Evaluating Phenotypic and Transcriptomic Responses Induced by Low-Level VOCs in Zebrafish: Benzene as an Example
Abstract
:1. Introduction
2. Methods
2.1. Fish Husbandry
2.2. Chemical Exposure
2.2.1. The Stock Chemicals and Dilutions
2.2.2. Exposures
2.3. Measurements of Body Burden
2.4. Abnormality and Mortality Screening
2.5. Behavioral Screening
2.6. RNA Isolation
2.7. Transcriptomic Analysis
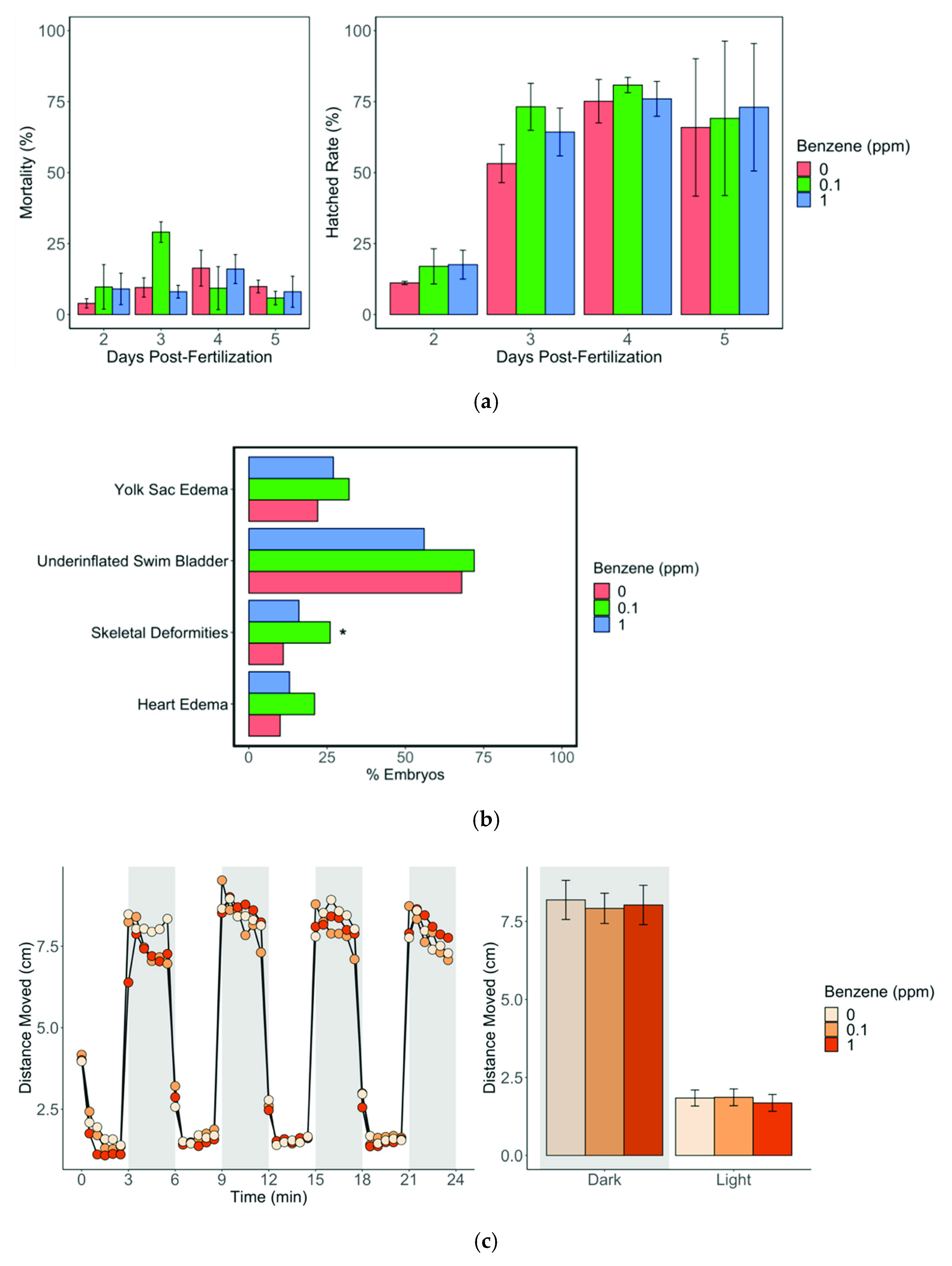
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKenzie, L.M.; Witter, R.Z.; Newman, L.S.; Adgate, J.L. Human Health Risk Assessment of Air Emissions from Development of Unconventional Natural Gas Resources. Sci. Total Environ. 2012, 424, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Xu, X.; Grgicak-Mannion, A.; Brook, J.; Wheeler, A. Multi-Season, Multi-Year Concentrations and Correlations amongst the BTEX Group of VOCs in an Urbanized Industrial City. Atmos. Environ. 2012, 61, 305–315. [Google Scholar] [CrossRef]
- Miri, M.; Rostami Aghdam Shendi, M.; Ghaffari, H.R.; Ebrahimi Aval, H.; Ahmadi, E.; Taban, E.; Gholizadeh, A.; Yazdani Aval, M.; Mohammadi, A.; Azari, A. Investigation of Outdoor BTEX: Concentration, Variations, Sources, Spatial Distribution, and Risk Assessment. Chemosphere 2016, 163, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Sources and Fates of BTEX in the General Environment and Its Distribution in Coastal Cities of China. J. Environ. Sci. Public Health 2017, 1, 86–106. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.J.; Runge-Morris, M.; Cassidy-Bushrow, A.E.; Straughen, J.K.; Dittrich, T.M.; Baker, T.R.; Petriello, M.C.; Mor, G.; Ruden, D.M.; O’leary, B.F.; et al. A Review of Volatile Organic Compound Contamination in Post-Industrial Urban Centers: Reproductive Health Implications Using a Detroit Lens. Int. J. Environ. Res. Public Health 2020, 17, 8755. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.J.; Hannigan, J.H.; Bowen, S.E. Effects of Inhaled Combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an Environmental Exposure Model. Environ. Toxicol. Pharmacol. 2021, 81, 103518. [Google Scholar] [CrossRef]
- Bahadar, H.; Mostafalou, S.; Abdollahi, M. Current Understandings and Perspectives on Non-Cancer Health Effects of Benzene: A Global Concern. Toxicol. Appl. Pharmacol. 2014, 276, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Slama, R.; Thiebaugeorges, O.; Goua, V.; Aussel, L.; Sacco, P.; Bohet, A.; Forhan, A.; Ducot, B.; Annesi-Maesano, I.; Heinrich, J. Maternal Personal Exposure to Airborne Benzene and Intrauterine Growth. Environ. Health Perspect. 2009, 117, 1313–1321. [Google Scholar] [CrossRef]
