Impact of Particles on Pulmonary Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
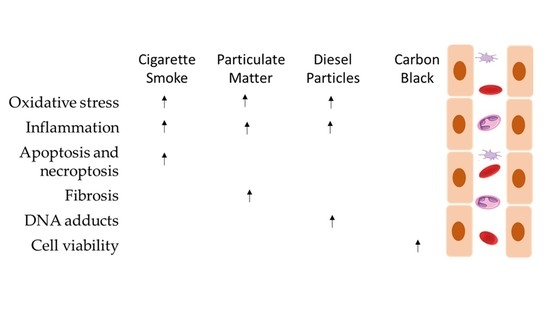
3. Results
4. Discussion
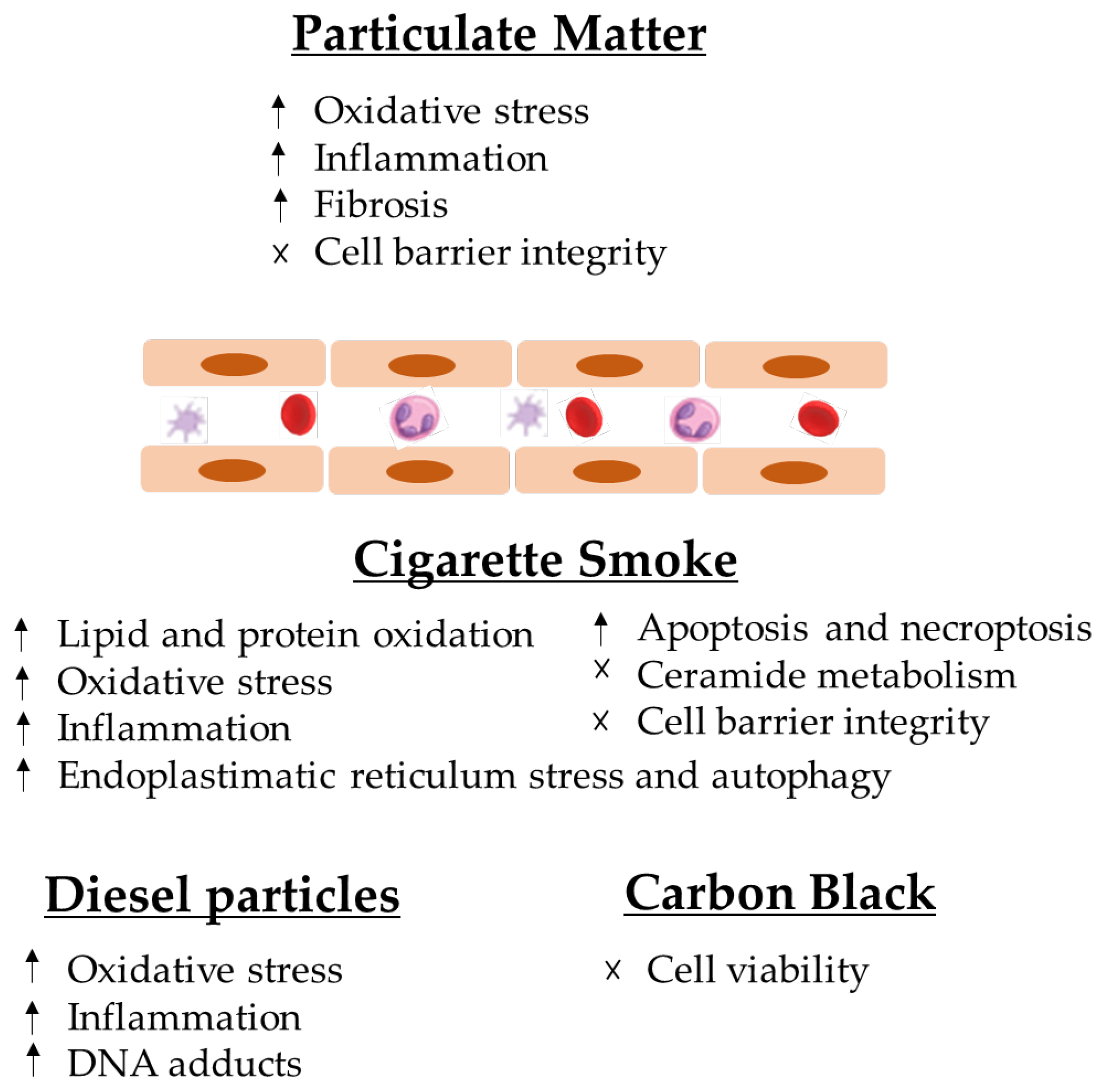
4.1. Particulate Matter
4.1.1. Cigarette Smoke
4.1.2. Diesel Particles
4.1.3. Carbon Black
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nwanaji-Enwerem, J.C.; Bind, M.-A.; Dai, L.; Oulhote, Y.; Colicino, E.; Di, Q.; Just, A.C.; Hou, L.; Vokonas, P.; Coull, B.A.; et al. Editor’s Highlight: Modifying Role of Endothelial Function Gene Variants on the Association of Long-Term PM2.5 Exposure with Blood DNA Methylation Age: The VA Normative Aging Study. Toxicol. Sci. 2017, 158, 116–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, S.; Pusadkar, V.; McDonald, J.; Mirpuri, J.; Azad, R.K.; Goven, A.; Lund, A.K. Traffic Generated Emissions Alter the Lung Microbiota by Promoting the Expansion of Proteobacteria in C57Bl/6 Mice Placed on a High-Fat Diet. Ecotoxicol. Environ. Saf. 2021, 213, 112035. [Google Scholar] [CrossRef] [PubMed]
- Marques-Ramos, A.; Candeias, M.M.; Menezes, J.; Lacerda, R.; Willcocks, M.; Teixeira, A.; Locker, N.; Romao, L. Cap-Independent Translation Ensures MTOR Expression and Function upon Protein Synthesis Inhibition. RNA 2017, 23, 1712–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlemann, M.; Zeidler, R.; Lang, S.; Mack, B.; Münz, M.; Gires, O. Carcinoma-Associated EIF3i Overexpression Facilitates MTOR-Dependent Growth Transformation. Mol. Carcinog. 2006, 45, 957–967. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Y.; Gao, Y.; Li, C.; Zhou, L.; Qi, W.; Zhang, Y.; Ye, L. Effects of Different Components of PM2.5 on the Expression Levels of NF-ΚB Family Gene MRNA and Inflammatory Molecules in Human Macrophage. Int. J. Environ. Res. Public Health 2019, 16, 1408. [Google Scholar] [CrossRef] [Green Version]
- Alain, T.; Morita, M.; Fonseca, B.D.; Yanagiya, A.; Siddiqui, N.; Bhat, M.; Zammit, D.; Marcus, V.; Metrakos, P.; Voyer, L.-A.; et al. EIF4E/4E-BP Ratio Predicts the Efficacy of MTOR Targeted Therapies. Cancer Res. 2012, 72, 6468–6476. [Google Scholar] [CrossRef] [Green Version]
- Appenzeller-Herzog, C.; Hall, M.N. Bidirectional Crosstalk between Endoplasmic Reticulum Stress and MTOR Signaling. Trends Cell Biol. 2012, 22, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.G.; Cambier, S.; Hennen, J.; Legay, S.; Serchi, T.; Nelissen, I.; Chary, A.; Moschini, E.; Krein, A.; Blömeke, B.; et al. Endothelial Responses of the Alveolar Barrier in Vitro in a Dose-Controlled Exposure to Diesel Exhaust Particulate Matter. Part. Fibre Toxicol. 2017, 14, 7. [Google Scholar] [CrossRef] [Green Version]
- Karki, P.; Meliton, A.; Sitikov, A.; Tian, Y.; Ohmura, T.; Birukova, A.A. Microtubule Destabilization Caused by Particulate Matter Contributes to Lung Endothelial Barrier Dysfunction and Inflammation. Cell. Signal. 2019, 53, 246–255. [Google Scholar] [CrossRef]
- Spira, A.; Beane, J.; Shah, V.; Liu, G.; Schembri, F.; Yang, X.; Palma, J.; Brody, J.S. Effects of Cigarette Smoke on the Human Airway Epithelial Cell Transcriptome. Proc. Natl. Acad. Sci. USA 2004, 101, 10143–10148. [Google Scholar] [CrossRef] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 1,3-Butadiene, Ethylene Oxide and Vinyl Halides (Vinyl Fluoride, Vinyl Chloride and Vinyl Bromide). IARC Monogr. Eval. Carcinog. Risks Hum. 2008, 97, 3–471. [Google Scholar]
