Evaluation of the Toxicity and Sublethal Effects of Acetamiprid and Dinotefuran on the Predator Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects and Plant Material
2.2. Insecticides
2.3. Toxicity Bioassay
2.4. Evaluation of Sublethal Effects of the Insecticides on Second Instar Larvae
2.5. Data Analysis
3. Results
3.1. Acetamiprid and Dinotefuran Toxicity on Second Instar Larvae of C. pallens
3.2. Sublethal Effects of Acetamiprid and Dinotefuran on C. pallens
3.2.1. Effects on the Developmental Period, Longevity, and Reproduction of C. pallens
3.2.2. Effect of Acetamiprid and Dinotefuran on the Population Growth Parameters of C. pallens
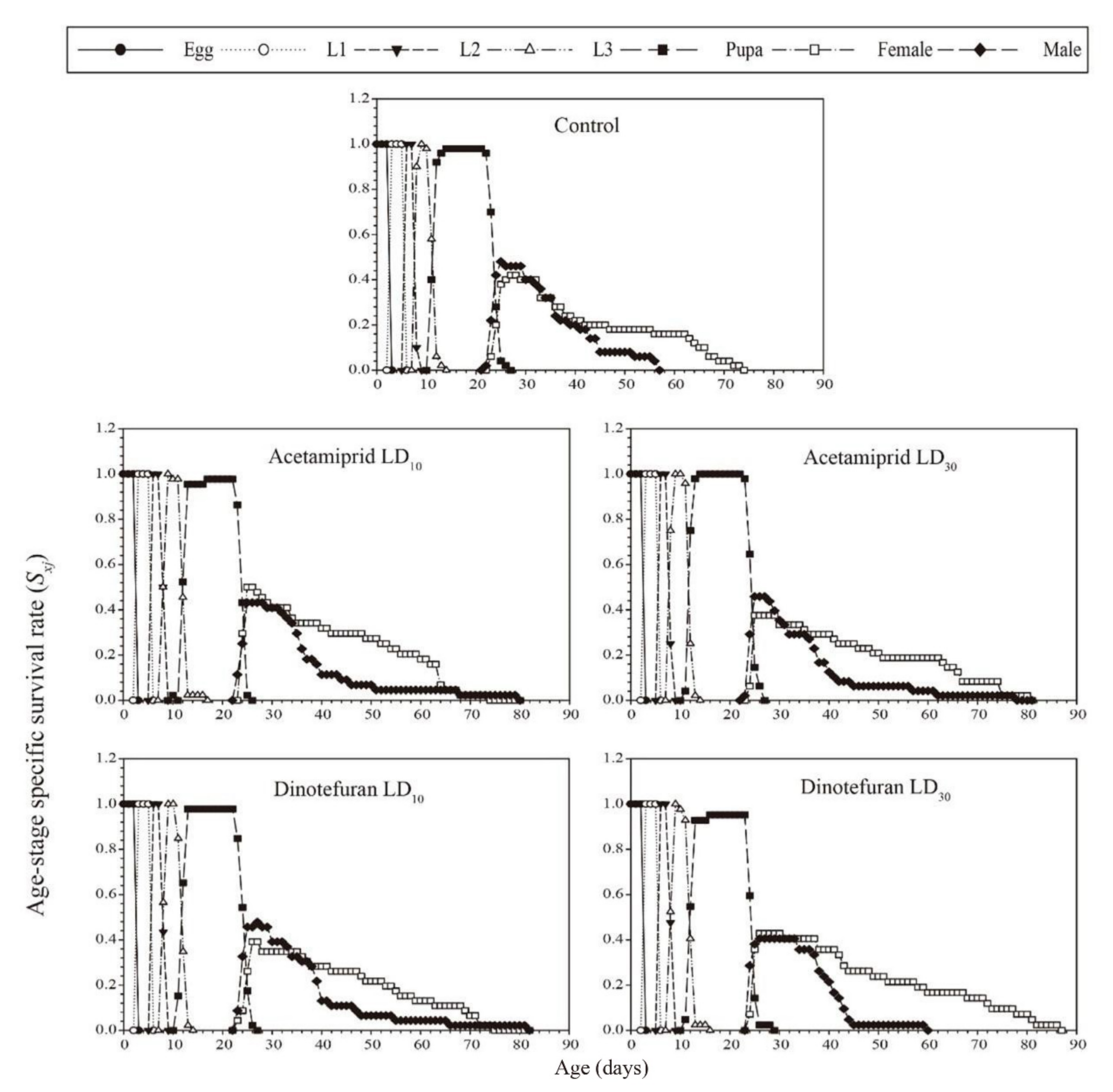
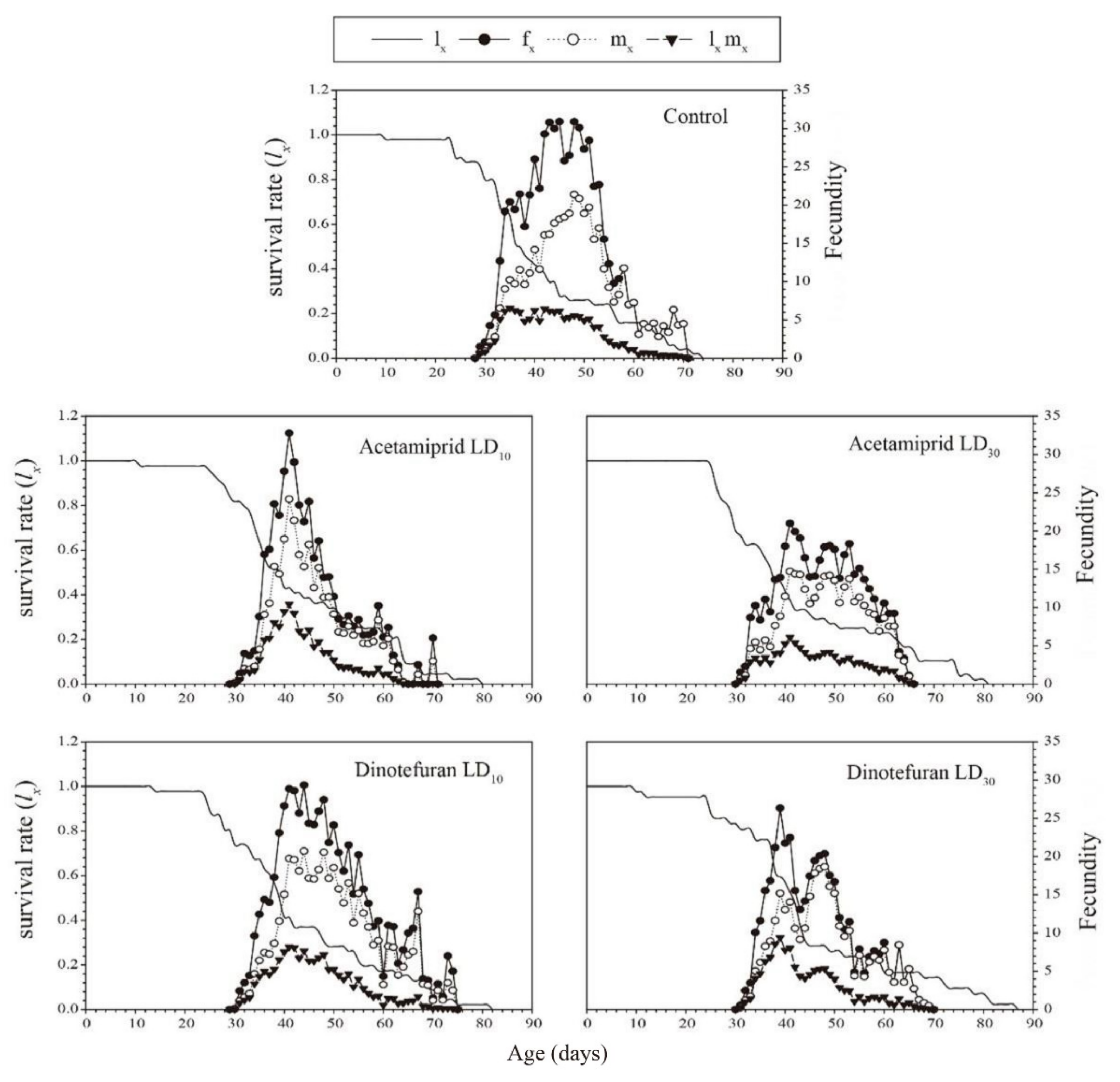
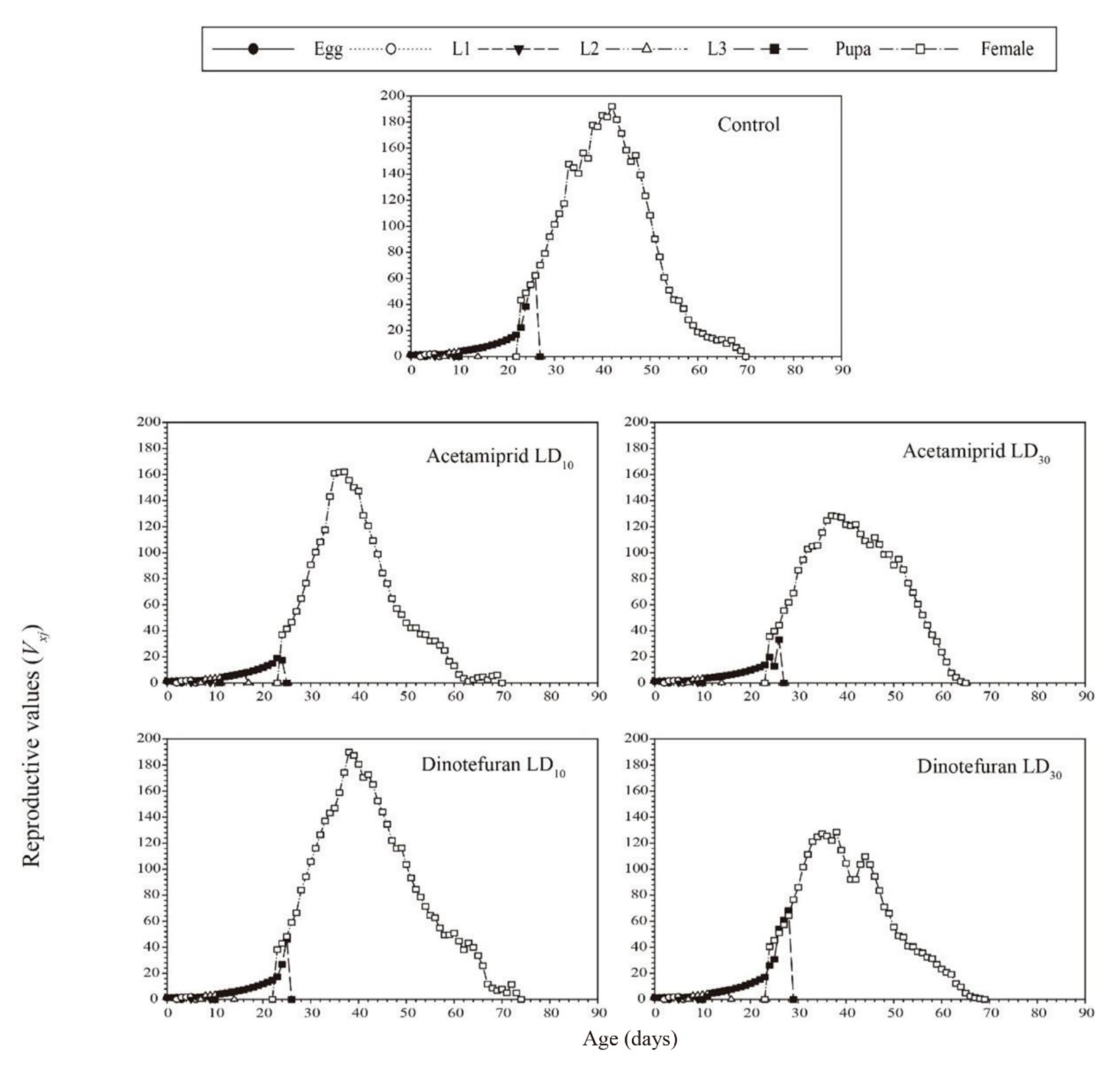
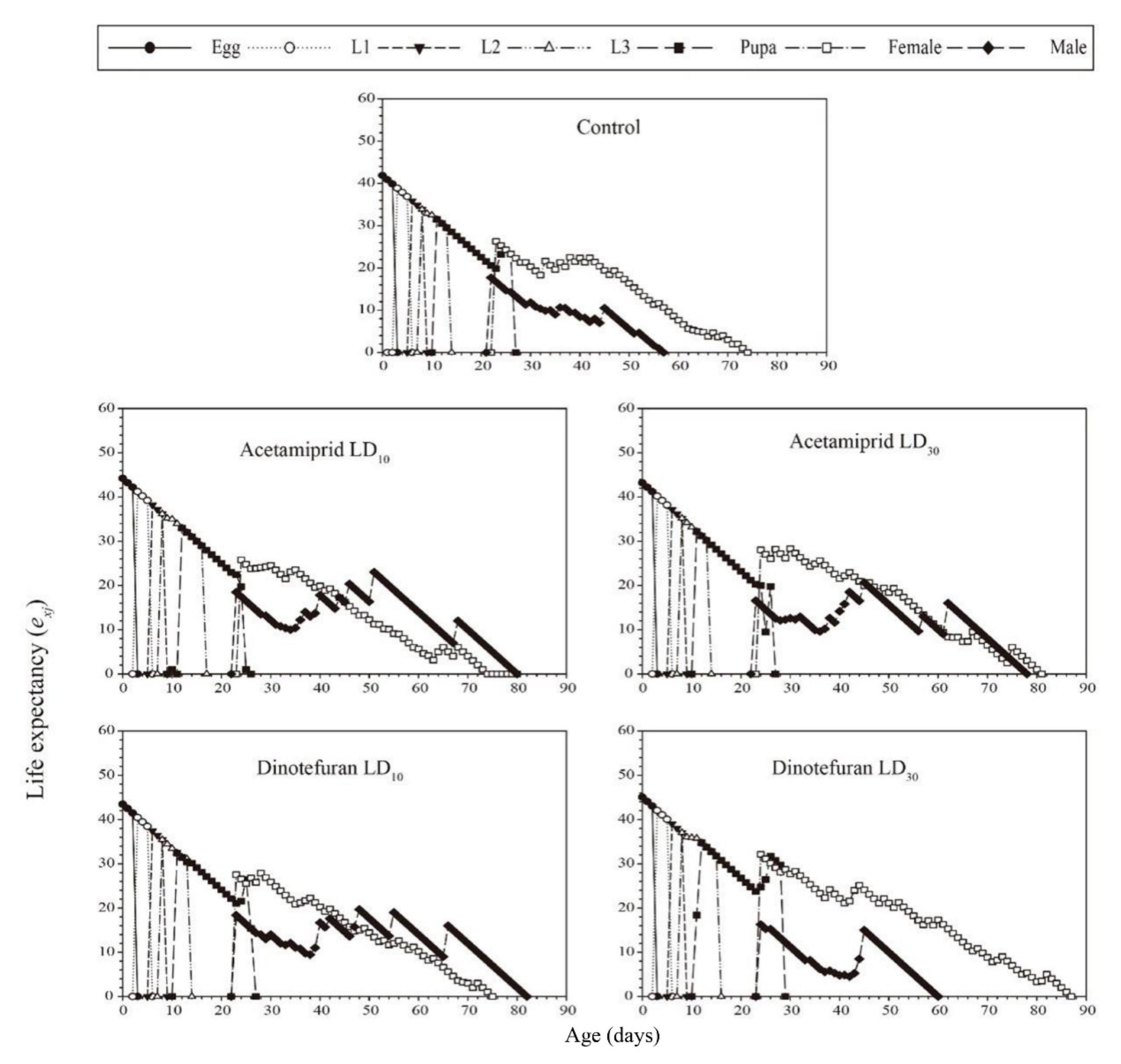
3.3. Effects of Acetamiprid and Dinotefuran on C. pallens Demographic Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luna, R.F.; Bestete, L.R.; Torres, J.B.; da Silva-Torres, C.S.A. Predation and behavioral changes in the neotropical lacewing Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae) exposed to lambda-cyhalothrin. Ecotoxicology 2018, 27, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R. Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Z.; Chang, C.H.; Lou, J.L.; Zhao, M.R.; Lu, C. Potential human exposures to neonicotinoid insecticides: A review. Environ. Pollut. 2018, 236, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Zanardi, O.Z.; Bordini, G.P.; Franco, A.A.; de Morais, M.R.; Yamamoto, P.T. Spraying pyrethroid and neonicotinoid insecticides can induce outbreaks of Panonychus citri (Trombidiformes: Tetranychidae) in citrus groves. Exp. Appl. Acarol. 2018, 76, 339–354. [Google Scholar] [CrossRef]
