Effects of Phthalate Mixtures on Ovarian Folliculogenesis and Steroidogenesis
Abstract
1. Introduction
2. Methods
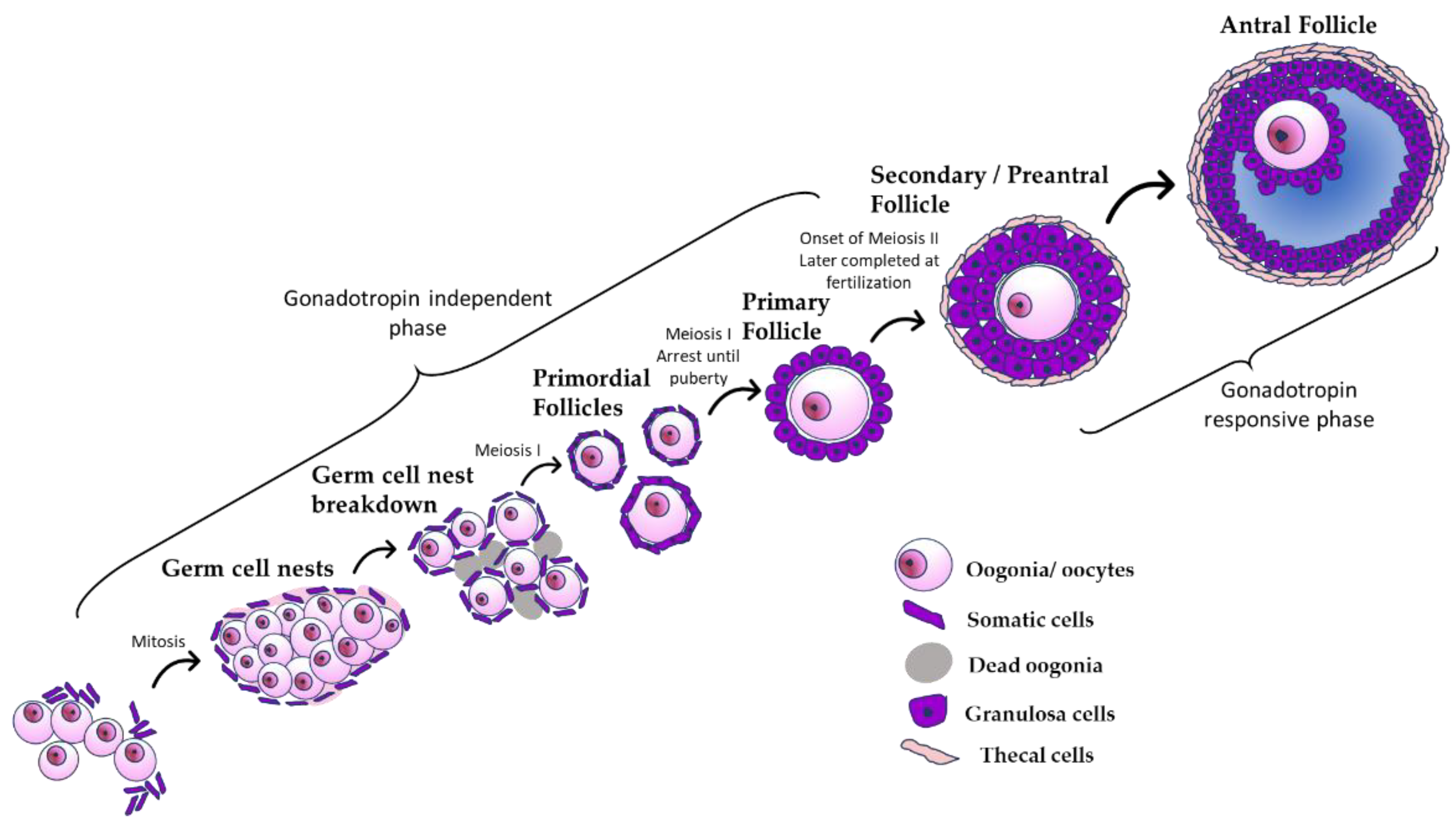
3. Development of the Ovary
4. Steroidogenesis
5. Phthalates
6. Effects of Single Phthalate and Phthalate Mixture Exposure on the Ovary In Vitro
7. Effects of Postnatal Single Phthalate and Phthalate Mixture Exposure on Ovarian Function and Female Reproduction
| Reference | Exposure | Main Findings |
|---|---|---|
| Li et al., 2016 [39] | Mouse (CD-1) Prepubertal exposure from PND 5–15 to DEHP (0, 20, and 40 µg/kg/day) via intraperitoneal exposure every 5 days; tissues collected at PND 20 |
|
| Liu et al., 2021 [40] | Mouse (ICR) Lactating mice exposed to DEHP until nursing mice reached PND 21 (20 and 40 µg/kg/day) via oral dosing; tissues collected at PND 21 |
|
| Hannon et al., 2014 [41] | Mouse (CD-1) Adult exposure to DEHP (20 µg/kg/day–750 mg/kg/day daily) for 10 or 30 days via oral dosing; tissues collected following the dosing period | 10-day exposure:
|
| Hannon et al., 2016 [42] | Mouse (CD-1) Adult exposure to DEHP (20 µg/kg/day–500 mg/kg/day) for 10 days via oral dosing; tissues collected at 6 and 9 months | Six months postdosing:
|
| Chiang et al., 2020 [43] | Mouse (CD-1) Adult exposure to DEHP (20 µg/kg/day–200 mg/kg/day) and DiNP (20 µg/kg/day–200 mg/kg/day) for 10 days via oral dosing; tissues collected at 12, 15, and 18 months | 12 months postdosing:
|
| Xu et al., 2010 [37] | Rat (SD) Young rat exposed to DEHP and B[a]P alone or as a mixture (B[a]P: 5 mg/kg/day and 10 mg/kg/day; DEHP: 300 mg/kg/day and 600 mg/kg/day; B[a]P + DEHP: 5 mg/kg/day + 300 mg/kg/day and 10 mg/kg/day and 600 mg/kg/day) on alternate days for 60 days via oral gavage; tissues collected at 60 days |
|
| Adam et al., 2021 [38] | Mouse (C57BL/6J) Adult exposure to DEHP (5 µg/kg/day and 50 µg/kg/day) or a mixture of phthalates for 6 weeks via oral dosing; tissues collected after week 7 |