- Das, S.K. Harmful Health Effects of Cigarette Smoking. Mol. Cell. Biochem. 2003, 253, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.O.; Davids, L.M.; Marnewick, J.L. Diesel Exhaust Particles and Endothelial Cells Dysfunction: An Update. Toxicol. In Vitro 2016, 32, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Alayev, A.; Holz, M.K. MTOR Signaling for Biological Control and Cancer. J. Cell. Physiol. 2013, 228, 1658–1664. [Google Scholar] [CrossRef] [Green Version]
- Long, C.M.; Nascarella, M.A.; Valberg, P.A. Carbon Black vs. Black Carbon and Other Airborne Materials Containing Elemental Carbon: Physical and Chemical Distinctions. Environ. Pollut. 2013, 181, 271–286. [Google Scholar] [CrossRef] [Green Version]
- Casanova, A.; Gomis-Berenguer, A.; Canizares, A.; Simon, P.; Calzada, D.; Ania, C.O. Carbon Black as Conductive Additive and Structural Director of Porous Carbon Gels. Materials 2020, 13, 217. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, N.R.; Pojana, G.; White, P.; Møller, P.; Cohn, C.A.; Korsholm, K.S.; Vogel, U.; Marcomini, A.; Loft, S.; Wallin, H. Genotoxicity, Cytotoxicity, and Reactive Oxygen Species Induced by Single-walled Carbon Nanotubes and C60 Fullerenes in the FE1-MutaTM Mouse Lung Epithelial Cells. Environ. Mol. Mutagen. 2008, 49, 476–487. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiyoshi, K.; Oshio, S.; Takano, H.; Takeda, K.; Ichinose, T. Effects of Fetal Exposure to Carbon Nanoparticles on Reproductive Function in Male Offspring. Fertil. Steril. 2010, 93, 1695–1699. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [Green Version]
- Giebe, S.; Cockcroft, N.; Hewitt, K.; Brux, M.; Hofmann, A.; Morawietz, H.; Brunssen, C. Cigarette Smoke Extract Counteracts Atheroprotective Effects of High Laminar Flow on Endothelial Function. Redox Biol. 2017, 12, 776–786. [Google Scholar] [CrossRef]
- Vesterdal, L.K.; Folkmann, J.K.; Jacobsen, N.R.; Sheykhzade, M.; Wallin, H.; Loft, S.; Møller, P. Pulmonary Exposure to Carbon Black Nanoparticles and Vascular Effects. Part. Fibre Toxicol. 2010, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garland, C.J.; Hiley, C.R.; Dora, K.A. EDHF: Spreading the Influence of the Endothelium: EDHF and Arterial Dilatation. Br. J. Pharmacol. 2011, 164, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Malik, A.B. Signaling Mechanisms Regulating Endothelial Permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef] [PubMed]
- Tailor, A.; Cooper, D.; Granger, D.N. Platelet–Vessel Wall Interactions in the Microcirculation. Microcirculation 2005, 12, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.; Mrowietz, C.; Lendlein, A.; Jung, F. Interaction of Human Umbilical Vein Endothelial Cells (HUVEC) with Platelets in Vitro: Influence of Platelet Concentration and Reactivity. Clin. Hemorheol. Microcirc. 2013, 55, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial Cell Control of Thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [Green Version]