- Dai, C.; Ricupero, M.; Puglisi, R.; Lu, Y.; Desneux, N.; Biondi, A.; Zappala, L. Can contamination by major systemic insecticides affect the voracity of the harlequin ladybird? Chemosphere 2020, 256, 126986. [Google Scholar] [CrossRef]
- Catae, A.F.; Roat, T.C.; Pratavieira, M.; Silva Menegasso, A.R.D.; Palma, M.S.; Malaspina, O. Exposure to a sublethal concentration of imidacloprid and the side effects on target and nontarget organs of Apis mellifera (Hymenoptera, Apidae). Ecotoxicology 2018, 27, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Ricupero, M.; Desneux, N.; Zappala, L.; Biondi, A. Target and non-target impact of systemic insecticides on a polyphagous aphid pest and its parasitoid. Chemosphere 2020, 247, 125728. [Google Scholar] [CrossRef]
- Yao, F.L.; Zheng, Y.; Zhao, J.W.; Desneux, N.; He, Y.X.; Weng, Q.Y. Lethal and sublethal effects of thiamethoxam on the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae) through different exposure routes. Chemosphere 2015, 128, 49–55. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Bethke, J.A. Impact of neonicotinoid insecticides on natural enemies in greenhouse and interiorscape environments. Pest Manag. Sci. 2011, 67, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Bucher, R.; Schafer, R.B.; Entling, M.H. Sublethal effects of imidacloprid on interactions in a tritrophic system of non-target species. Chemosphere 2015, 132, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J. A taxonomic review of the common green lacewing genus Chrysoperla (Neuroptera: Chrysopidae). Bull. Br. Mus. Entomol. 1994, 63, 137–210. Available online: https://biostor.org/reference/113516 (accessed on 4 May 2022).