|
| Ahmad et al., 2013 [45] | Rat (strain unknown) Young rats exposed to BBP (20 and 200 mg/kg/day) or DiBP (10 and 100 mg/kg/day) for three consecutive days via oral dosing; tissues collected on day 4 Study 2: Young rats exposed to BBP (20 and 200 mg/kg/day) or DiBP (10 and 100 mg/kg/day) from PND 21 for 20 days; tissues collected on PND 42 |
|
| Pocar et al., 2017 [44] | Mouse (CD-1) Prenatal exposure to DEHP (0.05 mg/kg/day, 5 mg/kg/day) from GD 0.5–PND 21 via chow; pups examined at PND 21, F1–F3 generation tissues collected at PND 42 |
|
8. Effects of Prenatal Single and Phthalate Mixture Exposure on the Ovary and Female Reproduction in Offspring
| Reference | Exposure | Main Findings |
|---|---|---|
| Zhou et al., 2017 [47] | Mouse (CD-1) Prenatal exposure from GD 10 to birth with a mixture of DEP, DEHP, DBP, DiBP, DiNP, and BzBP (20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, and 500 mg/kg/day) via oral dosing of pregnant dams; tissues collected at PND 60 |
|
| Gill et al., 2021 [49] | Mouse (CD-1) Prenatal exposure from GD 10 to birth with a mixture of DEP, DEHP, DBP, DiBP, DiNP, and BzBP (20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, and 500 mg/kg/day) via oral dosing of pregnant dams; tissues collected at PND 8 and 60, and at 3 months and 6 months |
|
| Brehm et al., 2021 [50] | Mouse (CD1) Prenatal exposure from GD 10 to birth with a mixture of DEP, DEHP, DBP, DiBP, DiNP, and BzBP (20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day via oral dosing of pregnant dams; tissues collected at 11 and 13 months | F1 generation:
|
| Hannas et al., 2013 [51] | Rat (SD) Prenatal exposure from GD 8–PND 3. to a mix of BBP, DBP, DEHP, DiBP, and DPeP (0, 65, 130, 260, 520, and 780 mg/kg/day) via oral gavage; pups examined at PND 2, tissues collected at PND 77 and PND 350 Part 2, Rat (SD) Prenatal exposure from GD 8–19, GD 8–13, or GD 14–19 to the mixture (520 mg/kg/day) via oral gavage dosing; tissues collected at PND 120 | Increased fetal mortality (780 mg/kg/day)