- Rounds, S.I.S.; Lu, Q.; Harrington, E.O.; Newton, J.; Casserly, B. Pulmonary Endothelial Cell Signaling and Function. Trans. Am. Clin. Climatol. Assoc. 2008, 119, 155–167; discussion 167-9. [Google Scholar]
- Michiels, C. Endothelial Cell Functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef]
- Gonzales, J.N.; Verin, A.D. Pulmonary Vascular Endothelial Cells. In Endothelial Dysfunction—Old Concepts and New Challenges; Lenasi, H., Ed.; InTech: Vienna, Austria, 2018; ISBN 978-1-78984-253-1. [Google Scholar]
- Millar, F.R.; Summers, C.; Griffiths, M.J.; Toshner, M.R.; Proudfoot, A.G. The Pulmonary Endothelium in Acute Respiratory Distress Syndrome: Insights and Therapeutic Opportunities. Thorax 2016, 71, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, L.; Zaidi, S.R.; Sammani, S.; Siegler, J.; Moreno-Vinasco, L.; Mathew, B.; Natarajan, V.; Garcia, J.G.N. Hydrogen Sulfide Attenuates Particulate Matter-Induced Human Lung Endothelial Barrier Disruption via Combined Reactive Oxygen Species Scavenging and Akt Activation. Am. J. Respir. Cell Mol. Biol. 2012, 47, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Karki, P.; Meliton, A.; Shah, A.; Tian, Y.; Ohmura, T.; Sarich, N.; Birukova, A.A.; Birukov, K.G. Role of Truncated Oxidized Phospholipids in Acute Endothelial Barrier Dysfunction Caused by Particulate Matter. PLoS ONE 2018, 13, e0206251. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, X.; Liu, X.; Zheng, J.; Chen, R.; Kan, H. Ambient Fine Particulate Matter Induce Toxicity in Lung Epithelial-Endothelial Co-Culture Models. Toxicol. Lett. 2019, 301, 133–145. [Google Scholar] [CrossRef]
- Cui, A.; Xiang, M.; Xu, M.; Lu, P.; Wang, S.; Zou, Y.; Qiao, K.; Jin, C.; Li, Y.; Lu, M.; et al. VCAM-1-Mediated Neutrophil Infiltration Exacerbates Ambient Fine Particle-Induced Lung Injury. Toxicol. Lett. 2019, 302, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Li, C.; Xu, J.; Ren, F.; Xu, X.; Liu, C.; Niu, B.; Li, F. LncRNA Gm16410 Regulates PM2.5-Induced Lung Endothelial-Mesenchymal Transition via the TGF-Β1/Smad3/p-Smad3 Pathway. Ecotoxicol. Environ. Saf. 2020, 205, 111327. [Google Scholar] [CrossRef]
- Tian, G.; Wang, J.; Lu, Z.; Wang, H.; Zhang, W.; Ding, W.; Zhang, F. Indirect Effect of PM1 on Endothelial Cells via Inducing the Release of Respiratory Inflammatory Cytokines. Toxicol. In Vitro 2019, 57, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chiang, E.T.; Moreno-Vinasco, L.; Lang, G.D.; Pendyala, S.; Samet, J.M.; Geyh, A.S.; Breysse, P.N.; Chillrud, S.N.; Natarajan, V.; et al. Particulate Matter Disrupts Human Lung Endothelial Barrier Integrity via ROS- and P38 MAPK-Dependent Pathways. Am. J. Respir. Cell Mol. Biol. 2010, 42, 442–449. [Google Scholar] [CrossRef]
- Mo, Y.; Wan, R.; Chien, S.; Tollerud, D.J.; Zhang, Q. Activation of Endothelial Cells after Exposure to Ambient Ultrafine Particles: The Role of NADPH Oxidase. Toxicol. Appl. Pharmacol. 2009, 236, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.; Mantecca, P.; Camatini, M.; Gualtieri, M. Effect of Nanoparticles and Environmental Particles on a Cocultures Model of the Air-Blood Barrier. BioMed Res. Int. 2013, 2013, 801214. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Tao, J.-Q.; Johncola, A.; Guo, W.; Caporale, A.; Langham, M.C.; Wehrli, F.W. Acute Exposure to E-Cigarettes Causes Inflammation and Pulmonary Endothelial Oxidative Stress in Nonsmoking, Healthy Young Subjects. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 317, L155–L166. [Google Scholar] [CrossRef]