- Chen, D.; Cho, S.-I.; Chen, C.; Wang, X.; Damokosh, A.I.; Ryan, L.; Smith, T.J.; Christiani, D.C.; Xu, X. Exposure to Benzene, Occupational Stress, and Reduced Birth Weight. Occup. Environ. Med. 2000, 57, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.R.; Yoo, C.I.; Lee, J.H.; Kim, S.-R.; Kim, Y. Hematological Changes of Children Exposed to Volatile Organic Compounds Containing Low Levels of Benzene. Sci. Total Environ. 2002, 299, 237–245. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Reddy, G.K. Health Effects of Benzene Exposure among Children Following a Flaring Incident at the British Petroleum Refinery in Texas City. Pediatr. Hematol. Oncol. 2014, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.A.; Reddy, G.K. Adverse Health Effects of Benzene Exposure among Children Following a Flaring Incident at the British Petroleum Refinery in Texas City. Clin. Pediatr. 2016, 55, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Kim, S.; Lan, Q.; Vermeulen, R.; Waidyanatha, S.; Zhang, L.; Li, G.; Yin, S.; Hayes, R.B.; Rothman, N.; et al. Evidence That Humans Metabolize Benzene via Two Pathways. Environ. Health Perspect. 2009, 117, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, K.R.; And, V.K.; Snyder, R. Correlation of Benzene Metabolism and Histological Lesions in Rainbow Trout (Salmo Gairdneri). Drug Metab. Rev. 1984, 15, 673–696. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A. Benzene’s Toxicity: A Consolidated Short Review of Human and Animal Studies by HA Khan. Hum. Exp. Toxicol. 2007, 26, 677–685. [Google Scholar] [CrossRef]
- Spatari, G.; Allegra, A.; Carrieri, M.; Pioggia, G.; Gangemi, S. Epigenetic Effects of Benzene in Hematologic Neoplasms: The Altered Gene Expression. Cancers 2021, 13, 2392. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Reddy, G.K. Health Risks Associated with Benzene Exposure in Children: A Systematic Review. Glob. Pediatr. Health 2018, 5, 2333794X18789275. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Trede, N.S.; Langenau, D.M.; Traver, D.; Look, A.T.; Zon, L.I. The Use of Zebrafish to Understand Immunity. Immunity 2004, 20, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Ciau-Uitz, A.; Monteiro, R.; Kirmizitas, A.; Patient, R. Developmental Hematopoiesis: Ontogeny, Genetic Programming and Conservation. Exp. Hematol. 2014, 42, 669–683. [Google Scholar] [CrossRef]
- Robertson, A.L.; Avagyan, S.; Gansner, J.M.; Zon, L.I. Understanding the Regulation of Vertebrate Hematopoiesis and Blood Disorders—Big Lessons from a Small Fish. FEBS Lett. 2016, 590, 4016–4033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockhammer, O.W.; Zakrzewska, A.; Hegedûs, Z.; Spaink, H.P.; Meijer, A.H. Transcriptome Profiling and Functional Analyses of the Zebrafish Embryonic Innate Immune Response to Salmonella Infection. J. Immunol. 2009, 182, 5641–5653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- U.S. Environmental Protection Agency. Method 8260C Volatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS); U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- Krauß, M.; Haucke, V. Phosphoinositide-metabolizing Enzymes at the Interface between Membrane Traffic and Cell Signalling. EMBO Rep. 2007, 8, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in Cell Regulation and Membrane Dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef]