- Tauber, M.J.; Tauber, C.A.; Daane, K.M.; Hagen, K.T.S. Commercialization of Predators: Recent Lessons from Green Lacewings (Neuroptera: Chrysopidae: Chrosoperla). Am. Entomol. 2000, 46, 26–38. [Google Scholar] [CrossRef]
- Brooks, S.J.; Barnard, P.C. The green lacewings of the world: A generic review (Neuroptera: Chrysopidae). Bull. Br. Mus. Nat. Hist. 1990, 59, 117–286. [Google Scholar]
- Uddin, J.; Holliday, N.J.; MacKay, P.A. Rearing lacewings, Chrysoperla carnea and Chrysopa oculata (Neuroptera: Chrysopidae), on prepupae of alfalfa leafcutting bee, Megachile rotundata (Hymenoptera: Megachilidae). Proc. Entomol. Soc. Manit. 2005, 61, 11–19. [Google Scholar]
- Zhang, F.; Wang, S.Q.; Luo, C.; Chen, Y.H.; Li, F. Effects of artificial diet and breeding methods on growth and development of Chryopa septempunctata Wesmael. Plant Prot. 2004, 30, 36–40. [Google Scholar] [CrossRef]
- Lin, R.; He, D.; Men, X.; Zheng, L.; Cheng, S.; Tao, L.; Yu, C. Sublethal and transgenerational effects of acetamiprid and imidacloprid on the predatory bug Orius sauteri (Poppius) (Hemiptera: Anthocoridae). Chemosphere 2020, 255, 126778. [Google Scholar] [CrossRef]
- Martinez, L.C.; Plata-Rueda, A.; Goncalves, W.G.; Freire, A.; Zanuncio, J.C.; Bozdogan, H.; Serrao, J.E. Toxicity and cytotoxicity of the insecticide imidacloprid in the midgut of the predatory bug, Podisus nigrispinus. Ecotoxicol. Environ. Saf. 2019, 167, 69–75. [Google Scholar] [CrossRef]
- Samia, R.R.; Gontijo, P.C.; Oliveira, R.L.; Carvalho, G.A. Sublethal and transgenerational effects of thiamethoxam applied to cotton seed on Chrysoperla externa and Harmonia axyridis. Pest Manag. Sci. 2019, 75, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Garzon, A.; Medina, P.; Amor, F.; Vinuela, E.; Budia, F. Toxicity