- Mizumura, K.; Justice, M.J.; Schweitzer, K.S.; Krishnan, S.; Bronova, I.; Berdyshev, E.V.; Hubbard, W.C.; Pewzner-Jung, Y.; Futerman, A.H.; Choi, A.M.K.; et al. Sphingolipid Regulation of Lung Epithelial Cell Mitophagy and Necroptosis during Cigarette Smoke Exposure. FASEB J. 2018, 32, 1880–1890. [Google Scholar] [CrossRef] [Green Version]
- Sakhatskyy, P.; Miranda, G.A.G.; Newton, J.; Lee, C.G.; Choudhary, G.; Vang, A.; Rounds, S.; Lu, Q. Cigarette Smoke-Induced Lung Endothelial Apoptosis and Emphysema Are Associated with Impairment of FAK and EIF2α. Microvasc. Res. 2014, 94, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, K.S.; Hatoum, H.; Brown, M.B.; Gupta, M.; Justice, M.J.; Beteck, B.; Van Demark, M.; Gu, Y.; Presson, R.G.; Hubbard, W.C.; et al. Mechanisms of Lung Endothelial Barrier Disruption Induced by Cigarette Smoke: Role of Oxidative Stress and Ceramides. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 301, L836–L846. [Google Scholar] [CrossRef]
- Bengalli, R.; Zerboni, A.; Marchetti, S.; Longhin, E.; Priola, M.; Camatini, M.; Mantecca, P. In Vitro Pulmonary and Vascular Effects Induced by Different Diesel Exhaust Particles. Toxicol. Lett. 2019, 306, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.; Longhin, E.; Marchetti, S.; Proverbio, M.C.; Battaglia, C.; Camatini, M. The Role of IL-6 Released from Pulmonary Epithelial Cells in Diesel UFP-Induced Endothelial Activation. Environ. Pollut. 2017, 231, 1314–1321. [Google Scholar] [CrossRef]
- Dinmohammadi, H.; Pirdel, Z.; Salarilak, L.; Hoylaerts, M.; Nejatbakhsh, R.; Biglari, A.; Jacquemin, M.; Shahani, T. Pure Ultra-Fine Carbon Particles Do Not Exert pro-Coagulation and Inflammatory Effects on Microvascular Endothelial Cells. Environ. Sci. Pollut. Res. 2019, 26, 991–999. [Google Scholar] [CrossRef]
- Zuo, H.; Faiz, A.; van den Berge, M.; Mudiyanselage, S.N.H.R.; Borghuis, T.; Timens, W.; Nikolaev, V.O.; Burgess, J.K.; Schmidt, M. Cigarette Smoke Exposure Alters Phosphodiesterases in Human Structural Lung Cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L59–L64. [Google Scholar] [CrossRef]
- Loscalzo, J. The Identification of Nitric Oxide as Endothelium-Derived Relaxing Factor. Circ. Res. 2013, 113, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Arnold, W.P.; Mittal, C.K.; Katsuki, S.; Murad, F. Nitric Oxide Activates Guanylate Cyclase and Increases Guanosine 3′:5′-Cyclic Monophosphate Levels in Various Tissue Preparations. Proc. Natl. Acad. Sci. USA 1977, 74, 3203–3207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizumura, K.; Cloonan, S.M.; Nakahira, K.; Bhashyam, A.R.; Cervo, M.; Kitada, T.; Glass, K.; Owen, C.A.; Mahmood, A.; Washko, G.R.; et al. Mitophagy-Dependent Necroptosis Contributes to the Pathogenesis of COPD. J. Clin. Investig. 