|
| Repouskou et al., 2019 [53] | Mouse (C57/BL6) Gestational exposure from GD 0.5 until birth to a mixture of MBP, MBzP, MEHP, and MNP (0 mg/kg/day, 0.26 mg/kg/day, 2.6 mg/kg/day, and 13 mg/kg/day) via food of pregnant dams; examined at PND 1; tissues collected at PND 21 and PND 90 |
|
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Front. Public Health 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Fourth National Report on Human Exposure to Environmental Chemicals; Department of Health and Human Services: Atlanta, GA, USA, 2009.
- Monget, P.; McNatty, K.; Monniaux, D. The Crazy Ovary. Genes 2021, 12, 928. [Google Scholar] [CrossRef]
- Rimon-Dahari, N.; Yerushalmi-Heinemann, L.; Alyagor, L.; Dekel, N. Ovarian Folliculogenesis. Results Probl. Cell Differ. 2016, 58, 167–190. [Google Scholar] [PubMed]
- Zhou, J.; Peng, X.; Mei, S. Autophagy in Ovarian Follicular Development and Atresia. Int. J. Biol. Sci. 2019, 15, 726–737. [Google Scholar] [CrossRef]
- Holesh, J.E.; Bass, A.N.; Lord, M. Physiology, Ovulation. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hannon, P.R.; Flaws, J.A. The Effects of Phthalates on the Ovary. Front. Endocrinol. (Lausanne) 2015, 6, 8. [Google Scholar] [CrossRef]
- Drummond, A.E. The role of steroids in follicular growth. Reprod. Biol. Endocrinol. 2006, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Toor, J.S.; Sikka, S.C. Chapter 59—Developmental and Reproductive Disorders—Role of Endocrine Disruptors in Testicular Toxicity. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1111–1121. [Google Scholar]
- Manna, P.R.; Stetson, C.L.; Slominski, A.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef]
- National Research Council Committee on the Health Risks of Phthalates. In Phthalates and Cumulative Risk Assessment: The Tasks Ahead; National Academies Press (US): Washington, DC, USA, 2008.
