Lanthanides Toxicity in Zebrafish Embryos Are Correlated to Their Atomic Number
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance
2.2. Chemical Preparation
2.3. Zebrafish Acute Toxicity Test (LC50)
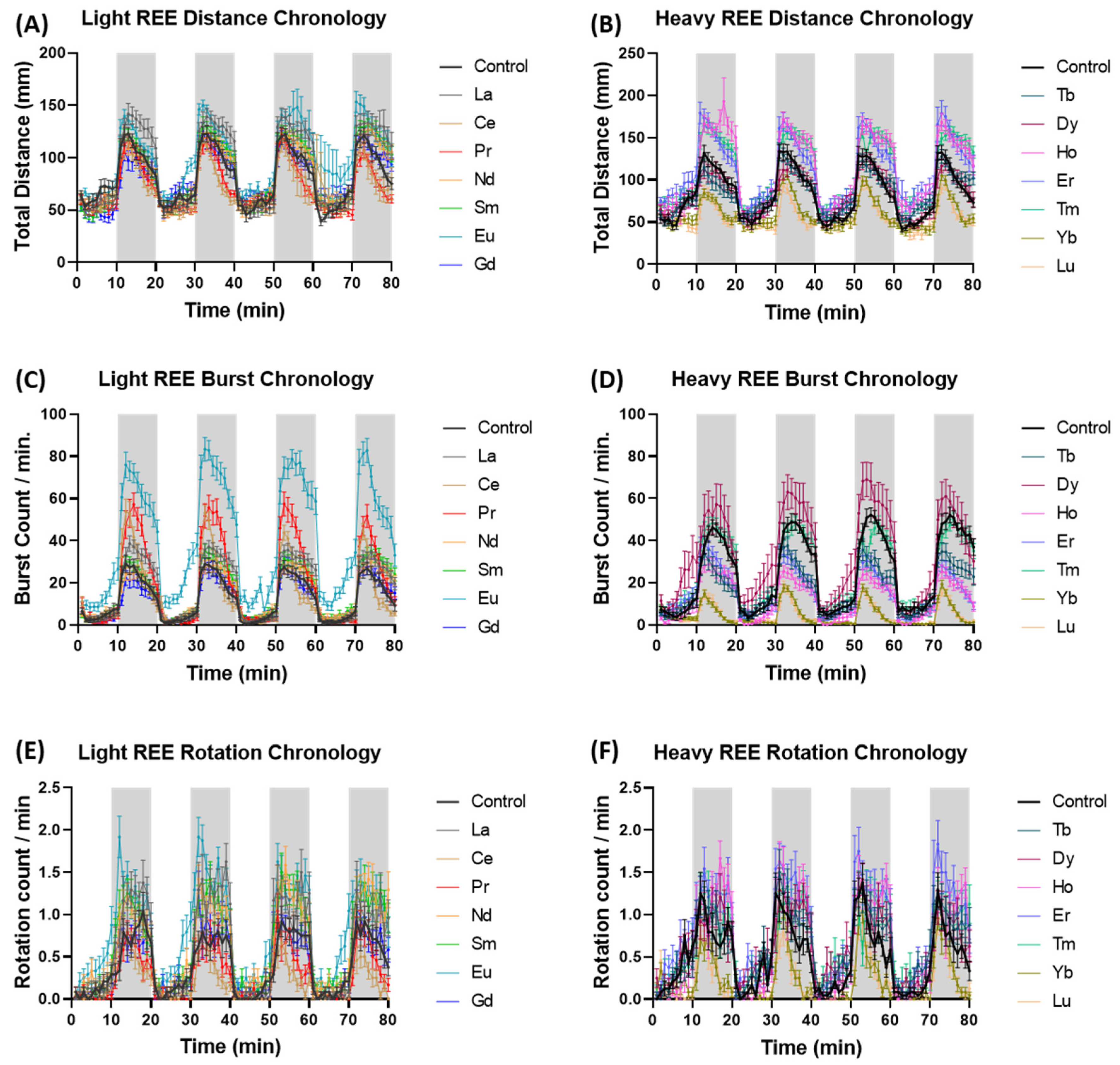
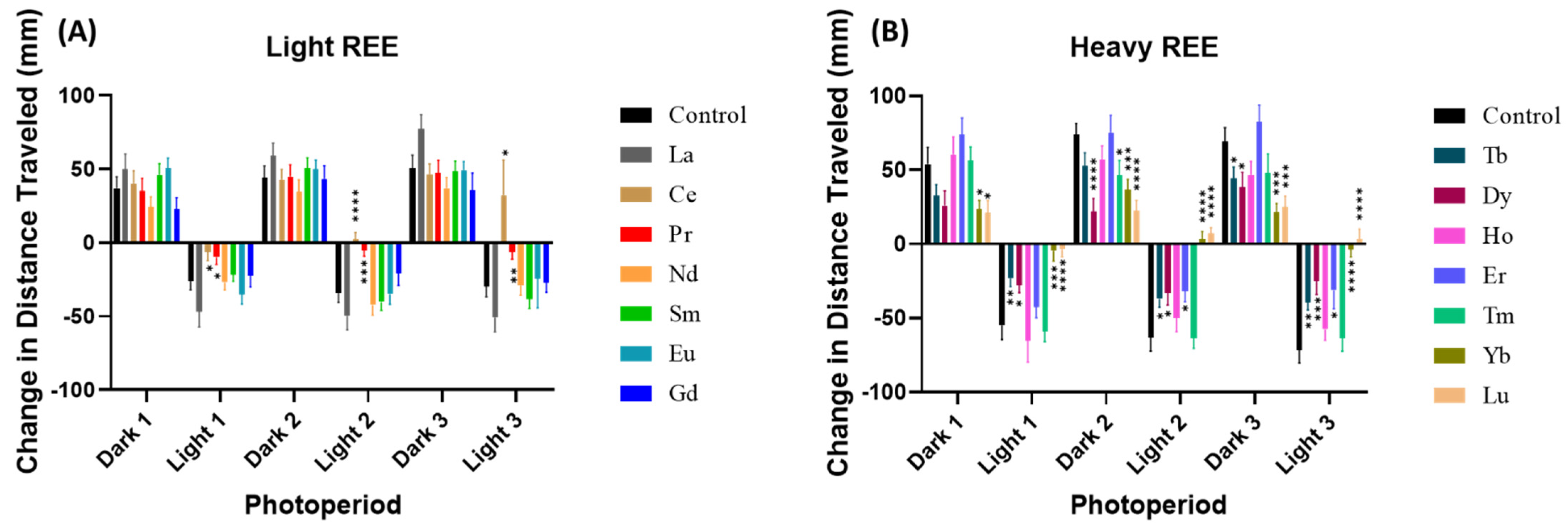
2.4. Zebrafish Locomotor Assay and Photo Motor Response (PMR) Test
2.5. Electronic Structures of Lanthanide Complexes
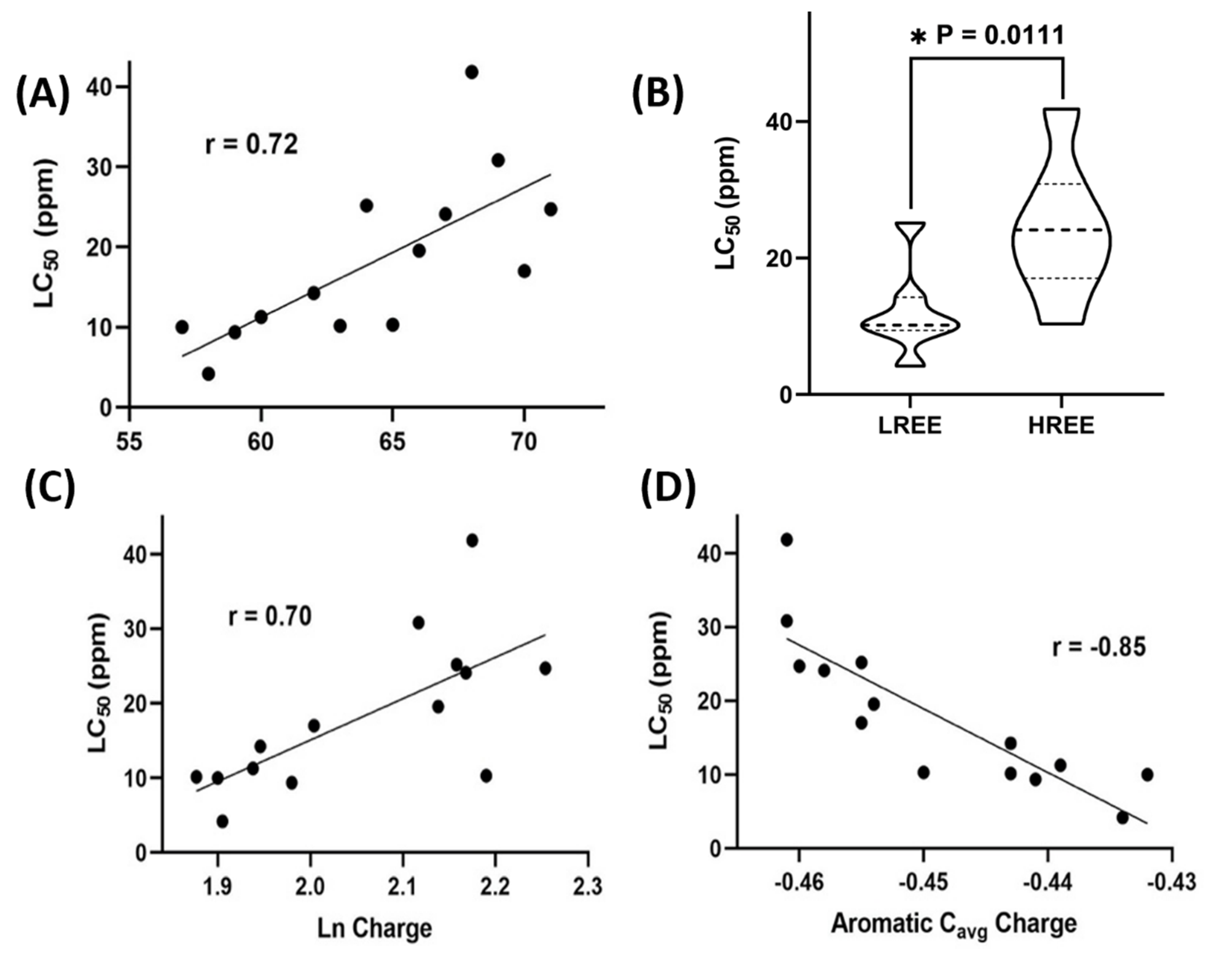
2.6. Statistics: Lanthanides’ Electronic Structures Correlates with REEs’ Toxicity
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakhmouradian, A.R.; Wall, F. Rare earth elements: Minerals, mines, magnets (and more). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Voncken, J.H.L. The Rare Earth Elements: An Introduction; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Castor, S.B. Rare earth elements. In Industrial Minerals & Rocks; Society for Mining, Metallurgy, and Exlporation, Inc. (SME): Colorado, CO, USA, 2006; pp. 769–792. [Google Scholar]
- Paul, J.; Campbell, G. Investigating Rare Earth Element Mine Development in EPA Region 8 and Potential Environmental Impacts; A National Service Center for Environmental Publications (NSCEP): Washington, DC, USA, 2011; 35p. [Google Scholar]
- Bresolin, B.M. Recovery of REEs from Exhausted Fluorescent Lamps. Master’s Thesis, University of Padua, Padua, Italy, 11 March 2015. [Google Scholar]
- Saravanan, R.; Rani, M.P. Metal and Alloy Bonding—An Experimental Analysis: Charge Density in Metals and Alloys; Springer Science & Business Media: London, UK, 2011. [Google Scholar]
- Jha, A.R. Rare Earth Materials: Properties and Applications; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Pushies, F.J. Special Ops-America’s Elite Forces in 21st Century Combat; Zenith Imprint: Minneapolis, MN, USA, 2003. [Google Scholar]