and sublethal effects of six insecticides to last instar larvae and adults of the biocontrol agents Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) and Adalia bipunctata (L.) (Coleoptera: Coccinellidae). Chemosphere 2015, 132, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-Table Analysis Incorporating Both Sexes and Variable Development Rates Among Individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2020. [Google Scholar]
- Nawaz, M.; Cai, W.; Jing, Z.; Zhou, X.; Mabubu, J.I.; Hua, H. Toxicity and sublethal effects of chlorantraniliprole on the development and fecundity of a non-specific predator, the multicolored Asian lady beetle, Harmonia axyridis (Pallas). Chemosphere 2017, 178, 496–503. [Google Scholar] [CrossRef]
- Mortl, M.; Vehovszky, A.; Klatyik, S.; Takacs, E.; Gyori, J.; Szekacs, A. Neonicotinoids: Spreading, translocation and aquatic toxicity. Int. J. Environ. Res. Public Health 2020, 17, 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bass, C.; Field, L.M. Neonicotinoids. Curr. Biol. 2018, 28, R772–R773. [Google Scholar] [CrossRef] [Green Version]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Margaritopoulos, J.T.; Kati, A.N.; Voudouris, C.C.; Skouras, P.J.; Tsitsipis, J.A. Long-term studies on the evolution of resistance of Myzus persicae (Hemiptera: Aphididae) to insecticides in Greece. Bull. Entomol. Res. 2020, 111, 1–16. [Google Scholar] [CrossRef]
- Rimoldi, F.; Fogel, M.N.; Ronco, A.E.; Schneider, M.I. Comparative susceptibility of two Neotropical predators, Eriopis connexa and Chrysoperla externa, to acetamiprid and pyriproxyfen: Short and long-term effects after egg exposure. Environ. Pollut. 2017, 231, 1042–1050. [Google Scholar] [CrossRef]
