), higher potassium (4.94 ± 1.21 vs. 3.78 ± 0.59 mEq/L, p < 0.00), higher blood sugar (181.13 ± 154.6 vs. 126.54 ± 52.77 mg/dL, p = 0.02), higher liver function test (AST 214 (76-2388) vs. 33 (21–62), p < 0.00; ALT 47 (19-131) vs. 24 (14–39) U/L, p = 0.012; total bilirubin 1.79 (0.8–3.5) vs. 0.6 (0.4–1) mg/dL, p = 0.00) and higher lactate (3.44 (2.71–9.24) vs. 2.23 (1.39–3.65) mmol/L, p = 0.01). The mean of all patients of pH, FiO2, PCO2 and HCO3 were 7.36 ± 0.1, 45.08 ± 33.11%, 41.5 ± 11.87 mmHg and 22.69 ± 5.28 mmol/L respectively. FiO2 (p = 0.00), PCO2 (p = 0.01) and HCO3 (p < 0.00) had significantly difference between the survivors and those who died.

Table 4.
Initial Laboratory data of the illicit drug users of survivors and mortality patients.
Thus, in summary, those who died were with deep coma, faster vital signs, more effort in respiration, more oxygen supply, more acidemia, acute kidney injury, hyperglycemia, and higher lactate level, indicating their more critical illness.
3.3. Multiple Logistic Regression
All the variables with a p-value < 0.1 were further analyzed through multiple logistic regression to identify independent predictors of in-hospital mortality. SBP < 90 mmHg (OR = 9.86), dyspnea (OR = 17.03), Sugar > 140 mg/dL (OR = 10.27) and HCO3 < 20.6 (OR = 13.35) mmHg was variable that remained in the model after logistic regression (Table 5). We compared the area under the receiver of operating characteristics (ROC) curve of these predictors (Figure 1). The areas under the curve of these predictors were: SBP < 90 mmHg, 0.67; dyspnea, 0.80; Sugar > 140, 0.74; HCO3 < 20.6, 0.79. The sensitivities, specificities, positive predictive value, negative predictive value, and accuracies of the above variables in predicting in-hospital mortality were 1, 0.82, 0.31, 1, and 0.83, respectively.

Table 5.
Logistic model for predicting the probability of mortality of drug users.
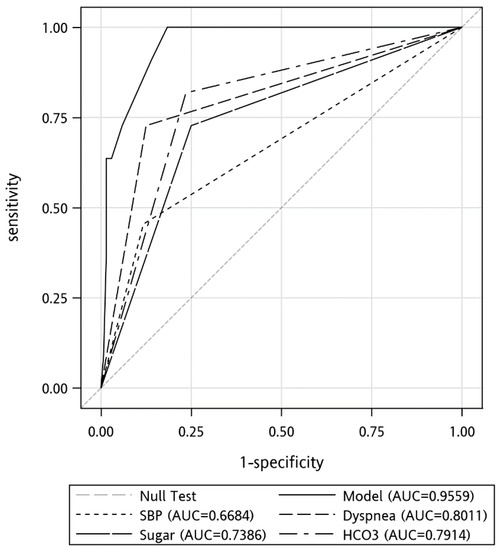
Figure 1.
Receiver operating characteristic (ROC) curves for the prediction of the in-hospital mortality.
3.4. Drugs Used in the Mortality Patients
The drugs used in the mortality group were as below. The patients of single-use of drugs were morphine (n = 5), methamphetamine (n = 4), meta-chlorophenyl piperazine (n = 2), and 4-chloromethcathinone (n = 1). The patients of multiple use of drugs were methamphetamine + morphine+ codeine (n = 1), norketamine + morphine (n = 1) and N-ethyl pentylone + methamphetamine (n = 1). Four of sixteen were detected both with traditional illicit drugs and NPS.