- Jastrow, R.; Brown, L. How to Make Nuclear Weapons Obsolete. Phys. Today 1985, 38, 75–78. [Google Scholar] [CrossRef]
- Malhotra, N.; Hsu, H.-S.; Liang, S.-T.; Roldan, M.J.M.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. An updated review of toxicity effect of the rare earth elements (REEs) on aquatic organisms. Animals 2020, 10, 1663. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.O.; Baccarelli, A. Metals and neurotoxicology. J. Nutr. 2007, 137, 2809–2813. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Orem, W.; Castranova, V.; Tatu, C.A.; Belkin, H.E.; Zheng, B.; Lerch, H.E.; Maharaj, S.V.; Bates, A.L. Health impacts of coal and coal use: Possible solutions. Int. J. Coal Geol. 2002, 50, 425–443. [Google Scholar] [CrossRef]
- Burton Jr, G.A. Assessing the toxicity of freshwater sediments. Environ. Toxicol. Chem. Int. J. 1991, 10, 1585–1627. [Google Scholar] [CrossRef]
- Yokel, R.A.; Lasley, S.M.; Dorman, D.C. The speciation of metals in mammals influences their toxicokinetics and toxicodynamics and therefore human health risk assessment. J. Toxicol. Environ. Health Part B 2006, 9, 63–85. [Google Scholar] [CrossRef]
- Schinzel, S.; Bindl, M.; Visseaux, M.; Chermette, H. Structural and electronic analysis of lanthanide complexes: Reactivity may not necessarily be independent of the identity of the lanthanide atom—A DFT study. J. Phys. Chem. A 2006, 110, 11324–11331. [Google Scholar] [CrossRef][Green Version]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997. [Google Scholar]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Dolg, M.; Stoll, H. Electronic structure calculations for molecules containing lanthanide atoms. Handb. Phys. Chem. Rare Earths 1996, 22, 607–729. [Google Scholar]