- Esquivel, C.J.; Martinez, E.J.; Baxter, R.; Trabanino, R.; Ranger, C.M.; Michel, A.; Canas, L.A. Thiamethoxam differentially impacts the survival of the generalist predators, Orius insidiosus (Hemiptera: Anthocoridae) and Hippodamia convergens (Coleoptera: Coccinellidae), when exposed via the food chain. J. Insect Sci. 2020, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Amarasekare, K.G.; Shearer, P.W. Comparing effects of insecticides on two green lacewings species, Chrysoperla johnsoni and Chrysoperla carnea (Neuroptera: Chrysopidae). J. Econ. Entomol. 2013, 106, 1126–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Zhang, Z.; Yu, X.; Ma, D.; Yu, C.; Liu, F.; Mu, W. Influence of lethal and sublethal exposure to clothianidin on the seven-spotted lady beetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae). Ecotoxicol. Environ. Saf. 2018, 161, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lin, R.; Lin, T.; You, Y.; Zeng, Z.; Zhou, X.; Zhou, Y.; Jiang, H.; Wei, H.; Fu, J.; et al. Effects of acetamiprid on life cycle development of predatory mite Amblyseius cucumeris (Acari: Phytoseiidae) after contact exposure. Chemosphere 2018, 210, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.X.; Zhu, Y.; Li, J.J.; Wang, N.M.; Yu, Q.T.; Xue, C.B. Acute lethal and sublethal effects of four insecticides on the lacewing (Chrysoperla sinica Tjeder). Chemosphere 2020, 250, 126321. [Google Scholar] [CrossRef]
- Valbon, W.R.; Hatano, E.; Oliveira, N.R.X.; Ataide, A.D.; Correa, M.J.M.; Gomes, S.F.; Martins, G.F.; Haddi, K.; Alvarenga, E.S.; Oliveira, E.E. Detrimental effects of pyriproxyfen on the detoxification and abilities of Belostoma anurum to prey upon Aedes aegypti larvae. Environ. Pollut. 2021, 284, 117130. [Google Scholar] [CrossRef]