4. Discussion
To the best of our knowledge, this study was the first cohort focused on clinical presentations and predictors of in-hospital mortality in illicit drug users in the NPS endemic era in Taiwan. Our results showed that the patients with higher heart rates, faster respiratory rates, lower systolic/diastolic/mean blood pressure, or GCS ≤ 8 were highly associated with in-hospital mortality. Lower platelet count, more severe renal injury with higher potassium level, higher blood sugar, more severe hepatic injury, and higher lactate acid level were also significantly related to in-hospital mortality. The independent predictors of in-hospital mortality were SBP < 90 mmHg, sugar > 140 mg/dL, dyspnea and HCO3 < 20.6 at the triage.
The overall mortality rate in our study was 4.5%. However, in French, 800 cases of NPS-related abuse or somatic complications were reported to the French Addict vigilance Network (DRAMES survey), including 71 fatal cases (9%) between 2009 and 2017 [3]. There were 3.2 NPS-related deaths per million inhabitants of countries reported in 2017 (range 0–19.8) and 4.9 per million inhabitants aged 15–64 (range 0–30.6) in five countries (Estonia, Finland, the UK, Sweden, and Turkey) [14]. Just as our study demonstrated, elderly-aged patients with substance abuse have higher mortality as described in the previous study [14]. Another retrospective study from the five Nordic countries: Denmark, Finland, Iceland, Norway, and Sweden, also showed many deaths numbers of addicts more than 45 years old [15].
Cardiovascular instability causing an increase in mortality in NPS intoxication patients was confirmed by our result of faster vital signs, but there have been little study into related topics. However, it is reasonable that patients with more severe cardiovascular effects such as hypotension or tachycardia would have a poorer prognosis, even death. Poor consciousness (GCS ≤ 8, 19% in this study) was also an important indicator of poor prognosis. These kinds of patients are prone to be intubated for airway protection. Sharon Essink et al. reported NPS intoxication symptoms in a prospective cohort that included patients with coma in seven cases (32%) and respiratory depression requiring mechanical ventilation in five cases (23%) [15]. Thus, when illicit drug users are in a deep coma, never hesitate to intubate the patients to have good supportive care.
The mortality patients had more severe metabolic acidosis. Metabolic acidosis indicated higher intoxication metabolites, direct cell injury, hypoxia, and possible increased lactate levels [16]. Clinically, acute metabolic acidosis decreases cardiac output, dilates arteries resulting in hypotension, alters oxygen delivery, decreases ATP production, causes a predisposition to arrhythmias, and impairment of the immune response [17]. The mortality patients also had significantly higher blood sugar levels. To our best knowledge, stress hyperglycemia describes a state of blood glucose deregulation during acute physiological stress, which is common in critically ill patients and appears to be a marker of disease severity [18,19]. It is an adaptive immune-neurohormonal response to physiological stress to increase metabolic substrates to struggling organs during a crisis [20]. In other words, if patients with NPS intoxication, higher hyperglycemia would be a poor sign for their prognosis due to more severe critical illness. Since blood glucose is easy and fast to obtain in the clinical setting, thus, blood glucose > 140 mg/dL may serve as an early warning sign in those severely ill illicit drug users. SBP < 90 mmHg indicated a poor prognosis, too. The most reliable explanation was that it caused shock status meaning poor perfusion and tissue hypoxia. Similarly, in another study, hypotension (mean arterial blood pressure of ≤ 59 mmHg) was identified as a predictor of mortality of elderly with acute poisoning in the ED [21]. Jayashree et al. reported hypotension at admission as the most significant predictor of death in children admitted to the ICU with acute intoxication [22].
In this study, we did not mainly focus on which type of NPS intoxication because many patients could present to the EDs with mixed or multiple drug intoxication. Instead, we attempted to identify patients with the highest risk of in-hospital mortality. Although the different characteristics of illicit drugs may vary in clinical presentations, it is quite impractical to treat patients until we confirm the culprit of illicit substances in the clinical setting. On the contrary, it is practical to know the red flag signs when patients are admitted to ED to treat them in priority and provide adequate, qualified, supportive care in advance and thus avoid poor outcomes. The four independent predictors may guide ED physicians to determine the initial severity of the patients with suspected illicit drug users and thus provide the intensive care treatment measurements.
