IVIVE: Facilitating the Use of In Vitro Toxicity Data in Risk Assessment and Decision Making
Abstract
1. Introduction
1.1. Multiple Definitions of IVIVE in Literature
1.2. Overview of Regulatory Applications of IVIVE
1.3. Introduction to the IVIVE Workgroup
2. Methods
3. Regulatory Application of IVIVE
4. Applications of IVIVE Approaches
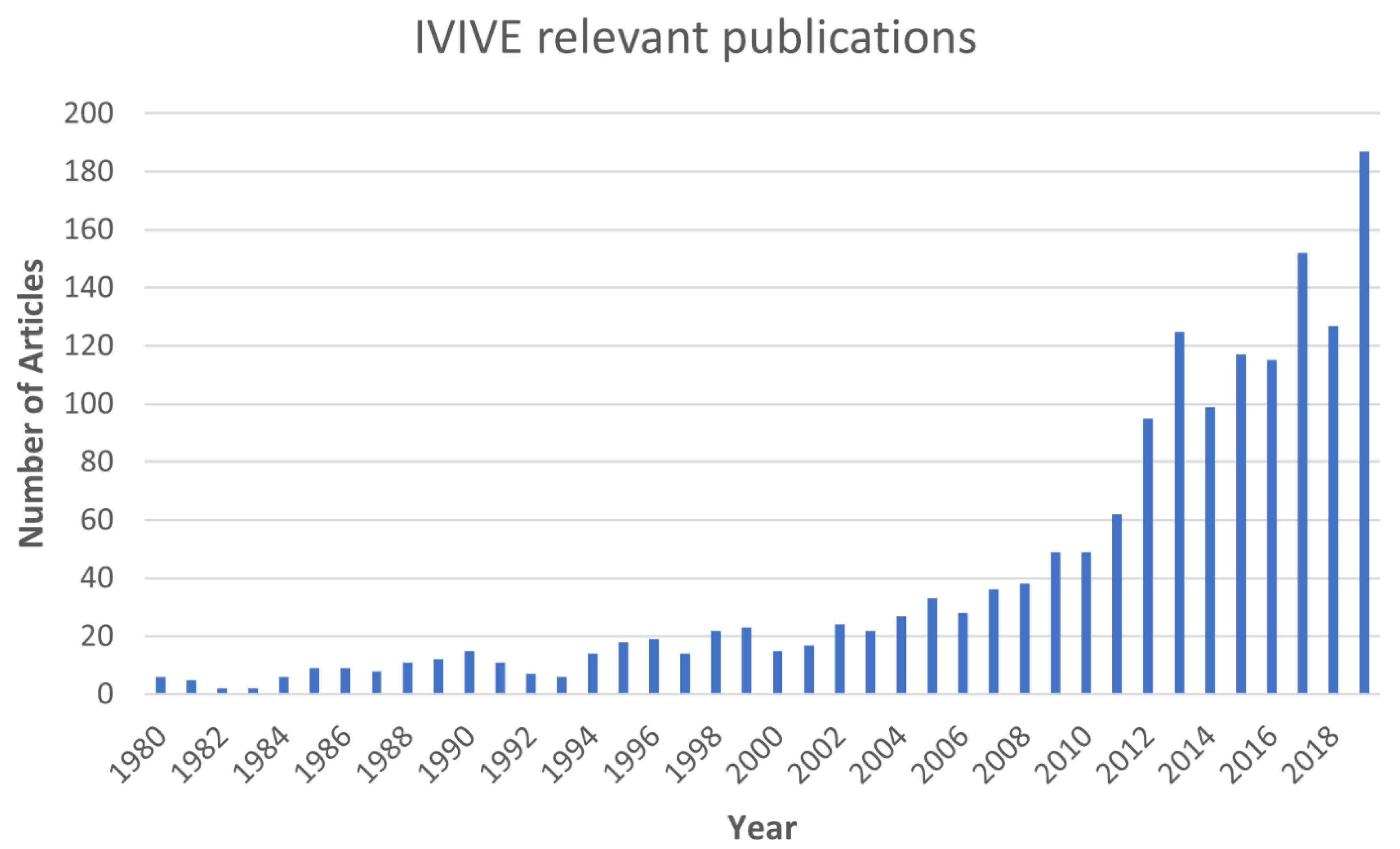
4.1. Review of IVIVE Literature
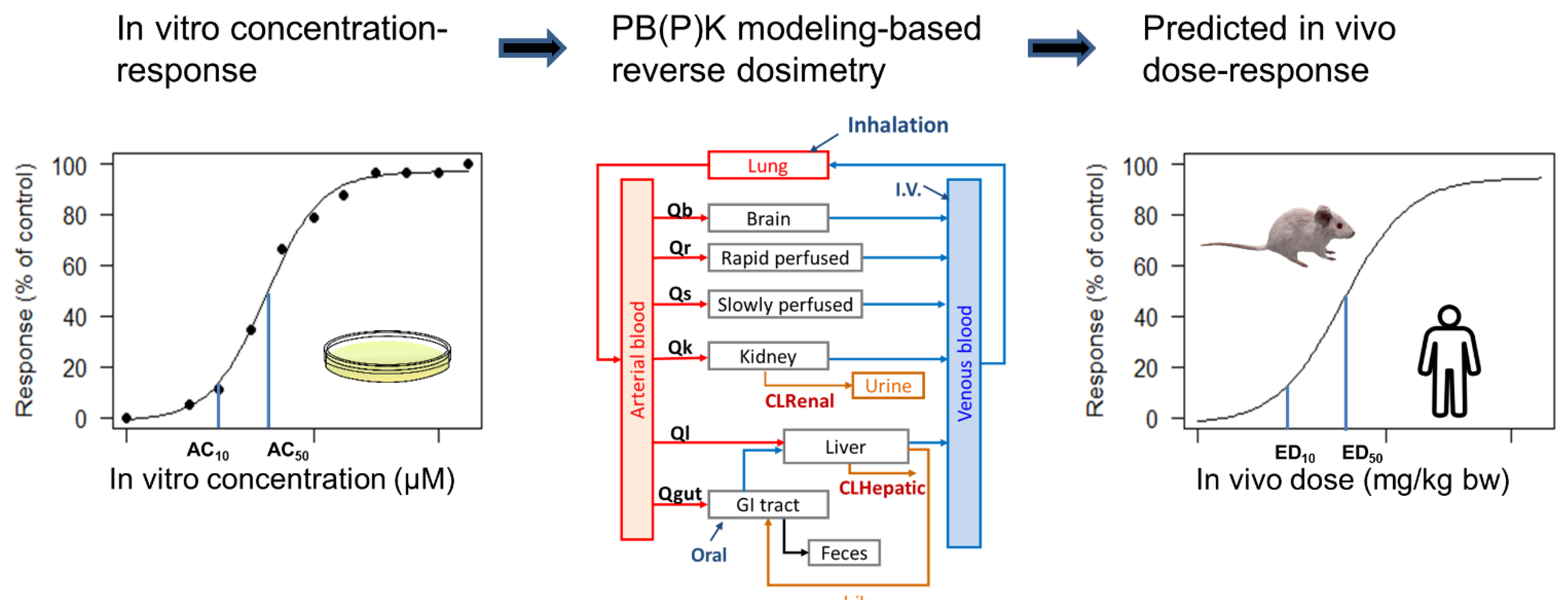
4.2. IVIVE of Dosimetry
4.2.1. Summary of Common Applications
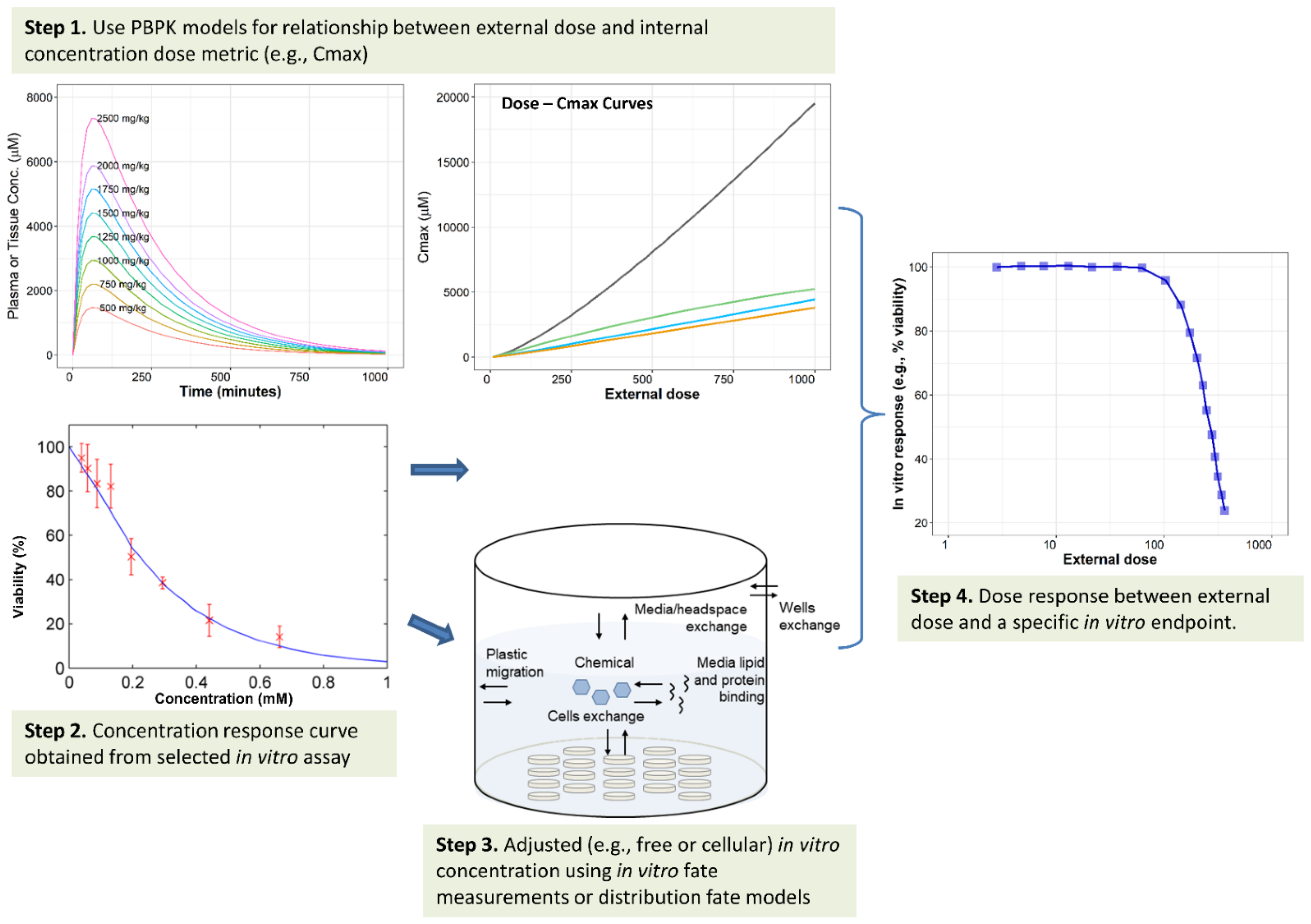
4.2.2. Challenges and Additional Considerations for IVIVE of Dosimetry
4.3. IVIVE of ADME Parameters
4.3.1. Summary of Common Applications
4.3.2. Evaluations and Additional Considerations for IVIVE of ADME Parameters
4.4. Employing IVIVE to Predict In Vivo Toxicity
5. Case Examples from the Literature
5.1. For Prioritization
5.2. Developmental Toxicity
5.3. Endocrine Effects
5.4. Case Examples of IVIVE of ADME Parameters
5.5. IVIVE Application to Engineered Nanomaterials (ENMs)
6. IVIVE Resources and Tools
6.1. Information Obtained from Literature and Agency’s Responses
6.2. Resources for Chemical Properties and In Vitro Data
6.2.1. Resources for Chemical Properties Data
6.2.2. Resources for In Vitro ADME Data (Reviews or Multiple Topics)
6.3. Models and Tools for PBPK Modeling and IVIVE
6.3.1. Resources Explicitly Designed to Support IVIVE of Dosimetry and Related Activities
6.3.2. Other Models and Tools for PBPK Modeling and IVIVE
7. Agency Needs, Areas of Research Needed, and Future Opportunities
7.1. Agency Needs, Gaps, and Uncertainty in IVIVE
7.2. Efforts to Address Needs and Future Opportunity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
FDA Disclaimer
NIST Disclaimer
Abbreviations
| 3Rs | to replace, reduce, and refine (or replacement, reduction, and refinement of) the use of animal models |
| ADME | absorption, distribution, metabolism and excretion |
| ATSDR | Agency for Toxic Substances and Disease Registry |
| AUC | area under curve |
| BMD | benchmark dose, the dose of a chemical that is required to achieve a predetermined response of a toxicological effect |
| BMD10 | derived benchmark dose that is associated with a 10% extra risk of adverse effect in the exposed test animals |
| BMDL10 | the lower bound of 95% confidence interval on BMD10 |
| CFSAN | FDA Center for Food Safety and Applied Nutrition |
| Cmax | the highest concentration of a chemical in the blood or a tissue after a dose is given |
| CPSC | U.S. Consumer Product Safety Commission |
| Css | steady-state concentration |
| DoD | U.S. Department of Defense |
| EAD | equivalent administered dose |
| ENM | engineered nanomaterial |
| EPA | U.S. Environmental Protection Agency |
| ER | estrogen receptor |
| EURL-ECVAM | European Union Reference Laboratory for Alternatives to Animal Testing |
| FDA | U.S. Food and Drug Administration |
| HTS | high throughput screening |
| HT-IVIVE | high throughput in vitro to in vivo extrapolation |
| ICCVAM | Interagency Coordinating Committee on the Validation of Alternative Methods |
| ICE | Integrated Chemical Environment |
| IVIVE | in vitro to in vivo extrapolation |
| IVIVE-WG | ICCVAM in vitro to in vivo Extrapolation Workgroup |
| LOAEL | low observed adverse effects level |
| log Kow | the n-octanol / water partition ratio or coefficient |
| NAM | new approach methodology |
| NIEHS | National Institute of Environmental Health Sciences |
| NIST | National Institute of Standards and Technology |
| NLM | U.S. National Library of Medicine |
| NOAEL | no observed adverse effect level |
| NOEL | no observed effect level |
| NTP | National Toxicology Program |
| OECD | Organisation for Economic Co-operation and Development |
| OED | oral equivalent dose |
| OPP | EPA Office of Pesticide Programs |
| QSAR | quantitative structure activity relationship |
| PD | pharmacodynamics |
| PK | pharmacokinetics |
| PBK | physiologically based kinetics |
| PBPK | physiologically based pharmacokinetics |
| PBTK | physiologically based toxicokinetics |
| PFOA | perfluorooctanoic acid |
| POD | point of departure |
| ToxCastTM | Toxicity forecaster |
| Tox21 | Toxicology in the 21st century |
References
- Prior, H.; Casey, W.; Kimber, I.; Whelan, M.; Sewell, F. Reflections on the Progress towards Non-Animal Methods for Acute Toxicity Testing of Chemicals. Regul. Toxicol. Pharmacol. 2019, 102, 30–33. [Google Scholar] [CrossRef] [PubMed]
- The Frank, R. Lautenberg Chemical Safety for the 21st Century Act; Pub.L. No: 114-182; codified at 15 U.S.C. § 2601 et seq; U.S. Government Publishing Office: Washington, DC, USA, 2016.