- Platas-Iglesias, C.; Roca-Sabio, A.; Regueiro-Figueroa, M.; Esteban-Gómez, D.; de Blas, A.; Rodríguez-Blas, T. Applications of density functional theory (DFT) to investigate the structural, spectroscopic and magnetic properties of lanthanide (III) complexes. Curr. Inorg. Chem. 2011, 1, 91–116. [Google Scholar] [CrossRef]
- Cotton, S.A. Establishing coordination numbers for the lanthanides in simple complexes. Comptes Rendus Chim. 2005, 8, 129–145. [Google Scholar] [CrossRef]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- van der Ven, L.T.; Van den Brandhof, E.-J.; Vos, J.H.; Power, D.M.; Wester, P.W. Effects of the antithyroid agent propylthiouracil in a partial life cycle assay with zebrafish. Environ. Sci. Technol. 2006, 40, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Van Eeden, F.; Schach, U.; Trowe, T.; Brand, M.; Furutani-Seiki, M.; Haffter, P.; Hammerschmidt, M.; Heisenberg, C.-P.; Jiang, Y.-J. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 1996, 123, 399–413. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Making waves: New developments in toxicology with the zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef]
- Bailey, J.; Oliveri, A.; Levin, E.D. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 14–23. [Google Scholar] [CrossRef]
- Hussain, A.; Audira, G.; Siregar, P.; Lin, Y.-C.; Villalobos, O.; Villaflores, O.; Wang, W.-D.; Hsiao, C.-D. Waterborne exposure of paclobutrazol at environmental relevant concentration induce locomotion hyperactivity in larvae and anxiolytic exploratory behavior in adult zebrafish. Int. J. Environ. Res. Public Health 2020, 17, 4632. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. JoVE 2012, 69, e4196. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, W.; Zhang, L.; He, X.; Ma, Y.; Liu, Y.; Chai, Z. Effects of rare earth elements La and Yb on the morphological and functional development of zebrafish embryos. J. Environ. Sci. 2012, 24, 209–213. [Google Scholar]