In this study, we also described the main drug classes’ clinical manifestations, symptoms, signs, and laboratory findings. The cathinone users were younger, had higher body temperature, experienced more palpitation, hallucination, agitation and delirium, higher creatinine levels, and hemoconcentration/dehydration. Opioid users had the smallest pupils and were more respiratory acidic because of the respiratory depression effects of opioids. Different illicit drug classes had different clinical manifestations, as we demonstrated here. Therefore, their specific clinical manifestations provide their value in diagnosis. However, in clinical settings, nearly half of the illicit drug users used multiple substances and the typical clinical manifestations of each drug class may be less observable. Regrettably, we cannot determine each of their prognostic factors of mortality in this study because of the small patient numbers of each illicit drug classes we had.
Our study has some limitations. First, our study was retrospective. Therefore, the study design had inherent limitations such as recall bias. Although we made an effort to remain objective, this study was inevitably limited by missing data. Second, the concomitant illicit drug use pattern may differ across countries or regions; therefore, this study’s findings should be interpreted cautiously. However, the compensated cardiovascular functions, the stress blood sugar level, and metabolic acidosis with lacticaemia all point us to a severely ill patient.
5. Conclusions
Regardless of the pattern of the use of the illicit drugs, patients with deep coma, unstable vital signs, metabolic acidosis, and stress blood sugar levels were independent predictors in illicit drug users in the NPS endemic in Taiwan. The recognition of these associated factors allows for risk stratification and determining the optimal treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics10070386/s1; Table S1: LC-MS/MS detection list of 110 drugs and metabolites. Table S2: Drugs use pattern. Table S3: The cathinones patients used.
Author Contributions
Conceptualization, C.-C.L.; methodology, C.-C.L.; software, S.-Y.G.; validation, C.-C.L. and H.-T.Y.; formal analysis, C.-C.L., H.-T.Y. and S.-Y.G.; investigation, C.-C.L., H.-T.Y., T.-I.W. and S.-Y.G.; resources, C.-C.L., S.-W.L., C.-C.F., J.-H.Y., Y.-C.C., T.-I.W. and Y.-J.S.; data curation, C.-C.L. and H.-Y.C.; writing—original draft preparation, C.-C.L. and H.-T.Y.; writing—review and editing, C.-C.L. and H.-T.Y.; supervision, C.-C.L.; project administration, C.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by Chang Gung Medical Foundation.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of Chang Gung Memorial Hospital (IRB No. 202100685B0 approval date is 10 May 2021).
Informed Consent Statement
Patient consent was waived because this was a retrospective study.
Data Availability Statement
Not applicable.
Acknowledgments
We also wish to thank Hsin-Yu Wang for her excellent secretary work and help in this study project.