- ICCVAM (Interagency Coordinating Committee on the Validation of Alternative Methods). A Strategic Roadmap for Establishing New Approaches to Evaluate the Safety of Chemicals and Medical Products in the United States; National Institute of Environmental Health Sciences: Durham, NC, USA, 2018.
- Hsieh, N.-H.; Chen, Z.; Rusyn, I.; Chiu, W.A. Risk Characterization and Probabilistic Concentration–Response Modeling of Complex Environmental Mixtures Using New Approach Methodologies (NAMs) Data from Organotypic in Vitro Human Stem Cell Assays. Environ. Health Perspect. 2021, 129, 17004. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Adeleye, Y.; Clewell, R.; Jennings, P.; Whelan, M. Moving Beyond Prioritization Toward True In Vitro Safety Assessment. Appl. Vitro Toxicol. 2016, 2, 67–73. [Google Scholar] [CrossRef]
- Hamon, J.; Renner, M.; Jamei, M.; Lukas, A.; Kopp-Schneider, A.; Bois, F.Y. Quantitative in Vitro to in Vivo Extrapolation of Tissues Toxicity. Toxicol. In Vitro 2015, 30, 203–216. [Google Scholar] [CrossRef]
- Meek, M.E.; Lipscomb, J.C. Gaining Acceptance for the Use of in Vitro Toxicity Assays and QIVIVE in Regulatory Risk Assessment. Toxicology 2015, 332, 112–123. [Google Scholar] [CrossRef]
- Clewell, H.J.; Campbell, J.L.; Van Landingham, C.; Franzen, A.; Yoon, M.; Dodd, D.E.; Andersen, M.E.; Gentry, P.R. Incorporation of in Vitro Metabolism Data and Physiologically Based Pharmacokinetic Modeling in a Risk Assessment for Chloroprene. Inhal. Toxicol. 2019, 31, 468–483. [Google Scholar] [CrossRef]
- Houston, J.B.; Carlile, D.J. Incorporation of in Vitro Drug Metabolism Data into Physiologically-Based Pharmacokinetic Models. Toxicol. In Vitro 1997, 11, 473–478. [Google Scholar] [CrossRef]
- Mallick, P. Utilizing in Vitro Transporter Data in IVIVE-PBPK: An Overview. ADMET DMPK 2017, 5, 201–211. [Google Scholar] [CrossRef][Green Version]
- Rostami-Hodjegan, A. Reverse Translation in PBPK and QSP: Going Backwards in Order to Go Forward with Confidence. Clin. Pharmacol. Ther. 2018, 103, 224–232. [Google Scholar] [CrossRef]
- Yoon, M.; Kedderis, G.L.; Yan, G.Z.; Clewell, H.J., 3rd. Use of in Vitro Data in Developing a Physiologically Based Pharmacokinetic Model: Carbaryl as a Case Study. Toxicology 2015, 332, 52–66. [Google Scholar] [CrossRef]
- Alqahtani, S.; Mohamed, L.A.; Kaddoumi, A. Experimental Models for Predicting Drug Absorption and Metabolism. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.M.; Benet, L.Z. In Vitro-In Vivo Extrapolation and Hepatic Clearance-Dependent Underprediction. J. Pharm. Sci. 2019, 108, 2500–2504. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kim, J.E.; Kim, D.D.; Yoon, I.S. In Vitro-in Vivo Extrapolation (IVIVE) for Predicting Human Intestinal Absorption and First-Pass Elimination of Drugs: Principles and Applications. Drug Dev. Ind. Pharm. 2014, 40, 989–998. [Google Scholar] [CrossRef]
- Ferguson, K.C.; Luo, Y.S.; Rusyn, I.; Chiu, W.A. Comparative Analysis of Rapid Equilibrium Dialysis (RED) and Solid Phase Micro-Extraction (SPME) Methods for In Vitro-In Vivo Extrapolation of Environmental Chemicals. Toxicol. In Vitro 2019, 60, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Moreau, M.; Efremenko, A.; Lake, B.G.; Wu, H.; Bruckner, J.V.; White, C.A.; Osimitz, T.G.; Creek, M.R.; Hinderliter, P.M.; et al. Evaluation of Age-Related Pyrethroid Pharmacokinetic Differences in Rats: Physiologically-Based Pharmacokinetic Model Development Using In Vitro Data and In Vitro to In Vivo Extrapolation. Toxicol. Sci. 2019, 169, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Wilk-Zasadna, I.; Bernasconi, C.; Pelkonen, O.; Coecke, S. Biotransformation in Vitro: An Essential Consideration in the Quantitative in Vitro-to-in Vivo Extrapolation (QIVIVE) of Toxicity Data. Toxicology 2015, 332, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.; Bouzom, F.; Scherrmann, J.-M.; Walther, B.; Decleves, X. Development of a Physiologically Based Pharmacokinetic Model for the Rat Central Nervous System and Determination of an in Vitro-in Vivo Scaling Methodology for the Blood-Brain Barrier Permeability of Two Transporter Substrates, Morphine and Oxycodone. J. Pharm. Sci. 2012, 101, 4277–4292. [Google Scholar] [CrossRef]
- Caruso, A.; Alvarez-Sanchez, R.; Hillebrecht, A.; Poirier, A.; Schuler, F.; Lave, T.; Funk, C.; Belli, S. PK/PD Assessment in CNS Drug Discovery: Prediction of CSF Concentration in Rodents for P-Glycoprotein Substrates and Application to in Vivo Potency Estimation. Biochem. Pharmacol. 2013, 85, 1684–1699. [Google Scholar] [CrossRef]
- Kunze, A.; Huwyler, J.; Poller, B.; Gutmann, H.; Camenisch, G. In Vitro-in Vivo Extrapolation Method to Predict Human Renal Clearance of Drugs. J. Pharm. Sci. 2014, 103, 994–1001. [Google Scholar] [CrossRef]
- Kusuhara, H.; Sugiyama, Y. In Vitro-in Vivo Extrapolation of Transporter-Mediated Clearance in the Liver and Kidney. Drug Metab. Pharmacokinet. 2009, 24, 37–52. [Google Scholar] [CrossRef]
- Leung, L.; Yang, X.; Strelevitz, T.J.; Montgomery, J.; Brown, M.F.; Zientek, M.A.; Banfield, C.; Gilbert, A.M.; Thorarensen, A.; Dowty, M.E. Clearance Prediction of Targeted Covalent Inhibitors by In Vitro-In Vivo Extrapolation of Hepatic and Extrahepatic Clearance Mechanisms. Drug Metab. Dispos. 2017, 45, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Coecke, S.; Pelkonen, O.; Leite, S.B.; Bernauer, U.; Bessems, J.G.; Bois, F.Y.; Gundert-Remy, U.; Loizou, G.; Testai, E.; Zaldivar, J.M. Toxicokinetics as a Key to the Integrated Toxicity Risk Assessment Based Primarily on Non-Animal Approaches. Toxicol. In Vitro 2013, 27, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, B.A. Quantitative in Vitro-to-in Vivo Extrapolation in a High-Throughput Environment. Toxicology 2015, 332, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Gargas, M.L.; Burgess, R.J.; Voisard, D.E.; Cason, G.H.; Andersen, M.E. Partition Coefficients of Low-Molecular-Weight Volatile Chemicals in Various Liquids and Tissues. Toxicol. Appl. Pharmacol. 1989, 98, 87–99. [Google Scholar] [CrossRef]
- Moxon, T.E.; Li, H.; Lee, M.Y.; Piechota, P.; Nicol, B.; Pickles, J.; Pendlington, R.; Sorrell, I.; Baltazar, M.T. Application of Physiologically Based Kinetic (PBK) Modelling in the next Generation Risk Assessment of Dermally Applied Consumer Products. Toxicol. In Vitro 2020, 63, 104746. [Google Scholar] [CrossRef]
- Yoon, M.; Campbell, J.L.; Andersen, M.E.; Clewell, H.J. Quantitative in Vitro to in Vivo Extrapolation of Cell-Based Toxicity Assay Results. Crit. Rev. Toxicol. 2012, 42, 633–652. [Google Scholar] [CrossRef]
- Bell, S.M.; Chang, X.; Wambaugh, J.F.; Allen, D.G.; Bartels, M.; Brouwer, K.L.R.; Casey, W.M.; Choksi, N.; Ferguson, S.S.; Fraczkiewicz, G.; et al. In Vitro to in Vivo Extrapolation for High Throughput Prioritization and Decision Making. Toxicol. In Vitro 2018, 47, 213–227. [Google Scholar] [CrossRef]
- Tan, Y.-M.; Liao, K.H.; Clewell, H.J. Reverse Dosimetry: Interpreting Trihalomethanes Biomonitoring Data Using Physiologically Based Pharmacokinetic Modeling. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 591–603. [Google Scholar] [CrossRef]
- Wetmore, B.A.; Wambaugh, J.F.; Ferguson, S.S.; Sochaski, M.A.; Rotroff, D.M.; Freeman, K.; Clewell, H.J., 3rd; Dix, D.J.; Andersen, M.E.; Houck, K.A.; et al. Integration of Dosimetry, Exposure, and High-Throughput Screening Data in Chemical Toxicity Assessment. Toxicol. Sci. 2012, 125, 157–174. [Google Scholar] [CrossRef]
- Blaauboer, B.J.; Boekelheide, K.; Clewell, H.J.; Daneshian, M.; Dingemans, M.M.L.; Goldberg, A.M.; Heneweer, M.; Jaworska, J.; Kramer, N.I.; Leist, M.; et al. The Use of Biomarkers of Toxicity for Integrating in Vitro Hazard Estimates into Risk Assessment for Humans. Altern. Anim. Exp. 2012, 29, 411–425. [Google Scholar] [CrossRef]
- Blaauboer, B.J. Biokinetic Modeling and in Vitro-in Vivo Extrapolations. J. Toxicol. Environ. Health-Part B Crit. Rev. 2010, 13, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.C.; Henneberger, L.; Schlichting, R.; Escher, B.I. How to Improve the Dosing of Chemicals in High-Throughput in Vitro Mammalian Cell Assays. Chem. Res. Toxicol. 2019, 32, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.C.; Henneberger, L.; Konig, M.; Bittermann, K.; Linden, L.; Goss, K.U.; Escher, B.I. Modeling Exposure in the Tox21 in Vitro Bioassays. Chem. Res. Toxicol. 2017, 30, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Groothuis, F.A.; Heringa, M.B.; Nicol, B.; Hermens, J.L.M.; Blaauboer, B.J.; Kramer, N.I. Dose Metric Considerations in in Vitro Assays to Improve Quantitative in Vitro–in Vivo Dose Extrapolations. Toxicology 2015, 332, 30–40. [Google Scholar] [CrossRef]
- Gulden, M.; Dierickx, P.; Seibert, H. Validation of a Prediction Model for Estimating Serum Concentrations of Chemicals Which Are Equivalent to Toxic Concentrations in Vitro. Toxicol. In Vitro 2006, 20, 1114–1124. [Google Scholar] [CrossRef]
- Hartung, T. Perspectives on In Vitro to In Vivo Extrapolations. Appl. In Vitro Toxicol. 2018, 4, 305–316. [Google Scholar] [CrossRef]
- Paini, A.; Sala Benito, J.V.; Bessems, J.; Worth, A.P. From in Vitro to in Vivo: Integration of the Virtual Cell Based Assay with Physiologically Based Kinetic Modelling. Toxicol. In Vitro 2017, 45, 241–248. [Google Scholar] [CrossRef]
- Poulin, P.; Burczynski, F.J.; Haddad, S. The Role of Extracellular Binding Proteins in the Cellular Uptake of Drugs: Impact on Quantitative In Vitro-to-In Vivo Extrapolations of Toxicity and Efficacy in Physiologically Based Pharmacokinetic-Pharmacodynamic Research. J. Pharm. Sci. 2016, 105, 497–508. [Google Scholar] [CrossRef]
- Sipes, N.S.; Wambaugh, J.F.; Pearce, R.; Auerbach, S.S.; Wetmore, B.A.; Hsieh, J.H.; Shapiro, A.J.; Svoboda, D.; DeVito, M.J.; Ferguson, S.S. An Intuitive Approach for Predicting Potential Human Health Risk with the Tox21 10k Library. Environ. Sci. Technol. 2017, 51, 10786–10796. [Google Scholar] [CrossRef]
- Leonard, J.A.; Tan, Y.-M.; Gilbert, M.; Isaacs, K.; El-Masri, H. Estimating Margin of Exposure to Thyroid Peroxidase Inhibitors Using High-Throughput in Vitro Data, High-Throughput Exposure Modeling, and Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling. Toxicol. Sci. 2016, 151, 57–70. [Google Scholar] [CrossRef]
- McNally, K.; Hogg, A.; Loizou, G. A Computational Workflow for Probabilistic Quantitative in Vitro to in Vivo Extrapolation. Front. Pharmacol. 2018, 9, 508. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Chen, L.; Rietjens, I. Role of Toxicokinetics and Alternative Testing Strategies in Pyrrolizidine Alkaloid Toxicity and Risk Assessment; State-of-the-Art and Future Perspectives. Food Chem. Toxicol. 2019, 131, 110572. [Google Scholar] [CrossRef] [PubMed]
- McMullen, P.D.; Andersen, M.E.; Cholewa, B.; Clewell, H.J.; Dunnick, K.M.; Hartman, J.K.; Mansouri, K.; Minto, M.S.; Nicolas, C.I.; Phillips, M.B.; et al. Evaluating Opportunities for Advancing the Use of Alternative Methods in Risk Assessment through the Development of Fit-for-Purpose in Vitro Assays. Toxicol. In Vitro 2018, 48, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Middleton, A.; Bhattacharya, S.; Conolly, R.B. Bridging the Data Gap From In Vitro Toxicity Testing to Chemical Safety Assessment Through Computational Modeling. Front. Public Health 2018, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.R.; Austin, C.P.; Kavlock, R.J.; Bucher, J.R. Improving the Human Hazard Characterization of Chemicals: A Tox21 Update. Environ. Health Perspect. 2013, 121, 756–765. [Google Scholar] [CrossRef]
- Kavlock, R.; Chandler, K.; Houck, K.; Hunter, S.; Judson, R.; Kleinstreuer, N.; Knudsen, T.; Martin, M.; Padilla, S.; Reif, D.; et al. Update on EPA’s ToxCast Program: Providing High Throughput Decision Support Tools for Chemical Risk Management. Chem. Res. Toxicol. 2012, 25, 1287–1302. [Google Scholar] [CrossRef]
- U.S. EPA. Use of High Throughput Assays and Computational Tools; Endocrine Disruptor Screening Program; Notice of Availability and Opportunity for Comment. Fed. Regist. 2015, 80, 35350–35355. [Google Scholar]
- OECD. Guidance Document on Good In Vitro Method Practices (GIVIMP); Series on Testing and Assessment No. 286; OECD Publishing: Paris, France, 2018; ISBN 978-92-64-31100-8. [Google Scholar]