- Calafat, A.M.; Ye, X.; Silva, M.J.; Kuklenyik, Z.; Needham, L.L. Human exposure assessment to environmental chemicals using biomonitoring. Int. J. Androl. 2006, 29, 166–171; discussion 181–185. [Google Scholar] [CrossRef]
- Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Kay, V.R.; Chambers, C.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol. 2013, 43, 200–219. [Google Scholar] [CrossRef]
- Koch, H.M.; Calafat, A.M. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.; Christensen, K.Y.; Manatunga, A.; Rudra, C.B.; Brock, J.W.; Small, C.M. Variability of phthalate monoester levels in daily first-morning urine from adult women: A pilot study. Rev. Environ. Health 2010, 25, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-C.; Liao, K.-W.; Chang, J.-W.; Chan, S.-H.; Lee, C.-C. Characterization of phthalates exposure and risk for cosmetics and perfume sales clerks. Environ. Pollut. 2018, 233, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, L.; Viganò, P.; Paci, E.; Capanna, S.; Alteri, A.; Campo, G.; Pigini, D.; De Rosa, M.; Tranfo, G.; Papaleo, B. Female Reproductive Health and Exposure to Phthalates and Bisphenol A: A Cross Sectional Study. Toxics 2021, 9, 299. [Google Scholar] [CrossRef]
- Zhou, C.; Flaws, J.A. Effects of an Environmentally Relevant Phthalate Mixture on Cultured Mouse Antral Follicles. Toxicol. Sci. 2017, 156, 217–229. [Google Scholar] [CrossRef]
- Land, K.L.; Lane, M.E.; Fugate, A.C.; Hannon, P.R. Ovulation is Inhibited by an Environmentally Relevant Phthalate Mixture in Mouse Antral Follicles In Vitro. Toxicol. Sci. 2021, 179, 195–205. [Google Scholar] [CrossRef]
- Hannon, P.R.; Brannick, K.E.; Wang, W.; Gupta, R.K.; Flaws, J.A. Di (2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 2015, 284, 42–53. [Google Scholar] [CrossRef]
- Rasmussen, L.M.; Sen, N.; Vera, J.C.; Liu, X.; Craig, Z.R. Effects of in vitro exposure to dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate on ovarian antral follicle growth and viability†. Biol. Reprod. 2017, 96, 1105–1117. [Google Scholar] [CrossRef]
- Wang, W.; Craig, Z.R.; Basavarajappa, M.S.; Gupta, R.K.; Flaws, J.A. Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol. Appl. Pharmacol. 2012, 258, 288–295. [Google Scholar] [CrossRef]
- Warner, G.R.; Meling, D.D.; De La Torre, K.M.; Wang, K.; Flaws, J.A. Environmentally relevant mixtures of phthalates and phthalate metabolites differentially alter the cell cycle and apoptosis in mouse neonatal ovaries†. Biol. Reprod. 2021, 104, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Mu, X.; Chen, X.; Geng, Y.; Zhang, Y.; Li, Q.; Gao, R.; Liu, T.; Wang, Y.; He, J. Dibutyl phthalate exposure disrupts the progression of meiotic prophase I by interfering with homologous recombination in fetal mouse oocytes. Environ. Pollut. 2019, 252, 388–398. [Google Scholar] [CrossRef]
- Liu, J.-C.; Yan, Z.-H.; Li, B.; Yan, H.-C.; De Felici, M.; Shen, W. Di (2-ethylhexyl) phthalate impairs primordial follicle assembly by increasing PDE3A expression in oocytes. Environ. Pollut. 2021, 270, 116088. [Google Scholar] [CrossRef]