- Banerjee, S.; Oneda, B.; Yap, L.; Jewell, D.; Matters, G.; Fitzpatrick, L.; Seibold, F.; Sterchi, E.; Ahmad, T.; Lottaz, D.; et al. MEP1A Allele for Meprin A Metalloprotease Is a Susceptibility Gene for Inflammatory Bowel Disease. Mucosal. Immunol. 2009, 2, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Schütte, A.; Hedrich, J.; Stöcker, W.; Becker-Pauly, C. Let It Flow: Morpholino Knockdown in Zebrafish Embryos Reveals a pro-Angiogenic Effect of the Metalloprotease Meprin A2. PLoS ONE 2010, 5, e8835. [Google Scholar] [CrossRef]
- Gautier, T.; Paul, C.; Deckert, V.; Desrumaux, C.; Klein, A.; Labbé, J.; Le Guern, N.; Athias, A.; Monier, S.; Hammann, A.; et al. Innate Immune Response Triggered by Triacyl Lipid a is Dependent on Phospholipid Transfer Protein (PLTP) Gene Expression. FASEB J. 2010, 24, 3544–3554. [Google Scholar] [CrossRef]
- Koch, B.E.V.; Stougaard, J.; Spaink, H.P. Spatial and Temporal Expression Patterns of Chitinase Genes in Developing Zebrafish Embryos. Gene Expr. Patterns 2014, 14, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.S.; Kim, T.H.; Lee, H.M.; Lee, S.H.; Lee, S.H.; Yoo, J.H.; Kim, Y.S.; Lee, S.H. Increased Expression of Acidic Mammalian Chitinase and Chitotriosidase in the Nasal Mucosa of Patients with Allergic Rhinitis. Laryngoscope 2010, 120, 870–875. [Google Scholar] [CrossRef]
- Alpern, D.; Langer, D.; Ballester, B.; Le Gras, S.; Romier, C.; Mengus, G.; Davidson, I. TAF4, a Subunit of Transcription Factor II D, Directs Promoter Occupancy of Nuclear Receptor HNF4A during Post-Natal Hepatocyte Differentiation. Elife 2014, 3, e03613. [Google Scholar] [CrossRef]
- Langer, D.; Martianov, I.; Alpern, D.; Rhinn, M.; Keime, C.; Dollé, P.; Mengus, G.; Davidson, I. Essential Role of the TFIID Subunit TAF4 in Murine Embryogenesis and Embryonic Stem Cell Differentiation. Nat. Commun. 2016, 7, 11063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krens, S.F.G.; Spaink, H.P.; Snaar-Jagalska, B.E. Functions of the MAPK Family in Vertebrate-Development. FEBS Lett. 2006, 580, 4984–4990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbueken, E.; Bars, C.; Ball, J.S.; Periz-Stanacev, J.; Marei, W.F.A.; Tochwin, A.; Gabriëls, I.J.; Michiels, E.D.G.; Stinckens, E.; Vergauwen, L.; et al. From MRNA Expression of Drug Disposition Genes to in Vivo Assessment of CYP-Mediated Biotransformation during Zebrafish Embryonic and Larval Development. Int. J. Mol. Sci. 2018, 19, 3976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaji, T.; Yamashita, N.; Umeda, H.; Zhang, S.; Mizoguchi, N.; Seki, M.; Kitazawa, T.; Teraoka, H. Cytochrome P450 Expression and Chemical Metabolic Activity before Full Liver Development in Zebrafish. Pharmaceuticals 2020, 13, 456. [Google Scholar] [CrossRef]
- Bolcome, R.E.; Sullivan, S.E.; Zeller, R.; Barker, A.P.; Collier, R.J.; Chan, J. Anthrax Lethal Toxin Induces Cell Death-Independent Permeability in Zebrafish Vasculature. Proc. Natl. Acad. Sci. USA 2008, 105, 2439–2444. [Google Scholar] [CrossRef] [Green Version]