- OECD. Overview of Concepts and Available Guidance Related to Integrated Approaches to Testing and Assessment (IATA); Series on Testing and Assessment No. 329; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Berggren, E.; White, A.; Ouedraogo, G.; Paini, A.; Richarz, A.-N.; Bois, F.Y.; Exner, T.; Leite, S.; van Grunsven, L.A.; Worth, A.; et al. Ab Initio Chemical Safety Assessment: A Workflow Based on Exposure Considerations and Non-Animal Methods. Comput. Toxicol. 2017, 4, 31–44. [Google Scholar] [CrossRef]
- OECD. Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. Case Study on the Use of Integrated Approaches to Testing and Assessment for Read-Across Based Filling of Developmental Toxicity Data Gap for Methyl Hexanoic Acid; Series on Testing and Assessment No. 325; OECD Publishing: Paris, France, 2020. [Google Scholar]
- OECD. OECD Series on Testing and Assessment No. 280: Guidance Document on the Determination of In Vitro Intrinsic Clearance Using Cryopreserved Hepatocytes (RT-HEP) or Liver S9 Sub-Cellular Fractions (RT-S9) from Rainbow Trout and Extrapolation to In Vivo Intrinsic Clearance; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Paini, A.; Joossens, E.; Bessems, J.; Desalegn, A.; Dorne, J.; Gosling, J.; Heringa, M.; Klaric, M.; Kramer, N.; Loizou, G.; et al. EURL ECVAM Workshop on New Generation of Physiologically-Based Kinetic Models in Risk Assessment; EUR 28794 EN; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Paini, A.; Leonard, J.A.; Joossens, E.; Bessems, J.G.M.; Desalegn, A.; Dorne, J.L.; Gosling, J.P.; Heringa, M.B.; Klaric, M.; Kliment, T.; et al. Next Generation Physiologically Based Kinetic (NG-PBK) Models in Support of Regulatory Decision Making. Comput. Toxicol. 2019, 9, 61–72. [Google Scholar] [CrossRef]
- Avila, A.M.; Bebenek, I.; Bonzo, J.A.; Bourcier, T.; Davis Bruno, K.L.; Carlson, D.B.; Dubinion, J.; Elayan, I.; Harrouk, W.; Lee, S.-L.; et al. An FDA/CDER Perspective on Nonclinical Testing Strategies: Classical Toxicology Approaches and New Approach Methodologies (NAMs). Regul. Toxicol. Pharmacol. 2020, 114, 104662. [Google Scholar] [CrossRef]
- Health Canada. Science Approach Document-Bioactivity Exposure Ratio: Application in Priority Setting and Risk Assessment. Available online: https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/science-approach-document-bioactivity-exposure-ratio-application-priority-setting-risk-assessment.html (accessed on 11 March 2021).
- Center for Drug Evaluation and Research. U.S. FDA Guidance Document. In Vitro Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry; FDA-2017-D-5961-0024; U.S. FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- Shen, J.; Swift, B.; Mamelok, R.; Pine, S.; Sinclair, J.; Attar, M. Design and Conduct Considerations for First-in-Human Trials. Clin. Transl. Sci. 2019, 12, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Durmowicz, A.G.; Lim, R.; Rogers, H.; Rosebraugh, C.J.; Chowdhury, B.A. The U.S. Food and Drug Administration’s Experience with Ivacaftor in Cystic Fibrosis. Establishing Efficacy Using In Vitro Data in Lieu of a Clinical Trial. Ann. Am. Thorac. Soc. 2018, 15, 1–2. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. A Proof-of-Concept Case Study Integrating Publicly Available Information to Screen Candidates for Chemical Prioritization under TSCA; EPA/600/R-21-106; U.S. Environmental Protection Agency: Washington, DC, USA, 2021. [CrossRef]
- U.S. EPA. Use of New Approach Methodologies to Derive Extrapolation Factors and Evaluate Developmental Neurotoxicity for Human Health Risk Assessment; EPA-HQ-OPP-2020-0263-0033; U.S. Environmental Protection Agency: Washington, DC, USA, 2020.
- U.S. EPA. New Approach Methods Work Plan (v2); EPA/600/X-21/209; U.S. Environmental Protection Agency: Washington, DC, USA, 2021.
- U.S. EPA. Administrator Memo Prioritizing Efforts to Reduce Animal Testing, September 10, 2019; U.S. Environmental Protection Agency: Washington, DC, USA, 2019.
- Turley, A.E.; Isaacs, K.K.; Wetmore, B.A.; Karmaus, A.L.; Embry, M.R.; Krishan, M. Incorporating New Approach Methodologies in Toxicity Testing and Exposure Assessment for Tiered Risk Assessment Using the RISK21 Approach: Case Studies on Food Contact Chemicals. Food Chem. Toxicol. 2019, 134, 110819. [Google Scholar] [CrossRef] [PubMed]
- Kleinstreuer, N.C.; Browne, P.; Chang, X.; Judson, R.; Casey, W.; Ceger, P.; Deisenroth, C.; Baker, N.; Markey, K.; Thomas, R.S. Evaluation of Androgen Assay Results Using a Curated Hershberger Database. Reprod. Toxicol. 2018, 81, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.; McLanahan, E.D.; Bushnell, P.J.; Hunter, E.S., 3rd; El-Masri, H. Species Extrapolation of Life-Stage Physiologically-Based Pharmacokinetic (PBPK) Models to Investigate the Developmental Toxicology of Ethanol Using in Vitro to in Vivo (IVIVE) Methods. Toxicol. Sci. 2015, 143, 512–535. [Google Scholar] [CrossRef] [PubMed]
- Cohen Hubal, E.A.; Wetmore, B.A.; Wambaugh, J.F.; El-Masri, H.; Sobus, J.R.; Bahadori, T. Advancing Internal Exposure and Physiologically-Based Toxicokinetic Modeling for 21st-Century Risk Assessments. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Paul Friedman, K.; Gagne, M.; Loo, L.-H.; Karamertzanis, P.; Netzeva, T.; Sobanski, T.; Franzosa, J.A.; Richard, A.M.; Lougee, R.R.; Gissi, A.; et al. Utility of In Vitro Bioactivity as a Lower Bound Estimate of In Vivo Adverse Effect Levels and in Risk-Based Prioritization. Toxicol. Sci. Off. J. Soc. Toxicol. 2020, 173, 202–225. [Google Scholar] [CrossRef]
- Rotroff, D.M.; Wetmore, B.A.; Dix, D.J.; Ferguson, S.S.; Clewell, H.J.; Houck, K.A.; Lecluyse, E.L.; Andersen, M.E.; Judson, R.S.; Smith, C.M.; et al. Incorporating Human Dosimetry and Exposure into High-Throughput in Vitro Toxicity Screening. Toxicol. Sci. 2010, 117, 348–358. [Google Scholar] [CrossRef]
- Wetmore, B.A.; Wambaugh, J.F.; Ferguson, S.S.; Li, L.; Clewell, H.J., 3rd; Judson, R.S.; Freeman, K.; Bao, W.; Sochaski, M.A.; Chu, T.M.; et al. Relative Impact of Incorporating Pharmacokinetics on Predicting in Vivo Hazard and Mode of Action from High-Throughput in Vitro Toxicity Assays. Toxicol. Sci. 2013, 132, 327–346. [Google Scholar] [CrossRef]
- Honda, G.S.; Pearce, R.G.; Pham, L.L.; Setzer, R.W.; Wetmore, B.A.; Sipes, N.S.; Gilbert, J.; Franz, B.; Thomas, R.S.; Wambaugh, J.F. Using the Concordance of in Vitro and in Vivo Data to Evaluate Extrapolation Assumptions. PLoS ONE 2019, 14, e0217564. [Google Scholar] [CrossRef]
- Ring, C.L.; Pearce, R.G.; Setzer, R.W.; Wetmore, B.A.; Wambaugh, J.F. Identifying Populations Sensitive to Environmental Chemicals by Simulating Toxicokinetic Variability. Environ. Int. 2017, 106, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Wambaugh, J.F.; Wetmore, B.A.; Ring, C.L.; Nicolas, C.I.; Pearce, R.G.; Honda, G.S.; Dinallo, R.; Angus, D.; Gilbert, J.; Sierra, T.; et al. Assessing Toxicokinetic Uncertainty and Variability in Risk Prioritization. Toxicol. Sci. 2019, 172, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Wambaugh, J.F.; Hughes, M.F.; Ring, C.L.; MacMillan, D.K.; Ford, J.; Fennell, T.R.; Black, S.R.; Snyder, R.W.; Sipes, N.S.; Wetmore, B.A.; et al. Evaluating In Vitro-In Vivo Extrapolation of Toxicokinetics. Toxicol. Sci. Off. J. Soc. Toxicol. 2018, 163, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, B.A.; Allen, B.; Clewell, H.J., 3rd; Parker, T.; Wambaugh, J.F.; Almond, L.M.; Sochaski, M.A.; Thomas, R.S. Incorporating Population Variability and Susceptible Subpopulations into Dosimetry for High-Throughput Toxicity Testing. Toxicol. Sci. 2014, 142, 210–224. [Google Scholar] [CrossRef]
- Dawson, D.E.; Ingle, B.L.; Phillips, K.A.; Nichols, J.W.; Wambaugh, J.F.; Tornero-Velez, R. Designing QSARs for Parameters of High-Throughput Toxicokinetic Models Using Open-Source Descriptors. Environ. Sci. Technol. 2021, 55, 6505–6517. [Google Scholar] [CrossRef]
- Ingle, B.L.; Veber, B.C.; Nichols, J.W.; Tornero-Velez, R. Informing the Human Plasma Protein Binding of Environmental Chemicals by Machine Learning in the Pharmaceutical Space: Applicability Domain and Limits of Predictability. J. Chem. Inf. Model. 2016, 56, 2243–2252. [Google Scholar] [CrossRef]
- Kenyon, E.M.; Eklund, C.; Pegram, R.A.; Lipscomb, J.C. Comparison of in Vivo Derived and Scaled in Vitro Metabolic Rate Constants for Several Volatile Organic Compounds (VOCs). Toxicol. In Vitro 2020, 69, 105002. [Google Scholar] [CrossRef]
- Nichols, J.W.; Ladd, M.A.; Fitzsimmons, P.N. Measurement of Kinetic Parameters for Biotransformation of Polycyclic Aromatic Hydrocarbons by Trout Liver S9 Fractions: Implications for Bioaccumulation Assessment. Appl. In Vitro Toxicol. 2018, 4, 365–378. [Google Scholar] [CrossRef]
- Nichols, J.W.; Fitzsimmons, P.N.; Burkhard, L.P. In Vitro-in Vivo Extrapolation of Quantitative Hepatic Biotransformation Data for Fish. II. Modeled Effects on Chemical Bioaccumulation. Environ. Toxicol. Chem. 2007, 26, 1304–1319. [Google Scholar] [CrossRef]
- Nichols, J.W.; Schultz, I.R.; Fitzsimmons, P.N. In Vitro-in Vivo Extrapolation of Quantitative Hepatic Biotransformation Data for Fish-I. A Review of Methods, and Strategies for Incorporating Intrinsic Clearance Estimates into Chemical Kinetic Models. Aquat. Toxicol. 2006, 78, 74–90. [Google Scholar] [CrossRef]
- Pradeep, P.; Patlewicz, G.; Pearce, R.; Wambaugh, J.; Wetmore, B.A.; Judson, R. Using Chemical Structure Information to Develop Predictive Models for in Vitro Toxicokinetic Parameters to Inform High-Throughput Risk-Assessment-ScienceDirect. Comput. Toxicol. 2020, 16, 100136. [Google Scholar] [CrossRef] [PubMed]
- Breen, M.; Ring, C.L.; Kreutz, A.; Goldsmith, M.-R.; Wambaugh, J.F. High-Throughput PBTK Models for in Vitro to in Vivo Extrapolation. Expert Opin. Drug Metab. Toxicol. 2021, 17, 903–921. [Google Scholar] [CrossRef] [PubMed]
- Linakis, M.W.; Sayre, R.R.; Pearce, R.G.; Sfeir, M.A.; Sipes, N.S.; Pangburn, H.A.; Gearhart, J.M.; Wambaugh, J.F. Development and Evaluation of a High Throughput Inhalation Model for Organic Chemicals. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Pearce, R.G.; Setzer, R.W.; Strope, C.L.; Sipes, N.S.; Wambaugh, J.F. Httk: R Package for High-Throughput Toxicokinetics. J. Stat. Softw. 2017, 79, 1–26. [Google Scholar] [CrossRef]
- Wambaugh, J.F.; Wetmore, B.A.; Pearce, R.; Strope, C.; Goldsmith, R.; Sluka, J.P.; Sedykh, A.; Tropsha, A.; Bosgra, S.; Shah, I.; et al. Toxicokinetic Triage for Environmental Chemicals. Toxicol. Sci. 2015, 147, 55–67. [Google Scholar] [CrossRef]
- Cote, I.; Andersen, M.; Ankley, G.T.; Barone, S.; Birnbaum, L.; Boekelheide, K.; Bois, F.Y.; Burgoon, L.; Chiu, W.A.; Crawford-Brown, D.; et al. The Next Generation of Risk Assessment Multi-Year Study—Highlights of Findings, Applications to Risk Assessment, and Future Directions. Environ. Health Perspect. 2016, 124, 1671–1682. [Google Scholar] [CrossRef]
- Judson, R.S.; Kavlock, R.J.; Setzer, R.W.; Cohen Hubal, E.A.; Martin, M.T.; Knudsen, T.B.; Houck, K.A.; Thomas, R.S.; Wetmore, B.A.; Dix, D.J. Estimating Toxicity-Related Biological Pathway Altering Doses for High-Throughput Chemical Risk Assessment. Chem. Res. Toxicol. 2011, 24, 451–462. [Google Scholar] [CrossRef]
- Thomas, R.S.; Bahadori, T.; Buckley, T.J.; Cowden, J.; Deisenroth, C.; Dionisio, K.L.; Frithsen, J.B.; Grulke, C.M.; Gwinn, M.R.; Harrill, J.A.; et al. The Next Generation Blueprint of Computational Toxicology at the U.S. Environmental Protection Agency. Toxicol. Sci. 2019, 169, 317–332. [Google Scholar] [CrossRef]
- Thomas, R.S.; Cheung, R.; Westphal, M.; Krewski, D.; Andersen, M.E. Risk Science in the 21st Century: A Data-Driven Framework for Incorporating New Technologies into Chemical Safety Assessment. Int. J. Risk Assess. Manag. 2017, 20, 88–108. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Madabushi, R.; Liu, Q.; Huang, S.-M.; Zineh, I. Model-Informed Drug Development: Current US Regulatory Practice and Future Considerations. Clin. Pharmacol. Ther. 2019, 105, 899–911. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Grimstein, M.; Fan, J.; Grillo, J.A.; Huang, S.-M.; Zhu, H.; Wang, Y. Application of PBPK Modeling and Simulation for Regulatory Decision Making and Its Impact on US Prescribing Information: An Update on the 2018-2019 Submissions to the US FDA’s Office of Clinical Pharmacology. J. Clin. Pharmacol. 2020, 60 (Suppl. 1), S160–S178. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research. U.S. FDA Guidance Document. Clinical Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry; FDA-2017-D-5961-0023; U.S. FDA: Silver Spring, MD, USA, 2020.