Conflicts of Interest
The authors declare no conflict of interest. The APC funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Weinstein, A.M.; Rosca, P.; Fattore, L.; London, E.D. Synthetic Cathinone and Cannabinoid Designer Drugs Pose a Major Risk for Public Health. Front. Psychiatry 2017, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Volkow, N.D. Abuse of New Psychoactive Substances: Threats and Solutions. Neuropsychopharmacology 2016, 41, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Batisse, A.; Eiden, C.; Peyriere, H.; Djezzar, S. Use of new psychoactive substances to mimic prescription drugs: The trend in France. Neurotoxicology 2020, 79, 20–24. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC). World Drug Report: Stimulants; United Nations Office on Drugs and Crime: Vienna, Austria, 2021. [Google Scholar]
- Chang, H.J. Health care systems in transition. II. Taiwan, Part II. The current status of HIV-AIDS in Taiwan. J. Public Health Med. 1998, 20, 11–15. [Google Scholar] [PubMed]
- Feng, L.Y.; Li, J.H. New psychoactive substances in Taiwan: Challenges and strategies. Curr. Opin. Psychiatry 2020, 33, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-M.; Tracy, D.K.; Huang, M.-C.; Pan, C.; Chen, L.-Y. Psychiatric Profiles and Clinical Manifestations of Cathinone Users: Case Series of Analytically Confirmed Cathinone Use in Taiwan. Addict. Addict. Disord. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Lin, C.C.; Weng, T.I.; Ng, C.J.; Shih, C.P.; Hsu, J.; Liao, Y.C.; Yang, C.C.; Fang, C.C. Emergency department visits due to new psychoactive substances and other illicit drugs in Taiwan: Preliminary results of the Taiwan Emergency Department Drug Abuse Surveillance (TEDAS) project. Clin. Toxicol. 2022, 60, 708–715. [Google Scholar] [CrossRef]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef]
- Flüchter, P.; Pajonk, F.G. Treatment of intoxication with new psychoactive substances and methamphetamine. Med. Monatsschr. Pharm. 2015, 38, 94–100. [Google Scholar]
- Wilkins, C. A critical first assessment of the new pre-market approval regime for new psychoactive substances (NPS) in New Zealand. Addiction 2014, 109, 1580–1586. [Google Scholar] [CrossRef]
- Mégarbane, B.; Oberlin, M.; Alvarez, J.C.; Balen, F.; Beaune, S.; Bédry, R.; Chauvin, A.; Claudet, I.; Danel, V.; Debaty, G.; et al. Management of pharmaceutical and recreational drug poisoning. Ann. Intensive Care 2020, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Bull. World Health Organ. 2007, 85, 867–872. [Google Scholar] [CrossRef] [PubMed]
- López-Pelayo, H.; Vicente, J.; Gallegos, A.; McAuley, A.; Büyük, Y.; White, M.; Giraudon, I. Mortality involving New Psychoactive Substances across Europe, 2016–2017. Emerg. Trends Drugs Addict. Health 2021, 1, 100016. [Google Scholar] [CrossRef]
- Essink, S.; Nugteren-van Lonkhuyzen, J.J.; van Riel, A.; Dekker, D.; Hondebrink, L. Significant toxicity following an increase in poisonings with designer benzodiazepines in the Netherlands between 2010 and 2020. Drug Alcohol Depend. 2022, 231, 109244. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, D.H.; Kim, T.; Lee, S.H.; Jeong, J.H.; Kim, S.C.; Park, Y.J.; Lim, D.; Kang, C. Anion gap and base deficit are predictors of mortality in acute pesticide poisoning. Hum. Exp. Toxicol. 2019, 38, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Madias, N.E. Metabolic acidosis: Pathophysiology, diagnosis and management. Nat. Rev. Nephrol. 2010, 6, 274–285. [Google Scholar] [CrossRef]
- Marik, P.E.; Bellomo, R. Stress hyperglycemia: An essential survival response! Crit. Care Med. 2013, 41, e93–e94. [Google Scholar] [CrossRef]
- Scheen, M.; Giraud, R.; Bendjelid, K. Stress hyperglycemia, cardiac glucotoxicity, and critically ill patient outcomes current clinical and pathophysiological evidence. Physiol. Rep. 2021, 9, e14713. [Google Scholar] [CrossRef]
- Mifsud, S.; Schembri, E.L.; Gruppetta, M. Stress-induced hyperglycaemia. Br. J. Hosp. Med. 2018, 79, 634–639. [Google Scholar] [CrossRef]
- Hu, Y.H.; Chou, H.L.; Lu, W.H.; Huang, H.H.; Yang, C.C.; Yen, D.H.; Kao, W.F.; Deng, J.F.; Huang, C.I. Features and prognostic factors for elderly with acute poisoning in the emergency department. J. Chin. Med. Assoc. 2010, 73, 78–87. [Google Scholar] [CrossRef][Green Version]
- Jayashree, M.; Singhi, S. Changing trends and predictors of outcome in patients with acute poisoning admitted to the intensive care. J. Trop. Pediatr. 2011, 57, 340–346. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).