- Dubé, M.; Auclair, J.; Hanana, H.; Turcotte, P.; Gagnon, C.; Gagné, F. Gene expression changes and toxicity of selected rare earth elements in rainbow trout juveniles. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 223, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hanana, H.; Taranu, Z.E.; Turcotte, P.; Gagnon, C.; Kowalczyk, J.; Gagné, F. Evaluation of general stress, detoxification pathways, and genotoxicity in rainbow trout exposed to rare earth elements dysprosium and lutetium. Ecotoxicol. Environ. Saf. 2021, 208, 111588. [Google Scholar] [CrossRef]
- Sojka, M.; Siepak, M.; Pietrewicz, K. Concentration of Rare Earth Elements in surface water and bottom sediments in Lake Wadag, Poland. J. Elem. 2019, 24, 125–140. [Google Scholar]
- Blaise, C.; Gagné, F.; Harwood, M.; Quinn, B.; Hanana, H. Ecotoxicity responses of the freshwater cnidarian Hydra attenuata to 11 rare earth elements. Ecotoxicol. Environ. Saf. 2018, 163, 486–491. [Google Scholar] [CrossRef]
- Steele, W.B.; Mole, R.A.; Brooks, B.W. Experimental protocol for examining behavioral response profiles in larval fish: Application to the neuro-stimulant caffeine. JoVE (J. Vis. Exp.) 2018, 137, e57938. [Google Scholar] [CrossRef]
- Suryanto, M.E.; Audira, G.; Uapipatanakul, B.; Hussain, A.; Saputra, F.; Siregar, P.; Chen, K.H.-C.; Hsiao, C.-D. Antidepressant screening demonstrated non-monotonic responses to amitriptyline, amoxapine and sertraline in locomotor activity assay in larval zebrafish. Cells 2021, 10, 738. [Google Scholar] [CrossRef]