- Center for Drug Evaluation and Research. U.S. FDA Guidance Document. Physiologically Based Pharmacokinetic Analyses—Format and Content Guidance for Industry; FDA-2016-D-3969-0018; U.S. FDA: Silver Spring, MD, USA, 2018.
- Center for Drug Evaluation and Research. U.S. FDA Guidance Document. Guidance for Industry Pulmonary Tuberculosis: Developing Drugs for Treatment; FDA-2013-D-1319-0002; U.S. FDA: Silver Spring, MD, USA, 2013.
- Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. U.S. FDA Guidance Document. S5(R3) Detection of Reproductive and Developmental Toxicity for Human Pharmaceuticals, Guidance for Industry; FDA-2017-D-5138-0014; U.S. FDA: Silver Spring, MD, USA, 2021.
- Louisse, J.; de Jong, E.; van de Sandt, J.J.M.; Blaauboer, B.J.; Woutersen, R.A.; Piersma, A.H.; Rietjens, I.M.C.M.; Verwei, M. The Use of in Vitro Toxicity Data and Physiologically Based Kinetic Modeling to Predict Dose-Response Curves for in Vivo Developmental Toxicity of Glycol Ethers in Rat and Man. Toxicol. Sci. 2010, 118, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, B.A.; Wambaugh, J.F.; Allen, B.; Ferguson, S.S.; Sochaski, M.A.; Setzer, R.W.; Houck, K.A.; Strope, C.L.; Cantwell, K.; Judson, R.S.; et al. Incorporating High-Throughput Exposure Predictions With Dosimetry-Adjusted In Vitro Bioactivity to Inform Chemical Toxicity Testing. Toxicol. Sci. 2015, 148, 121–136. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Series on Testing and Assessment No. 331: Guidance Document on the Characterisation, Validation and Reporting of Physiologically Based Kinetic (PBK) Models for Regulatory Purposes; OECD Series on Testing and Assessment; OECD Publishing: Paris, France, 2021. [Google Scholar]
- ECHA. Non-Animal Approaches-Current Status of Regulatory Applicability under the REACH, CLP and Biocidal Products Regulations; European Chemicals Agency: Helsinki, Finland, 2017; ISBN 978-92-9020-208-0. [Google Scholar]
- Scientific Committee on Consumer Safety. Guidance on the Safety Assessment of Nanomaterials in Cosmetics; European Commission: Luxembourg, 2020; ISBN 978-92-76-15271-2. [Google Scholar]
- National Research Council. Toxicity Testing in the 21st Century: A Vision and a Strategy; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Casey, W.; Chang, X.; Allen, D.; Ceger, P.; Choksi, N.; Hsieh, J.-H.; Wetmore, B.; Ferguson, S.; DeVito, M.; Sprankle, C.; et al. Evaluation and Optimization of Pharmacokinetic Models for In Vitro to in Vivo Extrapolation of Estrogenic Activity for Environmental Chemicals. Environ. Health Perspect. 2018, 126, 097001. [Google Scholar] [CrossRef]
- Aylward, L.L.; Hays, S.M. Consideration of Dosimetry in Evaluation of ToxCastTM Data. J. Appl. Toxicol. 2011, 31, 741–751. [Google Scholar] [CrossRef]
- Tonnelier, A.; Coecke, S.; Zaldivar, J.M. Screening of Chemicals for Human Bioaccumulative Potential with a Physiologically Based Toxicokinetic Model. Arch. Toxicol. 2012, 86, 393–403. [Google Scholar] [CrossRef]
- Louisse, J.; Beekmann, K.; Rietjens, I.M.C.M. Use of Physiologically Based Kinetic Modeling-Based Reverse Dosimetry to Predict in Vivo Toxicity from in Vitro Data. Chem. Res. Toxicol. 2017, 30, 114–125. [Google Scholar] [CrossRef]
- Louisse, J.; Bosgra, S.; Blaauboer, B.J.; Rietjens, I.; Verwei, M. Prediction of in Vivo Developmental Toxicity of All-Trans-Retinoic Acid Based on in Vitro Toxicity Data and in Silico Physiologically Based Kinetic Modeling. Arch. Toxicol. 2015, 89, 1135–1148. [Google Scholar] [CrossRef]
- Davidsen, A.B.; Mardal, M.; Holm, N.B.; Andreasen, A.K.; Johansen, S.S.; Noble, C.; Dalsgaard, P.; Linnet, K. Ketamine Analogues: Comparative Toxicokinetic in Vitro–in Vivo Extrapolation and Quantification of 2-Fluorodeschloroketamine in Forensic Blood and Hair Samples. J. Pharm. Biomed. Anal. 2020, 180, 113049. [Google Scholar] [CrossRef]
- Fay, K.A.; Villeneuve, D.L.; Swintek, J.; Edwards, S.W.; Nelms, M.D.; Blackwell, B.R.; Ankley, G.T. Differentiating Pathway-Specific from Nonspecific Effects in High-Throughput Toxicity Data: A Foundation for Prioritizing Adverse Outcome Pathway Development. Toxicol. Sci. 2018, 163, 500–515. [Google Scholar] [CrossRef]
- Judson, R.; Houck, K.; Martin, M.; Richard, A.M.; Knudsen, T.B.; Shah, I.; Little, S.; Wambaugh, J.; Woodrow Setzer, R.; Kothiya, P.; et al. Editor’s Highlight: Analysis of the Effects of Cell Stress and Cytotoxicity on In Vitro Assay Activity Across a Diverse Chemical and Assay Space. Toxicol. Sci. 2016, 152, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Paul Friedman, K.; Watt, E.D.; Hornung, M.W.; Hedge, J.M.; Judson, R.S.; Crofton, K.M.; Houck, K.A.; Simmons, S.O. Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicol. Sci. 2016, 151, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, Y.; Andersen, M.; Clewell, R.; Davies, M.; Dent, M.; Edwards, S.; Fowler, P.; Malcomber, S.; Nicol, B.; Scott, A.; et al. Implementing Toxicity Testing in the 21st Century (TT21C): Making Safety Decisions Using Toxicity Pathways, and Progress in a Prototype Risk Assessment. Toxicology 2015, 332, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, G.L.; Rostami-Hodjegan, A. Building Virtual Human Populations: Assessing the Propagation of Genetic Variability in Drug Metabolism to Pharmacokinetics and Pharmacodynamics. In Biosimulation in Drug Development; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 425–446. ISBN 978-3-527-62267-2. [Google Scholar]
- Edwards, S.W.; Preston, R.J. Systems Biology and Mode of Action Based Risk Assessment. Toxicol. Sci. 2008, 106, 312–318. [Google Scholar] [CrossRef]
- Rostami-Hodjegan, A. Translation of In Vitro Metabolic Data to Predict In Vivo Drug–Drug Interactions: IVIVE and Modeling and Simulations. In Enzyme- and Transporter-Based Drug-Drug Interactions: Progress and Future Challenges; Pang, K.S., Rodrigues, A.D., Peter, R.M., Eds.; Springer: New York, NY, USA, 2010; pp. 317–341. ISBN 978-1-4419-0840-7. [Google Scholar]
- Fabian, E.; Gomes, C.; Birk, B.; Williford, T.; Hernandez, T.R.; Haase, C.; Zbranek, R.; van Ravenzwaay, B.; Landsiedel, R. In Vitro-to-in Vivo Extrapolation (IVIVE) by PBTK Modeling for Animal-Free Risk Assessment Approaches of Potential Endocrine-Disrupting Compounds. Arch. Toxicol. 2019, 93, 401–416. [Google Scholar] [CrossRef]
- Lungu-Mitea, S.; Vogs, C.; Carlsson, G.; Montag, M.; Frieberg, K.; Oskarsson, A.; Lundqvist, J. Modeling Bioavailable Concentrations in Zebrafish Cell Lines and Embryos Increases the Correlation of Toxicity Potencies across Test Systems. Environ. Sci. Technol. 2021, 55, 447–457. [Google Scholar] [CrossRef]
- Sayre, R.R.; Wambaugh, J.F.; Grulke, C.M. Database of Pharmacokinetic Time-Series Data and Parameters for 144 Environmental Chemicals. Sci. Data 2020, 7, 122. [Google Scholar] [CrossRef]
- Groothuis, F.A.; Timmer, N.; Opsahl, E.; Nicol, B.; Droge, S.T.J.; Blaauboer, B.J.; Kramer, N.I. Influence of in Vitro Assay Setup on the Apparent Cytotoxic Potency of Benzalkonium Chlorides. Chem. Res. Toxicol. 2019, 32, 1103–1114. [Google Scholar] [CrossRef]
- Armitage, J.M.; Wania, F.; Arnot, J.A. Application of Mass Balance Models and the Chemical Activity Concept to Facilitate the Use of in Vitro Toxicity Data for Risk Assessment. Environ. Sci. Technol. 2014, 48, 9770–9779. [Google Scholar] [CrossRef]
- Kramer, N.I.; Hermens, J.L.M.; Schirmer, K. The Influence of Modes of Action and Physicochemical Properties of Chemicals on the Correlation between in Vitro and Acute Fish Toxicity Data. Toxicol. In Vitro 2009, 23, 1372–1379. [Google Scholar] [CrossRef]
- Zaldivar Comenges, J.M.; Joossens, E.; Benito, J.V.S.; Worth, A.; Paini, A. Theoretical and Mathematical Foundation of the Virtual Cell Based Assay—A Review. Toxicol. In Vitro 2016, 45, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Proença, S.; Escher, B.I.; Fischer, F.C.; Fisher, C.; Grégoire, S.; Hewitt, N.J.; Nicol, B.; Paini, A.; Kramer, N.I. Effective Exposure of Chemicals in in Vitro Cell Systems: A Review of Chemical Distribution Models. Toxicol. In Vitro 2021, 73, 105133. [Google Scholar] [CrossRef] [PubMed]
- Punt, A.; Schiffelers, M.J.; Jean Horbach, G.; van de Sandt, J.J.; Groothuis, G.M.; Rietjens, I.M.; Blaauboer, B.J. Evaluation of Research Activities and Research Needs to Increase the Impact and Applicability of Alternative Testing Strategies in Risk Assessment Practice. Regul. Toxicol. Pharmacol. 2011, 61, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ring, C.; Sipes, N.S.; Hsieh, J.-H.; Carberry, C.; Koval, L.E.; Klaren, W.D.; Harris, M.A.; Auerbach, S.S.; Rager, J.E. Predictive Modeling of Biological Responses in the Rat Liver Using in Vitro Tox21 Bioactivity: Benefits from High-Throughput Toxicokinetics. Comput. Toxicol. 2021, 18, 100166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H. Confidence Assessment of the Simcyp Time-Based Approach and a Static Mathematical Model in Predicting Clinical Drug-Drug Interactions for Mechanism-Based CYP3A Inhibitors. Drug Metab. Dispos. Biol. Fate Chem. 2010, 38, 1094–1104. [Google Scholar] [CrossRef]
- Yoon, M.; Efremenko, A.; Blaauboer, B.J.; Clewell, H.J. Evaluation of Simple in Vitro to in Vivo Extrapolation Approaches for Environmental Compounds. Toxicol. In Vitro 2014, 28, 164–170. [Google Scholar] [CrossRef]
- Kamiya, Y.; Otsuka, S.; Miura, T.; Yoshizawa, M.; Nakano, A.; Iwasaki, M.; Kobayashi, Y.; Shimizu, M.; Kitajima, M.; Shono, F.; et al. Physiologically Based Pharmacokinetic Models Predicting Renal and Hepatic Concentrations of Industrial Chemicals after Virtual Oral Doses in Rats. Chem. Res. Toxicol. 2020, 33, 1736–1751. [Google Scholar] [CrossRef]
- Rostami-Hodjegan, A. Physiologically Based Pharmacokinetics Joined with in Vitro-in Vivo Extrapolation of ADME: A Marriage under the Arch of Systems Pharmacology. Clin. Pharmacol. Ther. 2012, 92, 50–61. [Google Scholar] [CrossRef]
- Caldwell, J.C.; Evans, M.V.; Krishnan, K. Cutting Edge PBPK Models and Analyses: Providing the Basis for Future Modeling Efforts and Bridges to Emerging Toxicology Paradigms. J. Toxicol. 2012, 2012, 852384. [Google Scholar] [CrossRef]
- McLanahan, E.D.; El-Masri, H.A.; Sweeney, L.M.; Kopylev, L.Y.; Clewell, H.J.; Wambaugh, J.F.; Schlosser, P.M. Physiologically Based Pharmacokinetic Model Use in Risk Assessment—Why Being Published Is Not Enough. Toxicol. Sci. 2012, 126, 5–15. [Google Scholar] [CrossRef]
- Lukacova, V.; Woltosz, W.S.; Bolger, M.B. Prediction of Modified Release Pharmacokinetics and Pharmacodynamics from in Vitro, Immediate Release, and Intravenous Data. AAPS J. 2009, 11, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Bessems, J.G.; Loizou, G.; Krishnan, K.; Clewell, H.J., III; Bernasconi, C.; Bois, F.; Coecke, S.; Collnot, E.-M.; Diembeck, W.; Farcal, L.R.; et al. PBTK Modelling Platforms and Parameter Estimation Tools to Enable Animal-Free Risk Assessment Recommendations from a Joint EPAA-EURL ECVAM ADME Workshop. Regul. Toxicol. Pharmacol. 2014, 68, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Zhang, H.F.; Miah, M.K.; Caritis, S.N.; Venkataramanan, R. Physiologically Based Pharmacokinetic Approach Can Successfully Predict Pharmacokinetics of Citalopram in Different Patient Populations. J. Clin. Pharmacol. 2020, 60, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Kato, M.; Kudo, T.; Ito, K. In Vitro–in Vivo Extrapolation of Metabolic Clearance Using Human Liver Microsomes: Factors Showing Variability and Their Normalization. Xenobiotica 2020, 50, 1064–1075. [Google Scholar] [CrossRef]