- Rahmani, S.; Bandani, A.R. Sublethal concentrations of thiamethoxam adversely affect life table parameters of the aphid predator, Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae). Crop. Prot. 2013, 54, 168–175. [Google Scholar] [CrossRef]
- Rugno, G.R.; Zanardi, O.Z.; Parra, J.R.P.; Yamamoto, P.T. Lethal and sublethal toxicity of insecticides to the lacewing Ceraeochrysa Cubana. Neotrop. Entomol. 2019, 48, 162–170. [Google Scholar] [CrossRef]
- Zanardi, O.Z.; Bordini, G.P.; Franco, A.A.; Jacob, C.R.O.; Yamamoto, P.T. Sublethal effects of pyrethroid and neonicotinoid insecticides on Iphiseiodes zuluagai Denmark and Muma (Mesostigmata: Phytoseiidae). Ecotoxicology 2017, 26, 1188–1198. [Google Scholar] [CrossRef]
- Skouras, P.J.; Darras, A.I.; Mprokaki, M.; Demopoulos, V.; Margaritopoulos, J.T.; Delis, C.; Stathas, G.J. Toxicity, sublethal and low dose effects of imidacloprid and deltamethrin on the aphidophagous predator Ceratomegilla undecimnotata (Coleoptera: Coccinellidae). Insects 2021, 12, 696. [Google Scholar] [CrossRef]
- Skouras, P.J.; Brokaki, M.; Stathas, G.J.; Demopoulos, V.; Louloudakis, G.; Margaritopoulos, J.T. Lethal and sub-lethal effects of imidacloprid on the aphidophagous coccinellid Hippodamia variegata. Chemosphere 2019, 229, 392–400. [Google Scholar] [CrossRef] [PubMed]
| Insecticide | N a | Dose (ng a.i. per Insect) (95% CL) b | Slope ± SE c Data | χ2 d | ||
|---|---|---|---|---|---|---|
| LD10 | LD30 | LD50 | ||||
| Acetamiprid | 420 | 8.18 (5.74~10.44) | 16.84 (13.79~19.67) | 26.50 (22.95~30.48) | 4.30 ± 0.45 | 1.65 |
| Dinotefuran | 420 | 9.36 (7.55~10.96) | 15.01 (13.18~16.77) | 20.27 (18.29~22.36) | 6.55 ± 0.61 | 6.18 |