- Avraham-Davidi, I.; Ely, Y.; Pham, V.N.; Castranova, D.; Grunspan, M.; Malkinson, G.; Gibbs-Bar, L.; Mayseless, O.; Allmog, G.; Lo, B. ApoB-Containing Lipoproteins Regulate Angiogenesis by Modulating Expression of VEGF Receptor 1. Nat. Med. 2012, 18, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Cui, P. Complement System in Zebrafish. Dev. Comp. Immunol. 2014, 46, 3–10. [Google Scholar] [CrossRef]
- Chatterjee, B.; Banoth, B.; Mukherjee, T.; Taye, N.; Vijayaragavan, B.; Chattopadhyay, S.; Gomes, J.; Basak, S. Late-Phase Synthesis of IκBα Insulates the TLR4-Activated Canonical NF-ΚB Pathway from Noncanonical NF-ΚB Signaling in Macrophages. Sci. Signal. 2016, 9, ra120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Rubins, N.E.; Ahima, R.S.; Greenbaum, L.E.; Kaestner, K.H. Foxa2 Integrates the Transcriptional Response of the Hepatocyte to Fasting. Cell Metab. 2005, 2, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Wolfrum, C.; Shih, D.Q.; Kuwajima, S.; Norris, A.W.; Kahn, C.R.; Stoffel, M. Role of Foxa-2 in Adipocyte Metabolism and Differentiation. J. Clin. Investig. 2003, 112, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Dal-Pra, S.; Thisse, C.; Thisse, B. FoxA Transcription Factors Are Essential for the Development of Dorsal Axial Structures. Dev. Biol. 2011, 350, 484–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.-R.; Lu, Y.-F.; Lien, H.-W.; Huang, C.-J.; Hwang, S.-P.L. Foxa2 and Hif1ab Regulate Maturation of Intestinal Goblet Cells by Modulating Agr2 Expression in Zebrafish Embryos. Biochem. J. 2016, 473, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Phua, W.W.T.; Tan, W.R.; Yip, Y.S.; Hew, I.D.; Wee, J.W.K.; Cheng, H.S.; Leow, M.K.S.; Wahli, W.; Tan, N.S. PPARβ/δ Agonism Upregulates Forkhead Box A2 to Reduce Inflammation in C2C12 Myoblasts and in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, M.L.; Andrews, Z.B.; Duh, E.J.; Zechner, R.; Meikle, P.J.; Watt, M.J. Pigment Epithelium-Derived Factor Regulates Lipid Metabolism via Adipose Triglyceride Lipase. Diabetes 2011, 60, 1458–1466. [Google Scholar] [CrossRef] [Green Version]
- Shellenberger, T.D.; Mazumdar, A.; Henderson, Y.; Briggs, K.; Wang, M.; Chattopadhyay, C.; Jayakumar, A.; Frederick, M.; Clayman, G.L. Headpin: A Serpin with Endogenous and Exogenous Suppression of Angiogenesis. Cancer Res. 2005, 65, 11501–11509. [Google Scholar] [CrossRef] [Green Version]
- Goessling, W.; Sadler, K.C. Zebrafish: An Important Tool for Liver Disease Research. Gastroenterology 2015, 149, 1361–1377. [Google Scholar] [CrossRef] [Green Version]
- Turteltaub, K.W.; Mani, C. Benzene Metabolism in Rodents at Doses Relevant to Human Exposure from Urban Air. Res. Rep. Health Eff. Inst. 2003, 113, 1–26. [Google Scholar]
- Valcke, M.; Krishnan, K. Assessing the Impact of the Duration and Intensity of Inhalation Exposure on the Magnitude of the Variability of Internal Dose Metrics in Children and Adults. Inhal. Toxicol. 2011, 23, 863–877. [Google Scholar] [CrossRef]
- Sammett, D.; Lee, E.W.; Kocsis, J.J.; Snyder, R. Partial Hepatectomy Reduces Both Metabolism and Toxicity of Benzene. J. Toxicol. Environ. Health Part A Curr. Issues 1979, 5, 785–792. [Google Scholar] [CrossRef]