- Docci, L.; Klammers, F.; Ekiciler, A.; Molitor, B.; Umehara, K.; Walter, I.; Krähenbühl, S.; Parrott, N.; Fowler, S. In Vitro to In Vivo Extrapolation of Metabolic Clearance for UGT Substrates Using Short-Term Suspension and Long-Term Co-Cultured Human Hepatocytes. AAPS J. 2020, 22, 131. [Google Scholar] [CrossRef]
- Ginai, M.; Elsby, R.; Hewitt, C.J.; Surry, D.; Fenner, K.; Coopman, K. The Use of Bioreactors as in Vitro Models in Pharmaceutical Research. Drug Discov. Today 2013, 18, 922–935. [Google Scholar] [CrossRef]
- Prantil-Baun, R.; Novak, R.; Das, D.; Somayaji, M.R.; Przekwas, A.; Ingber, D.E. Physiologically Based Pharmacokinetic and Pharmacodynamic Analysis Enabled by Microfluidically Linked Organs-on-Chips. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 37–64. [Google Scholar] [CrossRef]
- Campbell, J.L.; Andersen, M.E.; Clewell, H.J. A Hybrid CFD-PBPK Model for Naphthalene in Rat and Human with IVIVE for Nasal Tissue Metabolism and Cross-Species Dosimetry. Inhal. Toxicol. 2014, 26, 333–344. [Google Scholar] [CrossRef]
- Jones, C.R.; Hatley, O.J.D.; Ungell, A.-L.; Hilgendorf, C.; Peters, S.A.; Rostami-Hodjegan, A. Gut Wall Metabolism. Application of Pre-Clinical Models for the Prediction of Human Drug Absorption and First-Pass Elimination. AAPS J. 2016, 18, 589–604. [Google Scholar] [CrossRef]
- Scotcher, D.; Billington, S.; Brown, J.; Jones, C.R.; Brown, C.D.A.; Rostami-Hodjegan, A.; Galetin, A. Microsomal and Cytosolic Scaling Factors in Dog and Human Kidney Cortex and Application for In Vitro-In Vivo Extrapolation of Renal Metabolic Clearance. Drug Metab. Dispos. 2017, 45, 556–568. [Google Scholar] [CrossRef]
- Yang, Y.; Himmelstein, M.W.; Clewell, H.J. Kinetic Modeling of Beta-Chloroprene Metabolism: Probabilistic in Vitro-in Vivo Extrapolation of Metabolism in the Lung, Liver and Kidneys of Mice, Rats and Humans. Toxicol. In Vitro 2012, 26, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Camenisch, G.P. Drug Disposition Classification Systems in Discovery and Development: A Comparative Review of the BDDCS, ECCS and ECCCS Concepts. Pharm. Res. 2016, 33, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Chowdhury, S.K.; Yucha, R.; Kelly, E.J.; Xiao, G. Emerging Kidney Models to Investigate Metabolism, Transport, and Toxicity of Drugs and Xenobiotics. Drug Metab. Dispos. Biol. Fate Chem. 2018, 46, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.; Bouzom, F.; Scherrmann, J.M.; Walther, B.; Decleves, X. Physiologically Based Pharmacokinetic Modelling of Drug Penetration across the Blood-Brain Barrier--towards a Mechanistic IVIVE-Based Approach. AAPS J. 2013, 15, 913–932. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.; Ye, M.; Nagar, S.; Korzekwa, K. Prediction of Tissue-Plasma Partition Coefficients Using Microsomal Partitioning: Incorporation into Physiologically Based Pharmacokinetic Models and Steady-State Volume of Distribution Predictions. Drug Metab. Dispos. 2019, 47, 1050–1060. [Google Scholar] [CrossRef]
- Jaroch, K.; Jaroch, A.; Bojko, B. Cell Cultures in Drug Discovery and Development: The Need of Reliable in Vitro-in Vivo Extrapolation for Pharmacodynamics and Pharmacokinetics Assessment. J. Pharm. Biomed. Anal. 2018, 147, 297–312. [Google Scholar] [CrossRef]
- Lu, J.; Goldsmith, M.-R.; Grulke, C.M.; Chang, D.T.; Brooks, R.D.; Leonard, J.A.; Phillips, M.B.; Hypes, E.D.; Fair, M.J.; Tornero-Velez, R.; et al. Developing a Physiologically-Based Pharmacokinetic Model Knowledgebase in Support of Provisional Model Construction. PLoS Comput. Biol. 2016, 12, e1004495. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Nagar, S.; Korzekwa, K. A Physiologically Based Pharmacokinetic Model to Predict the Pharmacokinetics of Highly Protein-Bound Drugs and Impact of Errors in Plasma Protein Binding. Biopharm. Drug Dispos. 2016, 37, 123–141. [Google Scholar] [CrossRef]
- Nagar, S.; Korzekwa, R.C.; Korzekwa, K. Continuous Intestinal Absorption Model Based on the Convection–Diffusion Equation. Mol. Pharm. 2017, 14, 3069–3086. [Google Scholar] [CrossRef]
- Williamson, B.; Harlfinger, S.; McGinnity, D.F. Evaluation of the Disconnect between Hepatocyte and Microsome Intrinsic Clearance and in Vitro in Vivo Extrapolation Performance. Drug Metab. Dispos. 2020, 48, 1137–1146. [Google Scholar] [CrossRef]
- Proctor, N.; Tucker, G.; Rostami-Hodjegan, A. Predicting Drug Clearance from Recombinantly Expressed CYPs: Intersystem Extrapolation Factors. Xenobiotica 2004, 34, 151–178. [Google Scholar] [CrossRef] [PubMed]
- Stadnicka-Michalak, J.; Tanneberger, K.; Schirmer, K.; Ashauer, R. Measured and Modeled Toxicokinetics in Cultured Fish Cells and Application to in Vitro-in Vivo Toxicity Extrapolation. PLoS ONE 2014, 9, e92303. [Google Scholar] [CrossRef] [PubMed]
- Black, S.R.; Nichols, J.W.; Fay, K.A.; Matten, S.R.; Lynn, S.G. Evaluation and Comparison of in Vitro Intrinsic Clearance Rates Measured Using Cryopreserved Hepatocytes from Humans, Rats, and Rainbow Trout. Toxicology 2021, 457, 152819. [Google Scholar] [CrossRef] [PubMed]
- Mebust, M.; Crawford-Brown, D.; Hofmann, W.; Schollnberger, H. Testing Extrapolation of a Biologically Based Exposure-Response Model from in Vitro to in Vivo Conditions. Regul. Toxicol. Pharmacol. 2002, 35, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Polak, S.; Wisniowska, B.; Fijorek, K.; Glinka, A.; Mendyk, A. In Vitro-in Vivo Extrapolation of Drug-Induced Proarrhythmia Predictions at the Population Level. Drug Discov. Today 2014, 19, 275–281. [Google Scholar] [CrossRef]
- Tong, S.; Sun, H.; Xue, C.; Chen, H.; Liu, J.; Yang, H.; Zhou, N.; Xiang, X.; Cai, W. Establishment and Assessment of a Novel in Vitro Bio-PK/PD System in Predicting the in Vivo Pharmacokinetics and Pharmacodynamics of Cyclophosphamide. Xenobiotica 2018, 48, 368–375. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, R.; Wen, Z.; Li, M. Narrowing the Gap between in Vitro and in Vivo Genetic Profiles by Deconvoluting Toxicogenomic Data in Silico. Front. Pharmacol. 2019, 10, 1489. [Google Scholar] [CrossRef]
- Kavlock, R.J.; Bahadori, T.; Barton-Maclaren, T.S.; Gwinn, M.R.; Rasenberg, M.; Thomas, R.S. Accelerating the Pace of Chemical Risk Assessment. Chem. Res. Toxicol. 2018, 31, 287–290. [Google Scholar] [CrossRef]
- Abdo, N.; Wetmore, B.A.; Chappell, G.A.; Shea, D.; Wright, F.A.; Rusyn, I. In Vitro Screening for Population Variability in Toxicity of Pesticide-Containing Mixtures. Environ. Int. 2015, 85, 147–155. [Google Scholar] [CrossRef]
- Testai, E.; Bechaux, C.; Buratti, F.M.; Darney, K.; Di Consiglio, E.; Kasteel, E.E.J.; Kramer, N.I.; Lautz, L.S.; Santori, N.; Skaperda, Z.-V.; et al. Modelling Human Variability in Toxicokinetic and Toxicodynamic Processes Using Bayesian Meta-Analysis, Physiologically-Based Modelling and in Vitro Systems. EFSA Support. Publ. 2021, 18, 6504E. [Google Scholar] [CrossRef]
- El-Masri, H.; Kleinstreuer, N.; Hines, R.N.; Adams, L.; Tal, T.; Isaacs, K.; Wetmore, B.A.; Tan, Y.M. Integration of Life-Stage Physiologically Based Pharmacokinetic Models with Adverse Outcome Pathways and Environmental Exposure Models to Screen for Environmental Hazards. Toxicol. Sci. 2016, 152, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Valdiviezo, A.; Luo, Y.-S.; Chen, Z.; Chiu, W.A.; Rusyn, I. Quantitative in Vitro-to-in Vivo Extrapolation for Mixtures: A Case Study of Superfund Priority List Pesticides. Toxicol. Sci. Off. J. Soc. Toxicol. 2021, 183, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Genschow, E.; Spielmann, H.; Scholz, G.; Seiler, A.; Brown, N.; Piersma, A.; Brady, M.; Clemann, N.; Huuskonen, H.; Paillard, F.; et al. The ECVAM International Validation Study on In Vitro Embryotoxicity Tests: Results of the Definitive Phase and Evaluation of Prediction Models. Altern. Lab. Anim. 2002, 30, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Louisse, J.; Verwei, M.; Woutersen, R.A.; Blaauboer, B.J.; Rietjens, I.M. Toward in Vitro Biomarkers for Developmental Toxicity and Their Extrapolation to the in Vivo Situation. Expert Opin. Drug Metab. Toxicol. 2012, 8, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.A.; Smith, A.M.; Egnash, L.A.; Conard, K.R.; West, P.R.; Burrier, R.E.; Donley, E.L.R.; Kirchner, F.R. Establishment and Assessment of a New Human Embryonic Stem Cell-Based Biomarker Assay for Developmental Toxicity Screening. Birth Defects Res. B Dev. Reprod. Toxicol. 2013, 98, 343–363. [Google Scholar] [CrossRef]
- Strikwold, M.; Spenkelink, B.; de Haan, L.H.J.; Woutersen, R.A.; Punt, A.; Rietjens, I. Integrating in Vitro Data and Physiologically Based Kinetic (PBK) Modelling to Assess the in Vivo Potential Developmental Toxicity of a Series of Phenols. Arch. Toxicol. 2017, 91, 2119–2133. [Google Scholar] [CrossRef]
- Strikwold, M.; Woutersen, R.A.; Spenkelink, B.; Punt, A.; Rietjens, I.M.C.M. Relative Embryotoxic Potency of P-Substituted Phenols in the Embryonic Stem Cell Test (EST) and Comparison to Their Toxic Potency in Vivo and in the Whole Embryo Culture (WEC) Assay. Toxicol. Lett. 2012, 213, 235–242. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Vervoort, J.; Rietjens, I.M.; van Ravenzwaay, B.; Louisse, J. Use of Physiologically Based Kinetic Modeling-Facilitated Reverse Dosimetry of in Vitro Toxicity Data for Prediction of in Vivo Developmental Toxicity of Tebuconazole in Rats. Toxicol. Lett. 2017, 266, 85–93. [Google Scholar] [CrossRef]
- Li, H.; Rietjens, I.M.C.M.; Louisse, J.; Blok, M.; Wang, X.; Snijders, L.; van Ravenzwaay, B. Use of the ES-D3 Cell Differentiation Assay, Combined with the BeWo Transport Model, to Predict Relative in Vivo Developmental Toxicity of Antifungal Compounds. Toxicol. In Vitro 2015, 29, 320–328. [Google Scholar] [CrossRef]
- Strikwold, M.; Spenkelink, B.; Woutersen, R.A.; Rietjens, I.; Punt, A. Development of a Combined In Vitro Physiologically Based Kinetic (PBK) and Monte Carlo Modelling Approach to Predict Interindividual Human Variation in Phenol-Induced Developmental Toxicity. Toxicol. Sci. 2017, 157, 365–376. [Google Scholar] [CrossRef]