| Stage or Development Period | Control | Acetamiprid LD10 | Acetamiprid LD30 | Dinotefuran LD10 | Dinotefuran LD30 |
|---|---|---|---|---|---|
| Developmental period (days) | |||||
| Second instar | 2.10 ± 0.04 c | 2.50 ± 0.51 a | 2.25 ± 0.44 b | 2.43 ± 0.50 ab | 2.48 ± 0.51 a |
| Third instar | 3.59 ± 0.08 b | 4.00 ± 0.81 a | 3.98 ± 0.568 a | 3.78 ± 0.59 ab | 3.86 ± 1.12 a |
| Pupa | 12.30 ± 0.11 b | 11.73 ± 0.12 c | 12.55 ± 0.01 ab | 12.68 ± 0.13 a | 12.32 ± 0.01 b |
| Preadult | 24.04 ± 0.14 b | 24.29 ± 0.10 b | 24.67 ± 0.12 a | 24.61 ± 0.16 ab | 24.84 ± 0.16 a |
| Adult longevity (days) | |||||
| Female | 49.29 ± 3.45 a | 49.77 ± 3.25 a | 52.36 ± 4.04 a | 51.37 ± 3.69 a | 56.21 ± 4.23 a |
| Male | 39.70 ± 1.80 a | 41.47 ± 2.89 a | 39.53 ± 2.56 a | 41.37 ± 2.65 a | 40.38 ± 1.57 a |
| Reproduction | |||||
| APOP (days) | 6.78 ± 0.45 b | 8.64 ± 0.76 a | 8.28 ± 0.81 ab | 7.91 ± 0.59 ab | 8.69 ± 0.66 a |
| TPOP (days) | 30.93 ± 0.50 b | 33.00 ± 0.79 a | 33.35 ± 0.94 a | 32.83 ± 0.72 a | 33.69 ± 0.65 a |
| Fecundity (eggs/female) | 328.33 ± 87.94 a | 255.92 ± 64.41 a | 248.72 ± 67.49 a | 355.08 ± 109.69 a | 273.87 ± 79.81 a |
| Reproductive days (days) | 18.07 ± 3.35 a | 17.57 ± 2.14 a | 16.51 ± 2.90 a | 20.67 ± 3.32 a | 16.54 ± 2.72 a |
| Population Parameters | Control | Acetamiprid LD10 | Acetamiprid LD30 | Dinotefuran LD10 | Dinotefuran LD30 |
|---|---|---|---|---|---|
| Preadult survival rate (%) | 90.01 ± 4.25 a | 93.18 ± 3.78 a | 87.48 ± 0.05 a | 89.14 ± 4.60 a | 88.10 ± 4.99 a |
| r, Intrinsic rate of increase (day−1) | 0.12 ± 0.01 a | 0.12 ± 0.01 a | 0.11 ± 0.01 a | 0.11 ± 0.01 a | 0.12 ± 0.01 a |
| R0, Net reproductive rate (offspring per individual) | 137.80 ± 42.93 a | 127.95 ± 37.24 a | 103.72 ± 32.78 a | 146.73 ± 51.57 a | 123.96 ± 41.38 a |