- Sheets, P.L.; Carlson, G.P. Kinetic Factors Involved in the Metabolism of Benzene in Mouse Lung and Liver. J. Toxicol. Environ. Health Part A 2004, 67, 421–430. [Google Scholar] [CrossRef]
- Fujii, R.; Yamashita, S.; Hibi, M.; Hirano, T. Asymmetric P38 Activation in Zebrafish: Its Possible Role in Symmetric and Synchronous Cleavage. J. Cell Biol. 2000, 150, 1335–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilbur, S.B.; Keith, S.; Faroon, O.; Wohlers, D. Toxicological Profile for Benzene; U.S. Department of Health & Human Services: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- Gao, A.; Yang, J.; Yang, G.; Niu, P.; Tian, L. Differential Gene Expression Profiling Analysis in Workers Occupationally Exposed to Benzene. Sci. Total Environ. 2014, 472, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Gut, I.; Nedelcheva, V.; Soucek, P.; Stopka, P.; Tichavská, B. Cytochromes P450 in Benzene Metabolism and Involvement of Their Metabolites and Reactive Oxygen Species in Toxicity. Environ. Health Perspect. 1996, 104 (Suppl. S6), 1211–1218. [Google Scholar] [PubMed] [Green Version]
- Thomas, R.; Hubbard, A.E.; McHale, C.M.; Zhang, L.; Rappaport, S.M.; Lan, Q.; Rothman, N.; Vermeulen, R.; Guyton, K.Z.; Jinot, J.; et al. Characterization of Changes in Gene Expression and Biochemical Pathways at Low Levels of Benzene Exposure. PLoS ONE 2014, 9, e91828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, C.L.; Kalasekar, S.M.; McCollum, C.W.; Riu, A.; Jonsson, P.; Lopez, J.; Swindell, E.C.; Bouhlatouf, A.; Balaguer, P.; Bondesson, M.; et al. Lxr Regulates Lipid Metabolic and Visual Perception Pathways during Zebrafish Development. Mol. Cell. Endocrinol. 2016, 419, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Cao, M.; Zhang, J.; Yang, W.; Wei, H.; Meng, X.; Yin, L.; Pu, Y. Benzene Exposure Alters Expression of Enzymes Involved in Fatty Acid β-Oxidation in Male C3H/He Mice. Int. J. Environ. Res. Public Health 2016, 13, 1068. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Xu, K.; Zhang, Q.; Jiang, X.; Man, Z.; Yin, L.; Zhang, J.; Pu, Y. Plasma Metabonomics Investigation Reveals Involvement of Fatty Acid Oxidation in Hematotoxicity in Chinese Benzene-Exposed Workers with Low White Blood Cell Count. Environ. Sci. Pollut. Res. 2018, 25, 32506–32514. [Google Scholar] [CrossRef]
- Ito, K.; Carracedo, A.; Weiss, D.; Arai, F.; Ala, U.; Avigan, D.E.; Schafer, Z.T.; Evans, R.M.; Suda, T.; Lee, C.-H. A PML–PPAR-δ Pathway for Fatty Acid Oxidation Regulates Hematopoietic Stem Cell Maintenance. Nat. Med. 2012, 18, 1350–1358. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Suda, T. Metabolic Requirements for the Maintenance of Self-Renewing Stem Cells. Nat. Rev. Mol. Cell Biol. 2014, 15, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, M.R.; Mirabilii, S.; Allegretti, M.; Licchetta, R.; Calarco, A.; Torrisi, M.R.; Foa, R.; Nicolai, R.; Peluso, G.; Tafuri, A. Targeting the Leukemia Cell Metabolism by the CPT1a Inhibition: Functional Preclinical Effects in Leukemias. Blood J. Am. Soc. Hematol. 2015, 126, 1925–1929. [Google Scholar] [CrossRef] [Green Version]