- Motamarri, P.; Nowak, M.R.; Leiter, K.; Knap, J.; Gavini, V. Higher-order adaptive finite-element methods for Kohn–Sham density functional theory. J. Comput. Phys. 2013, 253, 308–343. [Google Scholar] [CrossRef]
- Jin, S.; Huang, Y. A review on ecological toxicity of rare earth elements in soil. Asian J. Ecotoxicol. 2014, 9, 213–223. [Google Scholar]
- Borgmann, U.; Couillard, Y.; Doyle, P.; Dixon, D.G. Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness. Environ. Toxicol. Chem. Int. J. 2005, 24, 641–652. [Google Scholar] [CrossRef]
- Ortmann, J.; Altenburger, R.; Scholz, S.; Luckenbach, T. Photomotor response data analysis approach to assess chemical neurotoxicity with the zebrafish embryo. ALTEX-Altern. Anim. Exp. 2022, 39, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Lammer, E.; Kamp, H.; Hisgen, V.; Koch, M.; Reinhard, D.; Salinas, E.; Wendler, K.; Zok, S.; Braunbeck, T. Development of a flow-through system for the fish embryo toxicity test (FET) with the zebrafish (Danio rerio). Toxicol. Vitr. 2009, 23, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Z.; Chen, Z.; Ou, X.; Chen, J. Calculation of toxicity coefficient of potential ecological risk assessment of rare earth elements. Bull. Environ. Contam. Toxicol. 2020, 104, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Liu, Y. Metal pollution of potential hazards in water: Sedimentology method evaluation. Environ. Sci. Technol. 1989, 9, 16–25. [Google Scholar]
- Gonzalez, V.; Vignati, D.A.; Leyval, C.; Giamberini, L. Environmental fate and ecotoxicity of lanthanides: Are they a uniform group beyond chemistry? Environ. Int. 2014, 71, 148–157. [Google Scholar] [CrossRef]
- Alsop, D.; Wood, C.M. Metal uptake and acute toxicity in zebrafish: Common mechanisms across multiple metals. Aquat. Toxicol. 2011, 105, 385–393. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581. [Google Scholar] [CrossRef]