| T, Mean generation time (days) | 40.69 ± 0.84 a | 41.55 ± 0.72 a | 42.72 ± 1.06 a | 42.87 ± 1.13 a | 41.33 ± 0.78 a |
| λ, Finite rate of increase (day−1) | 1.13 ± 0.01 a | 1.12 ± 0.01 a | 1.11 ± 0.01 a | 1.12 ± 0.01 a | 1.12 ± 0.01 a |
| GRR, Gross reproduction rate (offspring/individual) | 422.18 ± 104.05 a | 314.43 ± 76.87 a | 314.04 ± 84.89 a | 440.44 ± 133.53 a | 321.88 ± 99.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Ren, X.; Ma, X.; Wang, D.; Hu, H.; Song, X.; Cui, J.; Ma, Y.; Yao, Y. Evaluation of the Toxicity and Sublethal Effects of Acetamiprid and Dinotefuran on the Predator Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). Toxics 2022, 10, 309. https://doi.org/10.3390/toxics10060309
Su Y, Ren X, Ma X, Wang D, Hu H, Song X, Cui J, Ma Y, Yao Y. Evaluation of the Toxicity and Sublethal Effects of Acetamiprid and Dinotefuran on the Predator Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). Toxics. 2022; 10(6):309. https://doi.org/10.3390/toxics10060309
Chicago/Turabian StyleSu, Yue, Xiangliang Ren, Xiaoyan Ma, Dan Wang, Hongyan Hu, Xianpeng Song, Jinjie Cui, Yan Ma, and Yongsheng Yao. 2022. "Evaluation of the Toxicity and Sublethal Effects of Acetamiprid and Dinotefuran on the Predator Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae)" Toxics 10, no. 6: 309. https://doi.org/10.3390/toxics10060309
APA StyleSu, Y., Ren, X., Ma, X., Wang, D., Hu, H., Song, X., Cui, J., Ma, Y., & Yao, Y. (2022). Evaluation of the Toxicity and Sublethal Effects of Acetamiprid and Dinotefuran on the Predator Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). Toxics, 10(6), 309. https://doi.org/10.3390/toxics10060309