- Liu, J.-C.; Lai, F.-N.; Li, L.; Sun, X.-F.; Cheng, S.-F.; Ge, W.; Wang, Y.-F.; Li, L.; Zhang, X.-F.; De Felici, M.; et al. Di (2-ethylhexyl) phthalate exposure impairs meiotic progression and DNA damage repair in fetal mouse oocytes in vitro. Cell Death Dis. 2017, 8, e2966. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, X.; Gao, R.; Geng, Y.; Liu, X.; Chen, X.; Wang, Y.; Ding, Y.; Wang, Y.; He, J. Foetal-neonatal exposure of Di (2-ethylhexyl) phthalate disrupts ovarian development in mice by inducing autophagy. J. Hazard. Mater. 2018, 358, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.R.; Li, Z.; Houde, M.L.; Atkinson, C.E.; Meling, D.D.; Chiang, C.; Flaws, J.A. Ovarian Metabolism of an Environmentally Relevant Phthalate Mixture. Toxicol. Sci. 2019, 169, 246–259. [Google Scholar] [CrossRef]
- Wang, W.; Craig, Z.; Basavarajappa, M.S.; Hafner, K.S.; Flaws, J.A. Mono-(2-Ethylhexyl) Phthalate Induces Oxidative Stress and Inhibits Growth of Mouse Ovarian Antral Follicles. Biol. Reprod. 2012, 87, 152. [Google Scholar] [CrossRef] [PubMed]
- Hannon, P.R.; Brannick, K.E.; Wang, W.; Flaws, J.A. Mono (2-Ethylhexyl) Phthalate Accelerates Early Folliculogenesis and Inhibits Steroidogenesis in Cultured Mouse Whole Ovaries and Antral Follicles. Biol. Reprod. 2015, 92, 120. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shui, S.; Yao, Y.; Sui, C.; Zhang, H. Ascorbic acid ameliorates dysregulated folliculogenesis induced by mono-(2-ethylhexyl) phthalate in neonatal mouse ovaries via reducing ovarian oxidative stress. Reprod. Domest. Anim. 2020, 55, 1418–1424. [Google Scholar] [CrossRef]
- Adir, M.; Combelles, C.M.; Mansur, A.; Ophir, L.; Hourvitz, A.; Orvieto, R.; Dor, J.; Machtinger, R. Dibutyl phthalate impairs steroidogenesis and a subset of LH-dependent genes in cultured human mural granulosa cell in vitro. Reprod. Toxicol. 2017, 69, 13–18. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, P.; Ye, Y.; Zhang, L.; Chen, M.; Hu, Y.; Gu, A.; Chen, S.; Wang, Y. NF-κB-vimentin is involved in steroidogenesis stimulated by mono-butyl phthalate in primary cultured ovarian granulosa cells. Toxicol Vitr. 2017, 45, 25–30. [Google Scholar] [CrossRef]
- Tripathi, A.; Pandey, V.; Sahu, A.; Singh, A.K.; Dubey, P.K. Encircling granulosa cells protects against di-(2-ethylhexyl) phthalate-induced apoptosis in rat oocytes cultured in vitro. Zygote 2019, 27, 203–213. [Google Scholar] [CrossRef]
- Xu, C.; Chen, J.-A.; Qiu, Z.; Zhao, Q.; Luo, J.; Yang, L.; Zeng, H.; Huang, Y.; Zhang, L.; Cao, J.; et al. Ovotoxicity and PPAR-mediated aromatase downregulation in female Sprague–Dawley rats following combined oral exposure to benzo[a]pyrene and di-(2-ethylhexyl) phthalate. Toxicol. Lett. 2010, 199, 323–332. [Google Scholar] [CrossRef]
- Adam, N.; Brusamonti, L.; Mhaouty-Kodja, S. Exposure of Adult Female Mice to Low Doses of di (2-ethylhexyl) Phthalate Alone or in an Environmental Phthalate Mixture: Evaluation of Reproductive Behavior and Underlying Neural Mechanisms. Environ. Health Perspect. 2021, 129, 17008. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.-C.; Lai, F.-N.; Liu, H.-Q.; Zhang, X.-F.; Dyce, P.W.; Shen, W.; Chen, H. Di (2-ethylhexyl) Phthalate Exposure Impairs Growth of Antral Follicle in Mice. PLoS ONE 2016, 11, e0148350. [Google Scholar] [CrossRef]
- Liu, J.-C.; Xing, C.-H.; Xu, Y.; Pan, Z.-N.; Zhang, H.-L.; Zhang, Y.; Sun, S.-C. DEHP exposure to lactating mice affects ovarian hormone production and antral follicle development of offspring. J. Hazard. Mater. 2021, 416, 125862. [Google Scholar] [CrossRef] [PubMed]