- Conley, J.M.; Hannas, B.R.; Furr, J.R.; Wilson, V.S.; Gray, L.E., Jr. A Demonstration of the Uncertainty in Predicting the Estrogenic Activity of Individual Chemicals and Mixtures from an in Vitro Estrogen Receptor Transcriptional Activation Assay (T47D-KBluc) to the in Vivo Uterotrophic Assay Using Oral Exposure. Toxicol. Sci. 2016, 153, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.N.; Laws, S.C.; Willett, K.; Schmieder, P.; Odum, J.; Bovee, T.F. In Vitro Metabolism and Bioavailability Tests for Endocrine Active Substances: What Is Needed next for Regulatory Purposes? Altex 2013, 30, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.S.; Magpantay, F.M.; Chickarmane, V.; Haskell, C.; Tania, N.; Taylor, J.; Xia, M.; Huang, R.; Rotroff, D.M.; Filer, D.L.; et al. Integrated Model of Chemical Perturbations of a Biological Pathway Using 18 In Vitro High-Throughput Screening Assays for the Estrogen Receptor. Toxicol. Sci. 2015, 148, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Kleinstreuer, N.; Ceger, P.; Hsieh, J.H.; Allen, D.; Warren, C.W. Application of Reverse Dosimetry to Compare In Vitro and In Vivo Estrogen Receptor Activity. Appl. In Vitro Toxicol. 2015, 1, 33–44. [Google Scholar] [CrossRef]
- Zhang, M.Y.; van Ravenzwaay, B.; Fabian, E.; Rietjens, I.; Louisse, J. Towards a Generic Physiologically Based Kinetic Model to Predict in Vivo Uterotrophic Responses in Rats by Reverse Dosimetry of in Vitro Estrogenicity Data. Arch. Toxicol. 2018, 92, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Beames, T.; Moreau, M.; Roberts, L.A.; Mansouri, K.; Haider, S.; Smeltz, M.; Nicolas, C.I.; Doheny, D.; Phillips, M.B.; Yoon, M.; et al. The Role of Fit-for-Purpose Assays within Tiered Testing Approaches: A Case Study Evaluating Prioritized Estrogen-Active Compounds in an in Vitro Human Uterotrophic Assay. Toxicol. Appl. Pharmacol. 2020, 387, 114774. [Google Scholar] [CrossRef]
- Punt, A.; Aartse, A.; Bovee, T.F.H.; Gerssen, A.; van Leeuwen, S.P.J.; Hoogenboom, R.; Peijnenburg, A. Quantitative in Vitro-to-in Vivo Extrapolation (QIVIVE) of Estrogenic and Anti-Androgenic Potencies of BPA and BADGE Analogues. Arch. Toxicol. 2019, 93, 1941–1953. [Google Scholar] [CrossRef]
- Jones, H.M.; Gardner, I.B.; Watson, K.J. Modelling and PBPK Simulation in Drug Discovery. AAPS J. 2009, 11, 155–166. [Google Scholar] [CrossRef]
- Malmborg, J.; Ploeger, B.A. Predicting Human Exposure of Active Drug after Oral Prodrug Administration, Using a Joined in Vitro/in Silico-in Vivo Extrapolation and Physiologically-Based Pharmacokinetic Modeling Approach. J. Pharmacol. Toxicol. Methods 2013, 67, 203–213. [Google Scholar] [CrossRef]
- Worley, R.R.; Fisher, J. Application of Physiologically-Based Pharmacokinetic Modeling to Explore the Role of Kidney Transporters in Renal Reabsorption of Perfluorooctanoic Acid in the Rat. Toxicol. Appl. Pharmacol. 2015, 289, 428–441. [Google Scholar] [CrossRef]
- Nakagawa, H.; Hirata, T.; Terada, T.; Jutabha, P.; Miura, D.; Harada, K.H.; Inoue, K.; Anzai, N.; Endou, H.; Inui, K.-I.; et al. Roles of Organic Anion Transporters in the Renal Excretion of Perfluorooctanoic Acid. Basic Clin. Pharmacol. Toxicol. 2008, 103, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Weaver, Y.M.; Ehresman, D.J.; Butenhoff, J.L.; Hagenbuch, B. Roles of Rat Renal Organic Anion Transporters in Transporting Perfluorinated Carboxylates with Different Chain Lengths. Toxicol. Sci. 2010, 113, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, A.; Ince, I.; Coboeken, K.; Eissing, T.; Hempel, G. A Physiologically Based Pharmacokinetic Model for Pregnant Women to Predict the Pharmacokinetics of Drugs Metabolized Via Several Enzymatic Pathways. Clin. Pharmacokinet. 2018, 57, 749–768. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, A.; Ince, I.; Meyer, M.; Willmann, S.; Eissing, T.; Hempel, G. Gestation-Specific Changes in the Anatomy and Physiology of Healthy Pregnant Women: An Extended Repository of Model Parameters for Physiologically Based Pharmacokinetic Modeling in Pregnancy. Clin. Pharmacokinet. 2017, 56, 1303–1330. [Google Scholar] [CrossRef]
- Johnson, T.N.; Rostami-Hodjegan, A. Resurgence in the Use of Physiologically Based Pharmacokinetic Models in Pediatric Clinical Pharmacology: Parallel Shift in Incorporating the Knowledge of Biological Elements and Increased Applicability to Drug Development and Clinical Practice. Paediatr. Anaesth. 2011, 21, 291–301. [Google Scholar] [CrossRef]
- Kapraun, D.F.; Wambaugh, J.F.; Setzer, R.W.; Judson, R.S. Empirical Models for Anatomical and Physiological Changes in a Human Mother and Fetus during Pregnancy and Gestation. PLoS ONE 2019, 14, e0215906. [Google Scholar] [CrossRef]
- Mallick, P.; Moreau, M.; Song, G.; Efremenko, A.Y.; Pendse, S.N.; Creek, M.R.; Osimitz, T.G.; Hines, R.N.; Hinderliter, P.; Clewell, H.J.; et al. Development and Application of a Life-Stage Physiologically Based Pharmacokinetic (PBPK) Model to the Assessment of Internal Dose of Pyrethroids in Humans. Toxicol. Sci. 2020, 173, 86–99. [Google Scholar] [CrossRef]
- Barter, Z.E.; Tucker, G.T.; Rowland-Yeo, K. Response to “Ethnic-Specific in Vitro-in Vivo Extrapolation and Physiologically Based Pharmacokinetic Approaches to Predict Cytochrome P450-Mediated Pharmacokinetics in Chinese Population: Opportunities and Challenges. ” Clin. Pharmacokinet. 2014, 53, 203. [Google Scholar] [CrossRef]
- Barter, Z.E.; Tucker, G.T.; Rowland-Yeo, K. Differences in Cytochrome P450-Mediated Pharmacokinetics between Chinese and Caucasian Populations Predicted by Mechanistic Physiologically Based Pharmacokinetic Modelling. Clin. Pharmacokinet. 2013, 52, 1085–1100. [Google Scholar] [CrossRef]
- Li, G.F.; Yu, G.; Liu, H.X.; Zheng, Q.S. Ethnic-Specific in Vitro-in Vivo Extrapolation and Physiologically Based Pharmacokinetic Approaches to Predict Cytochrome P450-Mediated Pharmacokinetics in the Chinese Population: Opportunities and Challenges. Clin. Pharmacokinet. 2014, 53, 197–202. [Google Scholar] [CrossRef]
- Reale, M.; Costantini, E.; Di Nicola, M.; D’Angelo, C.; Franchi, S.; D’Aurora, M.; Di Bari, M.; Orlando, V.; Galizia, S.; Ruggieri, S.; et al. Butyrylcholinesterase and Acetylcholinesterase Polymorphisms in Multiple Sclerosis Patients: Implication in Peripheral Inflammation. Sci. Rep. 2018, 8, 1319. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Ceger, P.; Allen, D.; Coyle, J.; Derk, R.; Garcia-Reyero, N.; Gordon, J.; Kleinstreuer, N.; Matheson, J.; McShan, D.; et al. U.S. Federal Agency Interests and Key Considerations for New Approach Methodologies for Nanomaterials. ALTEX-Altern. Anim. Exp. 2021, 39, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Sharma, M.; Clippinger, A.J.; Gordon, J.; Katz, A.; Laux, P.; Leibrock, L.B.; Luch, A.; Matheson, J.; Stucki, A.O.; et al. Use of Cause-and-Effect Analysis to Optimize the Reliability of In Vitro Inhalation Toxicity Measurements Using an Air–Liquid Interface. Chem. Res. Toxicol. 2021, 34, 1370–1385. [Google Scholar] [CrossRef]
- Leibrock, L.B.; Jungnickel, H.; Tentschert, J.; Katz, A.; Toman, B.; Petersen, E.J.; Bierkandt, F.S.; Singh, A.V.; Laux, P.; Luch, A. Parametric Optimization of an Air–Liquid Interface System for Flow-Through Inhalation Exposure to Nanoparticles: Assessing Dosimetry and Intracellular Uptake of CeO2 Nanoparticles. Nanomaterials 2020, 10, 2369. [Google Scholar] [CrossRef]
- Kooter, I.M.; Gröllers-Mulderij, M.; Steenhof, M.; Duistermaat, E.; van Acker, F.A.A.; Staal, Y.C.M.; Tromp, P.C.; Schoen, E.; Kuper, C.F.; van Someren, E. Cellular Effects in an In Vitro Human 3D Cellular Airway Model and A549/BEAS-2B In Vitro Cell Cultures Following Air Exposure to Cerium Oxide Particles at an Air–Liquid Interface. Appl. In Vitro Toxicol. 2016, 2, 56–66. [Google Scholar] [CrossRef]
- Lenz, A.-G.; Karg, E.; Brendel, E.; Hinze-Heyn, H.; Maier, K.L.; Eickelberg, O.; Stoeger, T.; Schmid, O. Inflammatory and Oxidative Stress Responses of an Alveolar Epithelial Cell Line to Airborne Zinc Oxide Nanoparticles at the Air-Liquid Interface: A Comparison with Conventional, Submerged Cell-Culture Conditions. Available online: https://www.hindawi.com/journals/bmri/2013/652632/ (accessed on 5 March 2021).
- Petersen, E.J.; Flores-Cervantes, D.X.; Bucheli, T.D.; Elliott, L.C.C.; Fagan, J.A.; Gogos, A.; Hanna, S.; Kägi, R.; Mansfield, E.; Bustos, A.R.M.; et al. Quantification of Carbon Nanotubes in Environmental Matrices: Current Capabilities, Case Studies, and Future Prospects. Environ. Sci. Technol. 2016, 50, 4587–4605. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Adeleye, A.S.; Sung, L.; Ho, K.T.; Burgess, R.M.; Petersen, E.J. Detection and Quantification of Graphene-Family Nanomaterials in the Environment. Environ. Sci. Technol. 2018, 52, 4491–4513. [Google Scholar] [CrossRef]
- Hinderliter, P.M.; Minard, K.R.; Orr, G.; Chrisler, W.B.; Thrall, B.D.; Pounds, J.G.; Teeguarden, J.G. ISDD: A Computational Model of Particle Sedimentation, Diffusion and Target Cell Dosimetry for in Vitro Toxicity Studies. Part. Fibre Toxicol. 2010, 7, 36. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Pirela, S.V.; Pal, A.; Liu, J.; Srebric, J.; Demokritou, P. Advanced Computational Modeling for in Vitro Nanomaterial Dosimetry. Part. Fibre Toxicol. 2015, 12, 32. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, Characterization, and in Vitro Dosimetry of Dispersed, Engineered Nanomaterials. Nat. Protoc. 2017, 12, 355–371. [Google Scholar] [CrossRef]
- Petersen, E.J.; Bustos, A.R.M.; Toman, B.; Johnson, M.E.; Ellefson, M.; Caceres, G.C.; Neuer, A.L.; Chan, Q.; Kemling, J.W.; Mader, B.; et al. Determining What Really Counts: Modeling and Measuring Nanoparticle Number Concentrations. Environ. Sci. Nano 2019, 6, 2876–2896. [Google Scholar] [CrossRef]
- Thomas, D.G.; Smith, J.N.; Thrall, B.D.; Baer, D.R.; Jolley, H.; Munusamy, P.; Kodali, V.; Demokritou, P.; Cohen, J.; Teeguarden, J.G. ISD3: A Particokinetic Model for Predicting the Combined Effects of Particle Sedimentation, Diffusion and Dissolution on Cellular Dosimetry for in Vitro Systems. Part. Fibre Toxicol. 2018, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Clippinger, A.J.; Allen, D.; Behrsing, H.; BéruBé, K.A.; Bolger, M.B.; Casey, W.; DeLorme, M.; Gaça, M.; Gehen, S.C.; Glover, K.; et al. Pathway-Based Predictive Approaches for Non-Animal Assessment of Acute Inhalation Toxicity. Toxicol. In Vitro 2018, 52, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Clippinger, A.J.; Ahluwalia, A.; Allen, D.; Bonner, J.C.; Casey, W.; Castranova, V.; David, R.M.; Halappanavar, S.; Hotchkiss, J.A.; Jarabek, A.M.; et al. Expert Consensus on an in Vitro Approach to Assess Pulmonary Fibrogenic Potential of Aerosolized Nanomaterials. Arch. Toxicol. 2016, 90, 1769–1783. [Google Scholar] [CrossRef]
- Ma-Hock, L.; Sauer, U.G.; Ruggiero, E.; Keller, J.-G.; Wohlleben, W.; Landsiedel, R. The Use of Nanomaterial In Vivo Organ Burden Data for In Vitro Dose Setting. Small 2021, 17, 2005725. [Google Scholar] [CrossRef]
- OECD. Test No. 413. Subchronic Inhalation Toxicity: 90-Day Study. In OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects; OECD Publishing: Paris, France, 2009. [Google Scholar]
- EPAA. European Partnership for Alternative Approaches to Animal Testing. Available online: https://ec.europa.eu/growth/sectors/chemicals/epaa_en (accessed on 14 April 2021).