- Samudio, I.; Harmancey, R.; Fiegl, M.; Kantarjian, H.; Konopleva, M.; Korchin, B.; Kaluarachchi, K.; Bornmann, W.; Duvvuri, S.; Taegtmeyer, H. Pharmacologic Inhibition of Fatty Acid Oxidation Sensitizes Human Leukemia Cells to Apoptosis Induction. J. Clin. Investig. 2010, 120, 142–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Guo, X.; Chen, Y.; Zhang, W.; Ren, J.; Gao, A. Association between Benzene Exposure, Serum Levels of Cytokines and Hematological Measures in Chinese Workers: A Cross-Sectional Study. Ecotoxicol. Environ. Saf. 2021, 207, 111562. [Google Scholar] [CrossRef] [PubMed]
- Cassidy-Bushrow, A.E.; Burmeister, C.; Birbeck, J.; Chen, Y.; Lamerato, L.; Lemke, L.D.; Li, J.; Mor, G.; O’Leary, B.F.; Peters, R.M.; et al. Ambient BTEX Exposure and Mid-Pregnancy Inflammatory Biomarkers in Pregnant African American Women. J. Reprod. Immunol. 2021, 145, 103305. [Google Scholar] [CrossRef]
- Minciullo, P.L.; Navarra, M.; Calapai, G.; Gangemi, S. Cytokine Network Involvement in Subjects Exposed to Benzene. J. Immunol. Res. 2014, 2014, 937987. Available online: https://www.hindawi.com/journals/jir/2014/937987/ (accessed on 10 May 2022). [CrossRef] [PubMed] [Green Version]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Okkenhaug, K. Signalling by the Phosphoinositide 3-Kinase Family in Immune Cells. Annu. Rev. Immunol. 2013, 31, 675–704. [Google Scholar] [CrossRef] [Green Version]
- Berbée, J.F.P.; Havekes, L.M.; Rensen, P.C.N. Apolipoproteins Modulate the Inflammatory Response to Lipopolysaccharide. J. Endotoxin Res. 2005, 11, 97–103. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-KappaB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Swaen, G.M.H.; van Amelsvoort, L.; Twisk, J.J.; Verstraeten, E.; Slootweg, R.; Collins, J.J.; Burns, C.J. Low Level Occupational Benzene Exposure and Hematological Parameters. Chem. Biol. Interact. 2010, 184, 94–100. [Google Scholar] [CrossRef]
- Schnatter, A.R.; Glass, D.C.; Tang, G.; Irons, R.D.; Rushton, L. Myelodysplastic Syndrome and Benzene Exposure among Petroleum Workers: An International Pooled Analysis. J. Natl. Cancer Inst. 2012, 104, 1724–1737. [Google Scholar] [CrossRef] [Green Version]
- Lan, Q.; Zhang, L.; Li, G.; Vermeulen, R.; Weinberg, R.S.; Dosemeci, M.; Rappaport, S.M.; Shen, M.; Alter, B.P.; Wu, Y.; et al. Hematotoxicity in Workers Exposed to Low Levels of Benzene. Science 2004, 306, 1774–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batterman, S.; Su, F.-C.; Li, S.; Mukherjee, B.; Jia, C. Personal Exposure to Mixtures of Volatile Organic Compounds: Modeling and Further Analysis of the RIOPA Data. Res. Rep. Health. Eff. Inst. 2014, 181, 3. [Google Scholar]
- Hernández, A.F.; Tsatsakis, A.M. Human Exposure to Chemical Mixtures: Challenges for the Integration of Toxicology with Epidemiology Data in Risk Assessment. Food Chem. Toxicol. 2017, 103, 188–193. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbol | Gene Name | 1 ppm | 0.1 ppm |
|---|---|---|---|
| Inflammatory response | |||