2014, 124, 3987–4003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrache, I.; Natarajan, V.; Zhen, L.; Medler, T.R.; Richter, A.T.; Cho, C.; Hubbard, W.C.; Berdyshev, E.V.; Tuder, R.M. Ceramide Upregulation Causes Pulmonary Cell Apoptosis and Emphysema-like Disease in Mice. Nat. Med. 2005, 11, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linkermann, A.; Green, D.R. Necroptosis. N. Engl. J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, S.; Erfinanda, L.; Bartz, C.; Kuebler, W.M. Novel Mechanisms Regulating Endothelial Barrier Function in the Pulmonary Microcirculation. J. Physiol. 2019, 597, 997–1021. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A. Functions of Ceramide in Coordinating Cellular Responses to Stress. Science 1996, 274, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ren, X.; Liu, J.; Guo, X.; Jiang, X.; Zhang, D.; Huang, Y.; Zhang, J. HSP27 Phosphorylation Protects against Endothelial Barrier Dysfunction under Burn Serum Challenge. Biochem. Biophys. Res. Commun. 2015, 463, 377–383. [Google Scholar] [CrossRef]
- Hiebl, B.; Peters, S.; Gemeinhardt, O.; Niehues, S.M.; Jung, F. Impact of Serum in Cell Culture Media on in Vitro Lactate Dehydrogenase (LDH) Release Determination. J. Cell. Biotechnol. 2017, 3, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Castejon, G.; Brough, D. Understanding the Mechanism of IL-1β Secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- van den Brule, S.; Rappe, M.; Ambroise, J.; Bouzin, C.; Dessy, C.; Paquot, A.; Muccioli, G.G.; Lison, D. Diesel Exhaust Particles Alter the Profile and Function of the Gut Microbiota upon Subchronic Oral Administration in Mice. Part. Fibre Toxicol. 2021, 18, 7. [Google Scholar] [CrossRef]
- Bai, H.; Wu, M.; Zhang, H.; Tang, G. Chronic Polycyclic Aromatic Hydrocarbon Exposure Causes DNA Damage and Genomic Instability in Lung Epithelial Cells. Oncotarget 2017, 8, 79034–79045. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Mangal, D.; Tacka, K.A.; Quinn, A.M.; Harvey, R.G.; Blair, I.A.; Penning, T.M. Evidence for the Aldo-Keto Reductase Pathway of Polycyclic Aromatic Trans-Dihydrodiol Activation in Human Lung A549 Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6846–6851. [Google Scholar] [CrossRef] [Green Version]
- Nebert, D.W.; Dalton, T.P. The Role of Cytochrome P450 Enzymes in Endogenous Signalling Pathways and Environmental Carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960. [Google Scholar] [CrossRef]
- Shimada, T.; Fujii-Kuriyama, Y. Metabolic Activation of Polycyclic Aromatic Hydrocarbons to Carcinogens by Cytochromes P450 1A1 And1B1. Cancer Sci. 2004, 95, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Moake, J.L. Factor VIII is Synthesized in Human Endothelial Cells, Packaged in Weibel-Palade Bodies and Secreted Bound to ULVWF Strings. PLoS ONE 2015, 10, e0140740. [Google Scholar] [CrossRef] [PubMed]
- Jośko, J.; Gwóźdź, B.; Jedrzejowska-Szypułka, H.; Hendryk, S. Vascular Endothelial Growth Factor (VEGF) and Its Effect on Angiogenesis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2000, 6, 1047–1052. [Google Scholar]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK Signalling Pathway and Tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchini, M.; Mannucci, P.M. Past, Present and Future of Hemophilia: A Narrative Review. Orphanet J. Rare Dis. 2012, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyvandi, F.; Garagiola, I.; Baronciani, L. Role of von Willebrand Factor in the Haemostasis. Blood Transfus. 2011, 9, s3–s8. [Google Scholar] [CrossRef]