- Pearson, R.G.; Songstad, J. Application of the principle of hard and soft acids and bases to organic chemistry. J. Am. Chem. Soc. 1967, 89, 1827–1836. [Google Scholar] [CrossRef]
- Toraishi, T.; Farkas, I.; Szabó, Z.; Grenthe, I. Complexation of Th (iv) and various lanthanides (iii) by glycolic acid; potentiometric, 13 C-NMR and EXAFS studies. J. Chem. Soc. Dalton Trans. 2002, 24, 3805–3812. [Google Scholar] [CrossRef]
- Wells, W.H.; Wells, V.L. The lanthanides, rare earth elements. Patty’s Toxicol. 2001, 20, 817–840. [Google Scholar]
- Barron, M.G. Bioaccumulation and bioconcentration in aquatic organisms. Handb. Ecotoxicol. 1995, 2, 652–662. [Google Scholar]
- MacMillan, G.A.; Clayden, M.G.; Chételat, J.; Richardson, M.C.; Ponton, D.E.; Perron, T.; Amyot, M. Environmental drivers of rare earth element bioaccumulation in freshwater zooplankton. Environ. Sci. Technol. 2018, 53, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Cardon, P.-Y.; Roques, O.; Caron, A.; Rosabal, M.; Fortin, C.; Amyot, M. Role of prey subcellular distribution on the bioaccumulation of yttrium (Y) in the rainbow trout. Environ. Pollut. 2020, 258, 113804. [Google Scholar] [CrossRef] [PubMed]
- Franke, C.; Studinger, G.; Berger, G.; Böhling, S.; Bruckmann, U.; Cohors-Fresenborg, D.; Jöhncke, U. The assessment of bioaccumulation. Chemosphere 1994, 29, 1501–1514. [Google Scholar] [CrossRef]
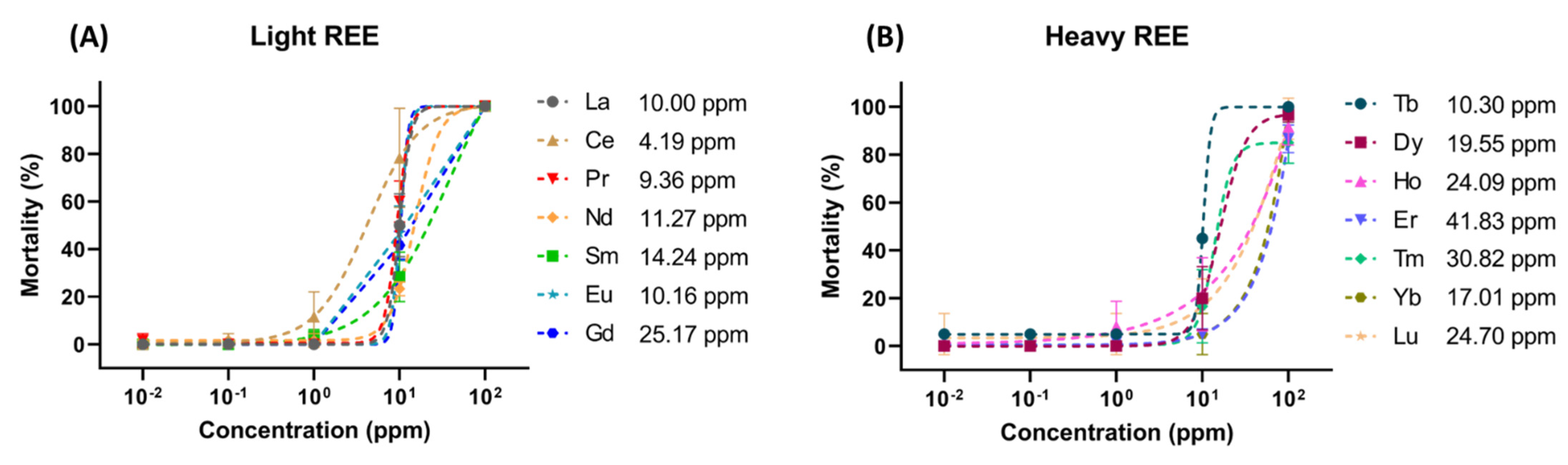

| REEs | LC50 (ppm) | Atomic Number | Valence Electron | HREE or LREE | Ln Milliken Charge | Aromatic Cavg Charge |
|---|---|---|---|---|---|---|
| La | 10 | 57 | 5d16s2 | LREE | 1.900 | −0.432 |
| Ce | 4.187 | 58 | 4f15d16s2 | LREE | 1.905 | −0.434 |