- Hannon, P.R.; Peretz, J.; Flaws, J.A. Daily Exposure to Di (2-ethylhexyl) Phthalate Alters Estrous Cyclicity and Accelerates Primordial Follicle Recruitment Potentially Via Dysregulation of the Phosphatidylinositol 3-Kinase Signaling Pathway in Adult Mice. Biol. Reprod. 2014, 90, 136. [Google Scholar] [CrossRef] [PubMed]
- Hannon, P.R.; Niermann, S.; Flaws, J.A. Acute Exposure to Di (2-Ethylhexyl) Phthalate in Adulthood Causes Adverse Reproductive Outcomes Later in Life and Accelerates Reproductive Aging in Female Mice. Toxicol. Sci. 2016, 150, 97–108. [Google Scholar] [CrossRef]
- Chiang, C.; Lewis, L.R.; Borkowski, G.; Flaws, J.A. Late-life consequences of short-term exposure to di (2-ethylhexyl) phthalate and diisononyl phthalate during adulthood in female mice. Reprod. Toxicol. 2020, 93, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Pocar, P.; Fiandanese, N.; Berrini, A.; Secchi, C.; Borromeo, V. Maternal exposure to di (2-ethylhexyl) phthalate (DEHP) promotes the transgenerational inheritance of adult-onset reproductive dysfunctions through the female germline in mice. Toxicol. Appl. Pharmacol. 2017, 322, 113–121. [Google Scholar] [CrossRef]
- Ahmad, R.; Verma, Y.; Gautam, A.K.; Kumar, S. Assessment of estrogenic potential of di-n-butyl phthalate and butyl benzyl phthalate in vivo. Toxicol. Ind. Health 2015, 31, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Yost, E.E.; Euling, S.Y.; Weaver, J.A.; Beverly, B.E.; Keshava, N.; Mudipalli, A.; Arzuaga, X.; Blessinger, T.; Dishaw, L.; Hotchkiss, A.; et al. Hazards of diisobutyl phthalate (DIBP) exposure: A systematic review of animal toxicology studies. Environ. Int. 2019, 125, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Gao, L.; Flaws, J.A. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol. Appl. Pharmacol. 2017, 318, 49–57. [Google Scholar] [CrossRef] [PubMed]
- NTP Center for the Evaluation of Risks to Human Reproduction. NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Di-Isononyl Phthalate (DINP); NIH: Bethesda, MD, USA, 2003.
- Gill, S.; Brehm, E.; Leon, K.; Chiu, J.; Meling, D.D.; Flaws, J.A. Prenatal exposure to an environmentally relevant phthalate mixture alters ovarian steroidogenesis and folliculogenesis in the F1 generation of adult female mice. Reprod. Toxicol. 2021, 106, 25–31. [Google Scholar] [CrossRef]
- Brehm, E.; Flaws, J.A. Prenatal exposure to a mixture of phthalates accelerates the age-related decline in reproductive capacity but may not affect direct biomarkers of ovarian aging in the F1 generation of female mice. Environ. Epigenetics 2021, 7, dvab010. [Google Scholar] [CrossRef]
- Hannas, B.R.; Howdeshell, K.L.; Furr, J.; Gray, L.E. In utero phthalate effects in the female rat: A model for MRKH syndrome. Toxicol. Lett. 2013, 223, 315–321. [Google Scholar] [CrossRef]
- Engel, A.; Buhrke, T.; Imber, F.; Jessel, S.; Seidel, A.; Völkel, W.; Lampen, A. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERα, ERβ, and AR. Toxicol. Lett. 2017, 277, 54–63. [Google Scholar] [CrossRef]
- Repouskou, A.; Panagiotidou, E.; Panagopoulou, L.; Bisting, P.L.; Tuck, A.R.; Sjödin, M.O.D.; Lindberg, J.; Bozas, E.; Rüegg, J.; Gennings, C.; et al. Gestational exposure to an epidemiologically defined mixture of phthalates leads to gonadal dysfunction in mouse offspring of both sexes. Sci. Rep. 2019, 9, 6424. [Google Scholar] [CrossRef]