- Borsig, L. Selectins in Cancer Immunity. Glycobiology 2018, 28, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Théorêt, J.-F.; Yacoub, D.; Hachem, A.; Gillis, M.-A.; Merhi, Y. P-Selectin Ligation Induces Platelet Activation and Enhances Microaggregate and Thrombus Formation. Thromb. Res. 2011, 128, 243–250. [Google Scholar] [CrossRef]
- El Ayadi, A.; Herndon, D.N.; Finnerty, C.C. Biomarkers in Burn Patient Care. In Total Burn Care; Elsevier: Amsterdam, The Netherlands, 2018; pp. 232–235.e2. ISBN 978-0-323-47661-4. [Google Scholar]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Valentijn, K.M.; Sadler, J.E.; Valentijn, J.A.; Voorberg, J.; Eikenboom, J. Functional Architecture of Weibel-Palade Bodies. Blood 2011, 117, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Margraf, A.; Zarbock, A. Platelets in Inflammation and Resolution. J. Immunol. 2019, 203, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Packham, M.; Mustard, J. Platelet Adhesion. Prog. Hemost. Thromb. 1984, 7, 211–288. [Google Scholar] [PubMed]
- Barosova, H.; Karakocak, B.B.; Septiadi, D.; Petri-Fink, A.; Stone, V.; Rothen-Rutishauser, B. An In Vitro Lung System to Assess the Proinflammatory Hazard of Carbon Nanotube Aerosols. Int. J. Mol. Sci. 2022, 21, 5335. [Google Scholar] [CrossRef] [PubMed]
- Offer, S.; Hartner, E.; Di Bucchianico, S.; Bisig, C.; Bauer, S.; Pantzke, J.; Zimmermann, E.J.; Cao, X.; Binder, S.; Kuhn, E.; et al. Effect of Atmospheric Aging on Soot Particle Toxicity in Lung Cell Models at the Air–Liquid Interface: Differential Toxicological Impacts of Biogenic and Anthropogenic Secondary Organic Aerosols (SOAs). Environ. Health Perspect. 2022, 130, 027003. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.R.; Dawoud, H.; Libby, P.; Bhatt, D.L.; Malinski, T.; Mason, R.P. Eicosapentaenoic acid (EPA) reduces inflammation and improves nitric oxide bioavailability in pulmonary endothelial cells following exposure to air pollution particles. J. Am. Coll. Cardiol. 2022, 79, 1758. [Google Scholar] [CrossRef]
- Eckhardt, C.M.; Baccarelli, A.A.; Wu, H. Environmental Exposures and Extracellular Vesicles: Indicators of Systemic Effects and Human Disease. Curr. Environ. Health Rep. 2022. [Google Scholar] [CrossRef]
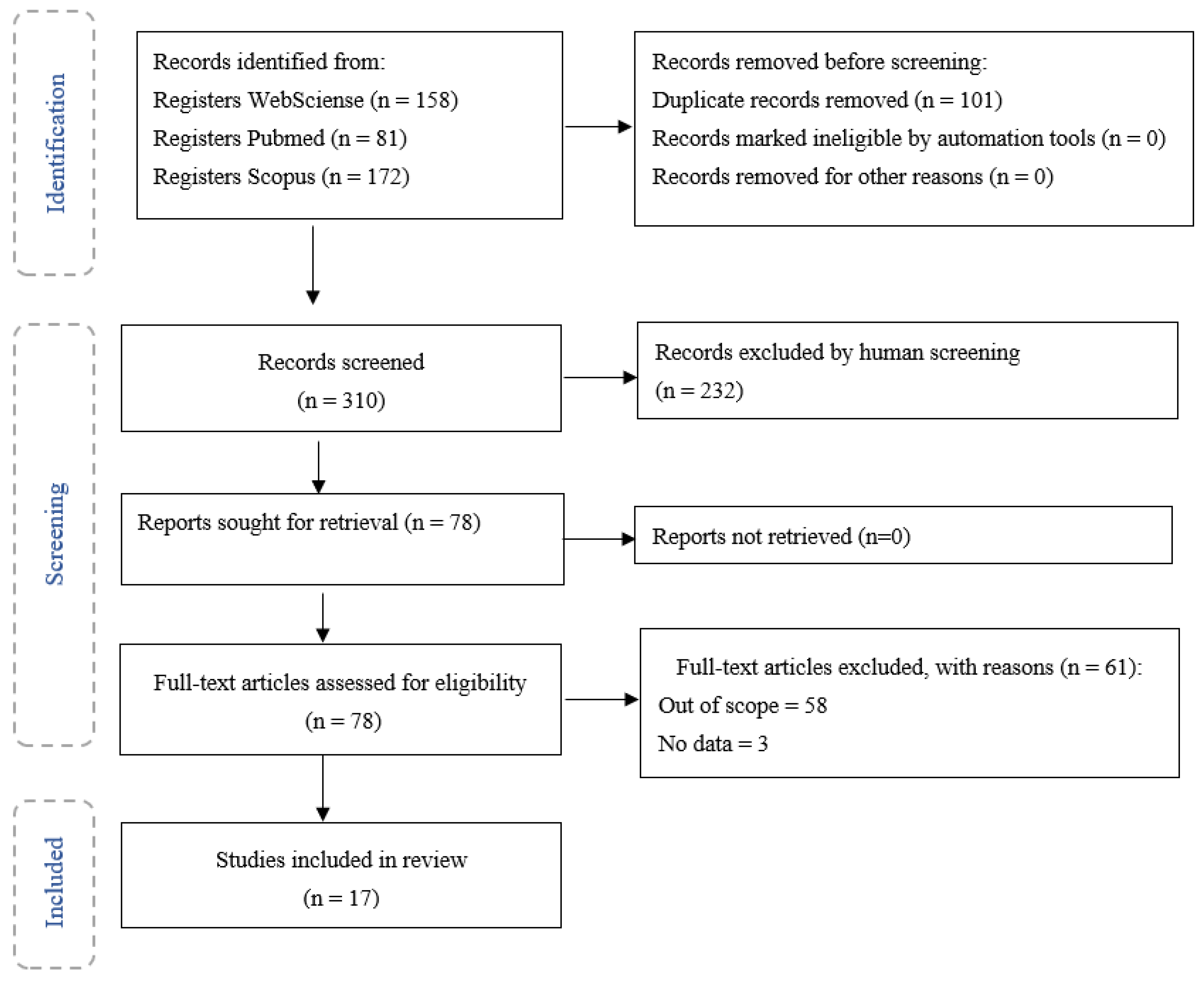
| Search Databases | Search Terms |
|---|---|
| Pubmed | “endothelial cells” [MeSH Terms] AND “lung” [MeSH Terms] AND (“particulate matter” [MeSH Terms] OR “particle*” [All Fields]) |
| Web of Science | “endothelial cells” AND “lung” AND (“particulate matter” OR “particle*”) |
| Scopus | “endothelial cells” AND “lung” AND (“particulate matter” OR “particle*”) |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Articles in English language | Articles in other languages |
| Articles published from 1 January 2000 to 6 March 2021 | Articles published prior to 2000 |
| Articles related to lung endothelial cells | Articles related to other cells |
| Articles related to particulate matter effects | Articles related to another organs |
| Articles on in vitro and/or in vivo studies | Articles related with other pollutants |
| Pollutant | Objective | Methodology | Results | Main Findings | |
|---|---|---|---|---|---|