| Pr | 9.36 | 59 | 4f36s2 | LREE | 1.980 | −0.441 |
| Nd | 11.27 | 60 | 4f46s2 | LREE | 1.938 | −0.439 |
| Sm | 14.24 | 62 | 4f66s2 | LREE | 1.946 | −0.443 |
| Eu | 10.16 | 63 | 4f76s2 | LREE | 1.877 | −0.443 |
| Gd | 25.17 | 64 | 4f75d16s2 | LREE | 2.158 | −0.455 |
| Tb | 10.3 | 65 | 4f96s2 | HREE | 2.190 | −0.45 |
| Dy | 19.55 | 66 | 4f106s2 | HREE | 2.138 | −0.454 |
| Ho | 24.09 | 67 | 4f116s2 | HREE | 2.168 | −0.458 |
| Er | 41.83 | 68 | 4f126s2 | HREE | 2.175 | −0.461 |
| Tm | 30.82 | 69 | 4f136s2 | HREE | 2.117 | −0.461 |
| Yb | 17.01 | 70 | 4f146s2 | HREE | 2.004 | −0.455 |
| Lu | 24.7 | 71 | 4f145d16s2 | HREE | 2.254 | −0.460 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Liu, R.-X.; Audira, G.; Suryanto, M.E.; Roldan, M.J.M.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. Lanthanides Toxicity in Zebrafish Embryos Are Correlated to Their Atomic Number. Toxics 2022, 10, 336. https://doi.org/10.3390/toxics10060336
Lin Y-T, Liu R-X, Audira G, Suryanto ME, Roldan MJM, Lee J-S, Ger T-R, Hsiao C-D. Lanthanides Toxicity in Zebrafish Embryos Are Correlated to Their Atomic Number. Toxics. 2022; 10(6):336. https://doi.org/10.3390/toxics10060336
Chicago/Turabian StyleLin, Ying-Ting, Rong-Xuan Liu, Gilbert Audira, Michael Edbert Suryanto, Marri Jmelou M. Roldan, Jiann-Shing Lee, Tzong-Rong Ger, and Chung-Der Hsiao. 2022. "Lanthanides Toxicity in Zebrafish Embryos Are Correlated to Their Atomic Number" Toxics 10, no. 6: 336. https://doi.org/10.3390/toxics10060336
APA StyleLin, Y.-T., Liu, R.-X., Audira, G., Suryanto, M. E., Roldan, M. J. M., Lee, J.-S., Ger, T.-R., & Hsiao, C.-D. (2022). Lanthanides Toxicity in Zebrafish Embryos Are Correlated to Their Atomic Number. Toxics, 10(6), 336. https://doi.org/10.3390/toxics10060336