- Cozigou, G.; Crozier, J.; Hendriksen, C.; Manou, I.; Ramirez-Hernandez, T.; Weissenhorn, R. The European Partnership for Alternative Approaches to Animal Testing (EPAA): Promoting Alternative Methods in Europe and Beyond. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 209–213. [Google Scholar]
- Kuepfer, L.; Niederalt, C.; Wendl, T.; Schlender, J.; Willmann, S.; Lippert, J.; Block, M.; Eissing, T.; Teutonico, D. Applied Concepts in PBPK Modeling: How to Build a PBPK/PD Model. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 516–531. [Google Scholar] [CrossRef]
- Sushko, I.; Novotarskyi, S.; Körner, R.; Pandey, A.K.; Rupp, M.; Teetz, W.; Brandmaier, S.; Abdelaziz, A.; Prokopenko, V.V.; Tanchuk, V.Y.; et al. Online Chemical Modeling Environment (OCHEM): Web Platform for Data Storage, Model Development and Publishing of Chemical Information. J. Comput.-Aided Mol. Des. 2011, 25, 533–554. [Google Scholar] [CrossRef]
- Pence, H.E.; Williams, A. ChemSpider: An Online Chemical Information Resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A Large-Scale Bioactivity Database for Drug Discovery. Nucleic Acids Res. 2012, 40, D1100–D1107. [Google Scholar] [CrossRef]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A Community Data Resource for Environmental Chemistry. J. Cheminform. 2017, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.M.; Williams, C.R. Distributed Structure-Searchable Toxicity (DSSTox) Public Database Network: A Proposal. Mutat. Res. 2002, 499, 27–52. [Google Scholar] [CrossRef]
- Grulke, C.M.; Williams, A.J.; Thillanadarajah, I.; Richard, A.M. EPA’s DSSTox Database: History of Development of a Curated Chemistry Resource Supporting Computational Toxicology Research. Comput. Toxicol. 2019, 12, 100096. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Ekins, S. A Quality Alert and Call for Improved Curation of Public Chemistry Databases. Drug Discov. Today 2011, 16, 747–750. [Google Scholar] [CrossRef]
- Kim, S. Exploring Chemical Information in PubChem. Curr. Protoc. 2021, 1, e217. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Madden, J.C.; Pawar, G.; Cronin, M.T.D.; Webb, S.; Tan, Y.-M.; Paini, A. In Silico Resources to Assist in the Development and Evaluation of Physiologically-Based Kinetic Models. Comput. Toxicol. 2019, 11, 33–49. [Google Scholar] [CrossRef]
- Pawar, G.; Madden, J.C.; Ebbrell, D.; Firman, J.W.; Cronin, M.T.D. In Silico Toxicology Data Resources to Support Read-Across and (Q)SAR. Front. Pharmacol. 2019, 10, 561. [Google Scholar] [CrossRef]
- Scotcher, D.; Jones, C.; Posada, M.; Rostami-Hodjegan, A.; Galetin, A. Key to Opening Kidney for In Vitro-In Vivo Extrapolation Entrance in Health and Disease: Part I: In Vitro Systems and Physiological Data. AAPS J. 2016, 18, 1067–1081. [Google Scholar] [CrossRef]
- Scotcher, D.; Jones, C.; Posada, M.; Galetin, A.; Rostami-Hodjegan, A. Key to Opening Kidney for In Vitro-In Vivo Extrapolation Entrance in Health and Disease: Part II: Mechanistic Models and In Vitro-In Vivo Extrapolation. AAPS J. 2016, 18, 1082–1094. [Google Scholar] [CrossRef]
- Clippinger, A.J.; Allen, D.; Jarabek, A.M.; Corvaro, M.; Gaça, M.; Gehen, S.; Hotchkiss, J.A.; Patlewicz, G.; Melbourne, J.; Hinderliter, P.; et al. Alternative Approaches for Acute Inhalation Toxicity Testing to Address Global Regulatory and Non-Regulatory Data Requirements: An International Workshop Report. Toxicol. In Vitro 2018, 48, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Chilton, M.L.; Sartini, A.; Gibson, L.; Barber, C.; Covey-Crump, L.; Przybylak, K.R.; Cronin, M.T.D.; Madden, J.C. Assessment and Reproducibility of Quantitative Structure-Activity Relationship Models by the Nonexpert. J. Chem. Inf. Model. 2018, 58, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, A.; Brandmaier, S.; Huijbregts, M.A.; Ragas, A.M.; Veltman, K.; Hendriks, A.J. QSARs for Estimating Intrinsic Hepatic Clearance of Organic Chemicals in Humans. Environ. Toxicol. Pharmacol. 2016, 42, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, A.; Brandmaier, S.; Huijbregts, M.A.J.; Ragas, A.M.J.; Veltman, K.; Hendriks, A.J. The Utilisation of Structural Descriptors to Predict Metabolic Constants of Xenobiotics in Mammals. Environ. Toxicol. Pharmacol. 2015, 39, 247–258. [Google Scholar] [CrossRef]
- Polasek, T.M.; Patel, F.; Jensen, B.P.; Sorich, M.J.; Wiese, M.D.; Doogue, M.P. Predicted Metabolic Drug Clearance with Increasing Adult Age. Br. J. Clin. Pharmacol. 2013, 75, 1019–1028. [Google Scholar] [CrossRef]
- Bhatt, D.K.; Gaedigk, A.; Pearce, R.E.; Leeder, J.S.; Prasad, B. Age-Dependent Protein Abundance of Cytosolic Alcohol and Aldehyde Dehydrogenases in Human Liver. Drug Metab. Dispos. Biol. Fate Chem. 2017, 45, 1044–1048. [Google Scholar] [CrossRef]
- Barter, Z.E.; Bayliss, M.K.; Beaune, P.H.; Boobis, A.R.; Carlile, D.J.; Edwards, R.J.; Houston, J.B.; Lake, B.G.; Lipscomb, J.C.; Pelkonen, O.R.; et al. Scaling Factors for the Extrapolation of in Vivo Metabolic Drug Clearance from in Vitro Data: Reaching a Consensus on Values of Human Microsomal Protein and Hepatocellularity per Gram of Liver. Curr. Drug Metab. 2007, 8, 33–45. [Google Scholar] [CrossRef]
- Lipscomb, J.C.; Poet, T.S. In Vitro Measurements of Metabolism for Application in Pharmacokinetic Modeling. Pharmacol. Ther. 2008, 118, 82–103. [Google Scholar] [CrossRef]
- Rostami-Hodjegan, A.; Tucker, G.T. Simulation and Prediction of in Vivo Drug Metabolism in Human Populations from in Vitro Data. Nat. Rev. Drug Discov. 2007, 6, 140–148. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, N.; Tian, X.; Liu, T.; Fang, Y.; Zhou, J.; Wen, Q.; Xu, B.; Qi, B.; Gao, J.; et al. Content and Activity of Human Liver Microsomal Protein and Prediction of Individual Hepatic Clearance in Vivo. Sci. Rep. 2015, 5, 17671. [Google Scholar] [CrossRef]
- Wilson, Z.E.; Rostami-Hodjegan, A.; Burn, J.L.; Tooley, A.; Boyle, J.; Ellis, S.W.; Tucker, G.T. Inter-Individual Variability in Levels of Human Microsomal Protein and Hepatocellularity per Gram of Liver. Br. J. Clin. Pharmacol. 2003, 56, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.E.; Cotton, C.A.; Edginton, A.N. Development of a Decision Tree to Classify the Most Accurate Tissue-Specific Tissue to Plasma Partition Coefficient Algorithm for a given Compound. J. Pharmacokinet. Pharmacodyn. 2014, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Buist, H.E.; de Wit-Bos, L.; Bouwman, T.; Vaes, W.H.J. Predicting Blood:Air Partition Coefficients Using Basic Physicochemical Properties. Regul. Toxicol. Pharmacol. 2012, 62, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.P.; Kenny, L.C. Comparison of Models for the Estimation of Biological Partition Coefficients. J. Toxicol. Environ. Health Part A 2002, 65, 897–931. [Google Scholar] [CrossRef] [PubMed]
- Sarigiannis, D.A.; Papadaki, K.; Kontoroupis, P.; Karakitsios, S.P. Development of QSARs for Parameterizing Physiology Based ToxicoKinetic Models. Food Chem. Toxicol. 2017, 106, 114–124. [Google Scholar] [CrossRef]
- Pearce, R.G.; Setzer, R.W.; Davis, J.L.; Wambaugh, J.F. Evaluation and Calibration of High-Throughput Predictions of Chemical Distribution to Tissues. J. Pharmacokinet. Pharmacodyn. 2017, 44, 549–565. [Google Scholar] [CrossRef]
- Bell, S.; Abedini, J.; Ceger, P.; Chang, X.; Cook, B.; Karmaus, A.L.; Lea, I.; Mansouri, K.; Phillips, J.; McAfee, E.; et al. An Integrated Chemical Environment with Tools for Chemical Safety Testing. Toxicol. In Vitro 2020, 67, 104916. [Google Scholar] [CrossRef]
- Krishna, S.; Berridge, B.; Kleinstreuer, N. High-Throughput Screening to Identify Chemical Cardiotoxic Potential. Chem. Res. Toxicol. 2021, 34, 566–583. [Google Scholar] [CrossRef]
- Mansouri, K.; Grulke, C.M.; Judson, R.S.; Williams, A.J. OPERA Models for Predicting Physicochemical Properties and Environmental Fate Endpoints. J. Cheminform. 2018, 10, 10. [Google Scholar] [CrossRef]
- Punt, A.; Pinckaers, N.; Peijnenburg, A.; Louisse, J. Development of a Web-Based Toolbox to Support Quantitative In-Vitro-to-In-Vivo Extrapolations (QIVIVE) within Nonanimal Testing Strategies. Chem. Res. Toxicol. 2021, 34, 460–472. [Google Scholar] [CrossRef]
- Richard, A.M.; Judson, R.S.; Houck, K.A.; Grulke, C.M.; Volarath, P.; Thillainadarajah, I.; Yang, C.; Rathman, J.; Martin, M.T.; Wambaugh, J.F.; et al. ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chem. Res. Toxicol. 2016, 29, 1225–1251. [Google Scholar] [CrossRef] [PubMed]
- Jamei, M.; Marciniak, S.; Feng, K.; Barnett, A.; Tucker, G.; Rostami-Hodjegan, A. The Simcyp® Population-Based ADME Simulator. Expert Opin. Drug Metab. Toxicol. 2009, 5, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, T.; Lakshminarayana, S.B.; Hu, W.; He, H. Practical Anticipation of Human Efficacious Doses and Pharmacokinetics Using in Vitro and Preclinical in Vivo Data. AAPS J. 2009, 11, 602–614. [Google Scholar] [CrossRef] [PubMed]
- Eissing, T.; Kuepfer, L.; Becker, C.; Block, M.; Coboeken, K.; Gaub, T.; Goerlitz, L.; Jaeger, J.; Loosen, R.; Ludewig, B.; et al. A Computational Systems Biology Software Platform for Multiscale Modeling and Simulation: Integrating Whole-Body Physiology, Disease Biology, and Molecular Reaction Networks. Front. Physiol. 2011, 2, 4. [Google Scholar] [CrossRef]
- Leahy, D.E. Integrating in Vitro ADMET Data through Generic Physiologically Based Pharmacokinetic Models. Expert Opin Drug Metab. Toxicol. 2006, 2, 619–628. [Google Scholar] [CrossRef]
- International Programme on Chemical Safety & Inter-Organization Programme for the Sound Management of Chemicals. Chemicals, I.-O.P. for the S.M. of Characterization and Application of Physiologically Based Phamacokinetic Models in Risk Assessment; IPCS harmonization project document; no. 9; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-4-150090-6. [Google Scholar]
- Pendse, S.N.; Efremenko, A.; Hack, C.E.; Moreau, M.; Mallick, P.; Dzierlenga, M.; Nicolas, C.I.; Yoon, M.; Clewell, H.J.; McMullen, P.D. Population Life-Course Exposure to Health Effects Model (PLETHEM): An R Package for PBPK Modeling. Comput. Toxicol. 2020, 13, 100115. [Google Scholar] [CrossRef]
- Lipscomb, J.C.; Kedderis, G.L. Incorporating Human Interindividual Biotransformation Variance in Health Risk Assessment. Sci. Total Environ. 2002, 288, 13–21. [Google Scholar] [CrossRef]
- U.S. EPA. Chemical Safety for Sustainability National Research Program: Strategic Research Action Plan 2019-2022; EPA 601 K20001; U.S. Environmental Protection Agency: Washington, DC, USA, 2020.