| [31] | PM | The importance of the protective role of hydrogen sulphide (H2S) on PM-induced human lung EC barrier disruption and pulmonary inflammation. | (In vitro) HLMVECs. (In vivo) Mice. |
|
|
| [32] | PM | To investigate if the PM challenge triggers the production of bioactive Tr-OxPLs by pulmonary EC. | (In vitro) Human pulmonary endothelial cells. (In vivo) PM administered as suspension in saline to mice. |
|
|
| [33] | PM2.5 | The stabilisation of a suitable in vitro model to investigate PM2.5-mediated toxicity in vascular endothelial cells. | (In vitro) The Transwell culture method was used on A549 cells in apical chamber and EA.hy926 cells in the basolateral chamber to establish bi-culture, while tri-culture systems consisting of co-culture (A549 cells and THP-1-differentiated macrophages) in the apical chamber and also EA.hy926 cells in the basolateral chamber. |
|
|
| [34] | PM2.5 | To determine if polymorphonuclear leukocyte PMNs exacerbate PM2.5-induced lung in-jury and the role of vascular cell adhesion molecule 1 (VCAM-1) in this process. | (In vivo) The association between blood PMNs and ambient PM2.5 levels on the previous day was analysed. Neutropenia was achieved by injecting mice with PMN-specific antibodies. The inhibition of PMN infiltration was achieved by pre-treating PMNs with soluble VCAM-1. |
|
|
| [35] | PM2.5 | A simulation of the state of PM2.5 in a real environment and chronic exposure to PM2.5 in mice was used to construct a lung injury model to detect whether exposure to PM2.5 could trigger EndMT in the lungs. | (In vivo) Balb/c Mice. (In vitro) Pulmonary vascular endothelial cells (PVECs). The toxicity of PM2.5 investigated by determining its effect on the growth of MHC cells using the MTT assay. |
|
|
| [36] | PM1 | The examination of PM1-induced inflammatory response in the respiratory system and the indirect effect on endothelial cell function. | (In vitro) Transwell co-culture model of EA.hy926 cells with BEAS-2B cells and macrophages. |
|
|
| [37] | Fine/ultrafine PM | To analyse if PM, via enhanced oxidative stress, disrupts lung endothelial cells’ (LECs’) barrier integrity, thereby enhancing organ dysfunction. | (In vitro) Cultivated human pulmonary artery ECs on gold electrodes and assessed TER. The investigation of the involvement of ROS and mitogen-activated protein kinase (MAPK) signalling pathways. |
|
|
| [38] | Ultrafine particles | To test if exposure to UFPs leads to endothelial cell O2 U−generation via NADPH oxidase and results in activation of endothelial cells. | (In vivo) Mice. (In vitro) MPMVEC. |
|
|
| [39] | Nanoparticles/PM10 | To report the effects of metal oxide NPs (CuO and TiO2) and of PM on an in vitro model of the ABB constituted by the type II epithelial cell line (NCI-H441) and the endothelial one (HPMECST1.6R). | (In vitro) HPMECs. |
|
|
| [40] | E-cigarette smoke | The evaluation of the acute response to aerosol inhalation of e-cigarettes in terms of oxidative stress and indices of endothelial activation in human pulmonary microvascular endothelial cells (HPMVECs). | (In vivo) Healthy subjects were subjected to e-cig challenge. Serum was monitored for markers of inflammation (C-reactive protein (CRP) and soluble intercellular adhesion molecule (sICAM)) and nitric oxide metabolites (NOx). |