- Brehm, E.; Zhou, C.; Gao, L.; Flaws, J.A. Prenatal exposure to an environmentally relevant phthalate mixture accelerates biomarkers of reproductive aging in a multiple and transgenerational manner in female mice. Reprod. Toxicol. 2020, 98, 260–268. [Google Scholar] [CrossRef]
- Brehm, E.; Rattan, S.; Gao, L.; Flaws, J.A. Prenatal Exposure to Di (2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice. Endocrinology 2018, 159, 795–809. [Google Scholar] [CrossRef]
- Rattan, S.; Brehm, E.; Gao, L.; Niermann, S.; Flaws, J.A. Prenatal exposure to di (2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biol. Reprod. 2018, 98, 130–145. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhang, T.; Han, Z.; Liu, J.-C.; Liu, Y.-P.; Ma, J.-Y.; Li, L.; Shen, W. Transgenerational inheritance of ovarian development deficiency induced by maternal diethylhexyl phthalate exposure. Reprod. Fertil. Dev. 2015, 27, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Niermann, S.; Rattan, S.; Brehm, E.; Flaws, J.A. Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod. Toxicol. 2015, 53, 23–32. [Google Scholar] [CrossRef]
- Meltzer, D.; Martinez–Arguelles, D.B.; Campioli, E.; Lee, S.; Papadopoulos, V. In utero exposure to the endocrine disruptor di (2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reprod. Toxicol. 2015, 51, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.; Brehm, E.; Gao, L.; Flaws, J.A. Di (2-Ethylhexyl) Phthalate Exposure During Prenatal Development Causes Adverse Transgenerational Effects on Female Fertility in Mice. Toxicol. Sci. 2018, 163, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Q.; Liu, W.; Yu, M.; Zhang, Z.; Cui, X. DEHP exposure in utero disturbs sex determination and is potentially linked with precocious puberty in female mice. Toxicol. Appl. Pharmacol. 2016, 307, 123–129. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Qin, X.-S.; Ge, W.; Ma, H.-G.; Sun, L.-L.; Hou, Z.-M.; Chen, H.; Chen, P.; Qin, G.-Q.; et al. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol. Biol. Rep. 2014, 41, 1227–1235. [Google Scholar] [CrossRef]
- Rattan, S.; Beers, H.K.; Kannan, A.; Ramakrishnan, A.; Brehm, E.; Bagchi, I.; Irudayaraj, J.; Flaws, J.A. Prenatal and ancestral exposure to di (2-ethylhexyl) phthalate alters gene expression and DNA methylation in mouse ovaries. Toxicol. Appl. Pharmacol. 2019, 379, 114629. [Google Scholar] [CrossRef]
- Mirihagalle, S.; You, T.; Suh, L.; Patel, C.; Gao, L.; Rattan, S.; Qiao, H. Prenatal exposure to di-(2-ethylhexyl) phthalate and high-fat diet synergistically disrupts mouse fetal oogenesis and affects folliculogenesis. Biol. Reprod. 2019, 100, 1561–1570. [Google Scholar] [CrossRef]
- Moyer, B.; Hixon, M.L. Reproductive effects in F1 adult females exposed in utero to moderate to high doses of mono-2-ethylhexylphthalate (MEHP). Reprod. Toxicol. 2012, 34, 43–50. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Vandenberg, L.N. Assessing dose–response relationships for endocrine disrupting chemicals (EDCs): A focus on non-monotonicity. Environ. Health 2015, 14, 42. [Google Scholar] [CrossRef] [PubMed]

| Reference | Exposure | Main Findings |
|---|---|---|
| Rattan et al., 2019 [63] | Mouse (CD-1) Prenatal exposure from GD 10.5 to birth to DEHP (20 μg/kg/day, 200 μg/kg/day, 500 mg/kg/day, or 750 mg/kg/day) via oral dosing of pregnant dam; F1–F3 generations euthanized at PND 21, and ovaries collected | F1 generation:
|
| Mirihagalle et al., 2019 [64] | Mouse (CD-1) Prenatal exposure from GD 10.5 to birth to DEHP (20 μg/kg/day DEHP + control diet or 20 μg/kg/day DEHP + high-fat diet) via diet of pregnant dam; estrous cyclicity and fertility monitored at 3, 6, and 9 months in F1–F3, tissues collected at PND 21 |
|
| Brehm et al., 2018 [55] | Mouse (CD-1) Prenatal exposure from GD 11 to birth to DEHP (20 µg/kg/day, 200 µg/kg/day, 500 mg/kg/day, and 750 mg/kg/day) via oral dosing of pregnant dam; ovaries collected from the F1–F3 generation at 12 months of age | F1 generation:
|
| Rattan et al., 2018 [56] | Mouse (CD-1) Prenatal exposure from GD 10.5 to birth to DEHP (20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, 500 mg/kg/day, 750 mg/kg/day) via oral dosing of pregnant dams; F1–F3 generations euthanized at PND 21, and ovaries collected at PND 1, 8, 21, and 60, and at 3, 6, and 9 months | F1 generation:
|
| Rattan et al., 2018 [60] | Mouse (CD-1) Prenatal exposure from GD 10.5 to birth to DEHP (20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, 500 mg/kg/day, 750 mg/kg/day) via oral dosing of pregnant dams; estrous cyclicity and fertility monitored at 3, 6, and 9 months in F1–F3 | F1 generation:
|
| Wang et al., 2016 [61] | Mouse (ICR) Prenatal exposure from GD 0.5 to birth to DEHP (0, 0.02, 0.2, 2, 20, or 200 mg/kg/day) via oral dosing of pregnant dams; ovaries from F1 collected at PND 1 and 2 |
|
| Zhang et al., 2015 [57] | Mouse (CD-1) Prenatal exposure from 0.5 to 18.5 dpc to DEHP (DEHP 40 µg/kg/day) via addition to drinking water of pregnant dams; tissues collected at PND 21 |
|
| Niermann et al., 2015 [58] | Mouse (CD-1) Prenatal exposure from GD 11 to birth to DEHP (20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, 500 mg/kg/day, or 750 mg/kg/day) via oral dosing of pregnant dams; ovaries collected PND 1, 8, 21, and 60 |
|
| Meltzer et al., 2015 [59] | Rat (SD) Prenatal exposure from GD 14 to birth to DEHP (1, 20, 50, or 300 mg of DEHP/kg/day) via gavage of pregnant dams; ovaries collected at PND 60–68 in each stage of estrus |
|
| Moyer., 2012 [65] | Mouse (C57/BL6) Prenatal exposure from GD 17–19 to MEHP (100, 500, or 1000 mg/kg) via oral dosing of pregnant dams; collected at PND 56 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fletcher, E.J.; Santacruz-Márquez, R.; Mourikes, V.E.; Neff, A.M.; Laws, M.J.; Flaws, J.A. Effects of Phthalate Mixtures on Ovarian Folliculogenesis and Steroidogenesis. Toxics 2022, 10, 251. https://doi.org/10.3390/toxics10050251
Fletcher EJ, Santacruz-Márquez R, Mourikes VE, Neff AM, Laws MJ, Flaws JA. Effects of Phthalate Mixtures on Ovarian Folliculogenesis and Steroidogenesis. Toxics. 2022; 10(5):251. https://doi.org/10.3390/toxics10050251
Chicago/Turabian StyleFletcher, Endia J., Ramsés Santacruz-Márquez, Vasiliki E. Mourikes, Alison M. Neff, Mary J. Laws, and Jodi A. Flaws. 2022. "Effects of Phthalate Mixtures on Ovarian Folliculogenesis and Steroidogenesis" Toxics 10, no. 5: 251. https://doi.org/10.3390/toxics10050251
APA StyleFletcher, E. J., Santacruz-Márquez, R., Mourikes, V. E., Neff, A. M., Laws, M. J., & Flaws, J. A. (2022). Effects of Phthalate Mixtures on Ovarian Folliculogenesis and Steroidogenesis. Toxics, 10(5), 251. https://doi.org/10.3390/toxics10050251