| pik3c2b | Phosphatidylinositol-4-phosphate 3-kinase, catalytic subunit type 2 beta | 2.8 | 1.85 |
| mep1a.2 | Meprin A, alpha (PABA peptide hydrolase), tandem duplicate 2 | 1.7 | 1.73 |
| chia.2 | Acidic chitinase 2 | 2.01 | 1.90 |
| chrnb2 | Cholinergic receptor, nicotinic, beta 2 | 0.59 | 0.54 |
| pltp | Phospholipid transfer protein | 0.57 | 0.58 |
| nfkbiaa | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha a | 1.70 | |
| apob | Apolipoprotein B | 1.70 | |
| si:ch1073-280e3.1 | Orthologous to human c2, complement c2 | 1.70 | |
| c3a.3 | Complement C3a, tandem duplicate 3 | 2.16 | |
| il15 | Interleukin 15 | 0.49 | |
| ifih1 | Interferon induced with helicase C domain 1 | 0.54 | |
| mapk11 | Mitogen-activated protein kinase 11 | 0.58 | |
| Hematological system development and function | |||
| acad11 | Acyl-CoA dehydrogenase family, member 11 | 3.20 | |
| antxr2 | ANTXR cell adhesion molecule 2 | 0.49 | |
| gabrb1 | Gamma-aminobutyric acid type A receptor subunit beta1 | 0.58 | |
| hic1 | HIC ZBTB transcriptional repressor 1 | 1.72 | |
| hsp90aa1.1 | Heat shock protein 90, alpha (cytosolic), class A member 1, tandem duplicate 1 | 1.82 | |
| kif21b | Kinesin family member 21B | 2.12 | |
| slc27a2b | Solute carrier family 27 member 2 | 1.72 | |
| slc9a5 | Solute carrier family 9 member A5 | 0.56 | |
| lancl2 | Lanthionine synthetase C-like 2 | 0.59 | |
| Lipid/fatty acid metabolism | |||
| mecr | Mitochondrial trans-2-enoyl-CoA reductase | 2.14 | 1.97 |
| cyp2k6 | Cytochrome P450, family 2, subfamily K, polypeptide 6 | 1.72 | |
| cyp2n13 | Cytochrome P450, family 2, subfamily N, polypeptide 13 | 1.84 | |
| hmgcra | 3-hydroxy-3-methylglutaryl-CoA reductase a | 1.94 | |
| serpinf1 | Serpin family F member 1 | 1.84 | |
| Embryogenesis | |||
| taf4a | TAF4A RNA polymerase II, TATA box binding protein (TBP)-associated factor | 0.50 | 0.52 |
| foxa2 | Forkhead box A2 | 1.74 | |
| tacr1a | Tachykinin receptor 1a | 0.58 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Blount, J.R.; Haimbaugh, A.; Heldman, S.; Shields, J.N.; Baker, T.R. Evaluating Phenotypic and Transcriptomic Responses Induced by Low-Level VOCs in Zebrafish: Benzene as an Example. Toxics 2022, 10, 351. https://doi.org/10.3390/toxics10070351
Wu C-C, Blount JR, Haimbaugh A, Heldman S, Shields JN, Baker TR. Evaluating Phenotypic and Transcriptomic Responses Induced by Low-Level VOCs in Zebrafish: Benzene as an Example. Toxics. 2022; 10(7):351. https://doi.org/10.3390/toxics10070351
Chicago/Turabian StyleWu, Chia-Chen, Jessica R. Blount, Alex Haimbaugh, Samantha Heldman, Jeremiah N. Shields, and Tracie R. Baker. 2022. "Evaluating Phenotypic and Transcriptomic Responses Induced by Low-Level VOCs in Zebrafish: Benzene as an Example" Toxics 10, no. 7: 351. https://doi.org/10.3390/toxics10070351
APA StyleWu, C.-C., Blount, J. R., Haimbaugh, A., Heldman, S., Shields, J. N., & Baker, T. R. (2022). Evaluating Phenotypic and Transcriptomic Responses Induced by Low-Level VOCs in Zebrafish: Benzene as an Example. Toxics, 10(7), 351. https://doi.org/10.3390/toxics10070351