|
|
| [41] | Cigarette smoke | The investigation of the role of ceramide (Cer) in CS-induced lung cell mitophagy and cell death. | (In vitro) Cer and DHC treatments and aqueous CS extract (100%). (In vivo) DBA2/Jmice exposed for 5 h/d, 5 d/ wk, for 4 mo; PTEN-induced kinase 1 (Pink1)-/- mice exposed to room air or CS for 2 h/d, 5 d/wk, for 6 mo; CerS2-/- exposed to room air or CS for 2 h/d, 5 d/wk, for 1 mo. |
|
|
| [42] | Cigarette smoke | A comparation between Focal Adhesion Kinase (FAK) activation in the lungs of highly and mildly susceptible mice after exposure to CS for three weeks. | (In vitro) Rat lung microvascular endothelial cells (LMVECs). (In vivo) Male C57BL/6 and FAK mice exposed to room air or CS for 3 weeks at 6 h per day and 4 days per week. |
|
|
| [43] | Cigarette smoke | The investigation of the occurrence and mechanisms by which soluble components of mainstream CS disrupt the lung endothelial cell barrier function. | (In vitro) Cultured primary rat microvascular cell monolayers. |
|
|
| [44] | Diesel exhaust particles | The investigation of LECs’ primary response on bronchial cells BEAS-2B exposed to three different DEPs. | (In vitro) Microvascular lung HPMEC cells and BEAS-2B placed in aco-culture model and conditioned media. |
|
|
| [8] | Diesel exhaust particulate matter (DEPM) | Researching the response of ECs to DEPM, using a complex 3D tetraculture model of the alveolar barrier. | (In vitro) Aerosol exposure of DEPM; viability evaluated at 6, 24 and 48 h after exposure. |
|
|
| [45] | Diesel UFP | The investigation of the endothelial cells’ activation through epithelial-released mediators’ role. | (in vitro) HPMEC-ST1.6R (EC) treated with 40% of media (epithelial cells diluted in M199 medium), HPMEC-ST1.6R cells exposure lasted for 24 h. |
|
|
| [46] | Carbon black (CB) and multi-walled carbon nanotubes (MWCNTs) | The investigation of industrial CB and CNTs’ potential toxicity in endothelial cells by measuring Coagulation factor VIII (FVIII) and the Von-Willebrand factor (VWF). | (In vitro) MECs, 48 h after reaching confluence, were treated with culture medium of either MWCNTs or CB with a solution of 0.1, 1, 10, or 100 μg/mL in. |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-Silva, M.; Cardoso, J.; Alemão, C.; Santos, S.; Monteiro, A.; Manteigas, V.; Marques-Ramos, A. Impact of Particles on Pulmonary Endothelial Cells. Toxics 2022, 10, 312. https://doi.org/10.3390/toxics10060312
Almeida-Silva M, Cardoso J, Alemão C, Santos S, Monteiro A, Manteigas V, Marques-Ramos A. Impact of Particles on Pulmonary Endothelial Cells. Toxics. 2022; 10(6):312. https://doi.org/10.3390/toxics10060312
Chicago/Turabian StyleAlmeida-Silva, Marina, Jéssica Cardoso, Catarina Alemão, Sara Santos, Ana Monteiro, Vítor Manteigas, and Ana Marques-Ramos. 2022. "Impact of Particles on Pulmonary Endothelial Cells" Toxics 10, no. 6: 312. https://doi.org/10.3390/toxics10060312
APA StyleAlmeida-Silva, M., Cardoso, J., Alemão, C., Santos, S., Monteiro, A., Manteigas, V., & Marques-Ramos, A. (2022). Impact of Particles on Pulmonary Endothelial Cells. Toxics, 10(6), 312. https://doi.org/10.3390/toxics10060312