- Yun, Y.E.; Tornero-Velez, R.; Purucker, S.T.; Chang, D.T.; Edginton, A.N. Evaluation of Quantitative Structure Property Relationship Algorithms for Predicting Plasma Protein Binding in Humans. Comput. Toxicol. 2021, 17, 100142. [Google Scholar] [CrossRef]
- Barnes, D.G.; Dourson, M. Reference Dose (RfD): Description and Use in Health Risk Assessments. Regul. Toxicol. Pharmacol. 1988, 8, 471–486. [Google Scholar] [CrossRef]
- Dourson, M.L.; Felter, S.P.; Robinson, D. Evolution of Science-Based Uncertainty Factors in Noncancer Risk Assessment. Regul. Toxicol. Pharmacol. 1996, 24, 108–120. [Google Scholar] [CrossRef]
- Dourson, M.L.; York, R.G. Advances in Assessing Ingredient Safety. Regul. Toxicol. Pharmacol. 2016, 79 (Suppl. 2), S112–S118. [Google Scholar] [CrossRef] [PubMed]
- Emami, J. In Vitro-in Vivo Correlation: From Theory to Applications. J. Pharm. Pharm. Sci. 2006, 9, 169–189. [Google Scholar] [PubMed]
- Casey, W.; Jacobs, A.; Maull, E.; Matheson, J.; Clarke, C.; Lowit, A. A New Path Forward: The Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) and National Toxicology Program’s Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 170–173. [Google Scholar] [PubMed]
- Hartung, T. Lessons Learned from Alternative Methods and Their Validation for a New Toxicology in the 21st Century. J. Toxicol. Environ. Health Part B Crit. Rev. 2010, 13, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Madabushi, R.; Wang, Y.; Zineh, I. A Holistic and Integrative Approach for Advancing Model-Informed Drug Development. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 9–11. [Google Scholar] [CrossRef]
- Yu, C.; Bashaw, E.D. Regulatory Perspective of Biomarker Bioanalysis during Drug Development. Bioanalysis 2019, 11, 607–610. [Google Scholar] [CrossRef]
- Shebley, M.; Sandhu, P.; Emami Riedmaier, A.; Jamei, M.; Narayanan, R.; Patel, A.; Peters, S.A.; Reddy, V.P.; Zheng, M.; de Zwart, L.; et al. Physiologically Based Pharmacokinetic Model Qualification and Reporting Procedures for Regulatory Submissions: A Consortium Perspective. Clin. Pharmacol. Ther. 2018, 104, 88–110. [Google Scholar] [CrossRef]
| Agency/Organization | Use of In Vitro to In Vivo Extrapolation (IVIVE) in Risk Characterization | Use of IVIVE or In Vitro Data Outside of Quantitative Risk Characterization |
|---|---|---|
| Agency for Toxic Substances and Disease Registry (ATSDR) | Application of IVIVE approaches would require the ability to derive health guidance values using high-throughput in vitro data. Several uncertainties and assumptions remain; hence, IVIVE is not used in health assessments. | In vitro data are used or potentially used as weight of evidence. |
| U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition (FDA/CFSAN) | Use IVIVE to develop physiologically based pharmacokinetic (PBPK) models, specifically to account for metabolism in the liver and transport in the kidney. | Not applicable (N/A) |
| FDA Center for Drug Evaluation and Research (FDA/CDER) | The role of IVIVE in risk assessment has generally been limited to relating in vitro human ether-à-go-go-related gene (hERG) channel assay results to the risk of QT prolongation and PBPK modeling. Following established decision trees in dedicated guidance [59], in vitro data can be used to predict drug–drug interactions and therefore dismiss the need for clinical trials. It is anticipated that appropriately constructed IVIVE algorithms will play a critical role in assessing the utility of new approach methodologies (NAMs) proposed to be used in risk assessment, which may include the support of starting dose selection in first-in-human trials of products using the Minimum Anticipated Biological Effect Level [60]. | In vitro data can predict efficacy of drugs and estimate doses to use with high potential in the field of rare diseases [61]. |
| Consumer Product Safety Commission (CPSC) | Has not used the approach but could use the information during any applicable risk evaluation; the approach could be used in a weight of evidence approach for risk assessments. | N/A |
| U.S. Environmental Protection Agency, Office of Pesticide Programs (EPA/OPP) | Use IVIVE to perform a rapid risk screening for chemicals without in vivo toxicity data [62] or to support a weight of evidence approach to identify data needs or to derive extrapolation factors [63]. | Identify chemicals that act on a common mechanism. |
| U.S. Department of Defense (DoD) | Various applications use IVIVE to derive human-relevant numbers to address operational human toxicity issues providing informed assessment of risk. This approach has also been used in a corroborative weight of evidence evaluation of hazard (comparisons across various data streams). | N/A |
| National Institute of Environmental Health Sciences, National Toxicology Program (NIEHS/NTP) | N/A | Perform hazard characterization. Use IVIVE to estimate external doses needed to achieve blood levels that equate to the identified in vitro potencies. The approach is applied to multiple species including human. |
| European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) | N/A—does not conduct regulatory risk assessments. | Development of case studies to explore and illustrate applicability of in vitro data and IVIVE. |
| Agency/Organization | Publications or Guidance Documents |
|---|---|
| ATSDR | ATSDR does not have guidance on IVIVE. |
| CPSC | CPSC has no guidance document related to IVIVE. There is a proposed Guidance on Alternative Test Methods and Integrated Testing Approaches, 86 FR 16704, 31 March 2021. |
| DoD | The DoD has no specific guidance on IVIVE implementation; however, other guidance frameworks are currently being developed. |
| EPA | Guidance Documents: [49,64,65] Publications grouped into the following categories: |
| NIEHS/NTP | Publications: [29,41,73,76] |
| FDA/CDER | Publications: [57,93,94] Guidance Documents:
|
| European Commission/EURL ECVAM | There is no specific guidance on IVIVE so far, but various approaches have been reviewed or explored [39,43,99,100].
|
| Health Canada | Science approach document on bioactivity exposure ratio: application in priority setting and risk assessment [58]. |
| Agency/Organization | Models or Software Tools |
|---|---|
| ATSDR | Models or software tools such as PBPK modeling have been used for dosimetric adjustments in the minimal risk level (MRL) determination process. |
| CPSC | There are no current plans to use models or software for facilitating IVIVE analysis and decision-making. |
| DoD | Current software use runs the spectrum of options. Current legacy software is used for PBPK (e.g., acslX for PBPK modeling); widely available software (e.g., R, also for PBPK modeling); high-throughput toxicokinetics (httk) R package; molecular docking and deep learning (TensorFlow); AOP wiki; STRING, REACTOME, OECD QSAR Toolbox, and BIOVIA software packages; and tools developed within image analysis tools for cell cultures. |
| EPA/ORD | Developed httk R package [87]; SimcypTM for PBPK modeling; PBPK model knowledgebase [150]; Database of PK time-series data and parameters [120]. |
| NIEHS/NTP | No decision-making. Use httk R package, GastroPlus & ADMET Predictor (Simulations Plus), as well as the Integrated Chemical Environment (ICE) tool. |
| European Commission/EURL ECVAM | No decision-making. Use httk R package (for the Accelerating the Pace of Chemical Risk Assessment [APCRA] project); Berkeley Madonna PBK model; explored application of the Wetmore IVIVE approach [31] and the BMD approach in a reverse dosimetry way; ongoing work from EFSA on the toxicokinetic plate and EPAA project on IVIVE. An IVIVE EPAA project [211,212] conducted by Health and Safety Executive, UK, is ongoing, which is based on work from McNally et al. [43] (led by G. Loizou). It will provide a tool to translate in vitro concentration–response relationships to in vivo dose–responses, determine in vivo benchmark dose (BMD) values from the translated data, and compare the predicted in vivo BMD to existing experimental BMD values used in chemical safety assessments by a regulatory agency. |
| In Vitro Assay Data Type | Data Summary | References |
|---|---|---|
| Overview or summary of in vitro and in silico data | Comparison of metabolic clearance assay systems; discussion of computational systems with built-in in vitro biochemical scaling | [29] |
| In vitro ADME methods overview | [135] | |
| Kidney enzymes, transporters, scaling factors | [143,225,226] | |
| This review has an emphasis on test systems and dosimetry in the respiratory tract. | [227] | |
| As part of an assessment of QSAR quality and reproducibility, 80 models of 31 ADME-related endpoints were identified. | [228] | |
| A summary table in the Supplementary Materials of Patel et al. [228] notes published sources for in vitro data and QSARs pertaining to oral absorption (7 data sets), distribution across the blood–brain barrier (1 data set), and metabolism data (8 data sets: in vitro metabolic clearance, Vmax, and Km). | [228] | |
| Summaries of resources of ADME data sets, models, and predictive software (designated as freely available or commercial products); while these tables do not emphasize in vitro data, these resources are well represented. | [223] | |
| Review of “high-throughput toxicokinetics”—the combination of in vitro chemical-specific methods with generic toxicokinetic models for IVIVE | [85] | |
| In vitro data: metabolism in hepatocytes, microsomes, and purified enzymes | Hepatocyte, microsomal, and purified (non-recombinant) hepatic enzyme data assembled by Pirovano et al. for QSAR development | [229,230] |
| Literature curated intrinsic clearance data from pooled hepatocyte suspensions for 1015 chemicals measured using human hepatocytes and 225 chemicals using rat hepatocytes. Included in R package “httk” | [87] | |
| In vitro scaling data for scaling liver metabolism | Age-specific data (5-year bins, for adult humans aged 20–95 years old) for microsomal protein content of liver and liver weight used in Simcyp | [231] |
| “Age-dependent protein abundance of cytosolic alcohol and aldehyde dehydrogenases in human liver.” (neonates to adults) | [232] | |
| Human hepatic microsomal protein yields and hepatocellularity collated from multiple sources. Weakly statistically significant inverse relationship to age; no relationship with gender, smoking, or alcohol consumption | [233] | |
| Human hepatic CYP content (total, and per isoform, for 7 isoforms; n = 60 subjects); rat and human hepatocyte numbers and microsomal protein yield | [234] | |
| Human hepatic CYP content central tendencies and variation (total and per isoform, 10 isoforms, 42–350 white subjects); reviews of data on impact of disease, age, sex, environment, and genetics on hepatic clearance | [235] | |
| Distribution of hepatic microsomal protein yields for 128 adult (Chinese) humans | [236] | |
| Human hepatic microsomal protein yields (20 adults from the United Kingdom) | [237] | |
| Hepatic metabolism scaling factors for rainbow trout (microsomal protein yield, hepatocellularity, liver S9 yield, and CYP content (CYP2M1, CYP2K1, and CYP3A27) | [83] | |
| Population variability in hepatocellularity, liver blood flow, liver volume and liver density for estimating in vivo hepatic clearance from in vitro data. Implemented in R package “httk” | [74] | |
| Partition coefficients (PCs) | A decision tree was described to choose the best predicted tissue partition coefficients for a certain physicochemical space, selecting among 6 algorithms, based on a 122-drug training set. | [238] |
| Reports Quantitative Property Relationship (QPPR) models for human and rat blood:air PCs for diverse volatile organic chemicals | [239] | |
| Examines and compares the relative accuracy, strengths, and limitations of 7 published models for human tissue–air and 10 models for tissue–blood PCs. The most accurate models for each category were identified. | [240] | |
| Reports a QSAR model for predicting physicochemical and biochemical properties of industrial chemicals of various groups | [241] | |
| Evaluation of QSAR predictions for 964 experimentally derived chemical–tissue PC combinations (143 chemicals, 12 tissues) with calibration and uncertainty quantification; Data and results are implemented in R package “httk”. | [242] |
| Agency/Organization | Agency Needs | Concerns on Gaps or Uncertainty |
|---|---|---|
| ATSDR |
|
|
| FDA/CFSAN | To establish a consistent approach for IVIVE. |
|
| FDA/CDER | IVIVE needed to support the qualification of NAM(s) associated with specific regulatory context(s) of use. | Concerns will depend on the context of use being addressed by a NAM being qualified and include:
|
| CPSC | The method needs to be effective for mixture risk assessment. | Demonstration of effectiveness for mixture risk assessment. |
| EPA/OPP |
|
|
| EPA Office of Pollution Prevention and Toxics (OPPT) | Determine plausible route(s) of exposure: dermal, inhalation, oral. | Many chemicals are considered rapidly with only structure and physicochemical properties available. No time for even in vitro measurements of TK. Must rely on QSAR. |
| EPA/ORD |
|
|
| DoD |
|
|
| NIEHS/NTP | Agency does not develop regulatory risk assessments. | The standard issues with IVIVE might be explored further, e.g., domain of applicability, parameter estimation, uncertainty, inter-individual variability, accuracy, sensitivity, and specificity. |
| European Commission/EURL ECVAM | Agency does not develop regulatory risk assessments. |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Tan, Y.-M.; Allen, D.G.; Bell, S.; Brown, P.C.; Browning, L.; Ceger, P.; Gearhart, J.; Hakkinen, P.J.; Kabadi, S.V.; et al. IVIVE: Facilitating the Use of In Vitro Toxicity Data in Risk Assessment and Decision Making. Toxics 2022, 10, 232. https://doi.org/10.3390/toxics10050232
Chang X, Tan Y-M, Allen DG, Bell S, Brown PC, Browning L, Ceger P, Gearhart J, Hakkinen PJ, Kabadi SV, et al. IVIVE: Facilitating the Use of In Vitro Toxicity Data in Risk Assessment and Decision Making. Toxics. 2022; 10(5):232. https://doi.org/10.3390/toxics10050232
Chicago/Turabian StyleChang, Xiaoqing, Yu-Mei Tan, David G. Allen, Shannon Bell, Paul C. Brown, Lauren Browning, Patricia Ceger, Jeffery Gearhart, Pertti J. Hakkinen, Shruti V. Kabadi, and et al. 2022. "IVIVE: Facilitating the Use of In Vitro Toxicity Data in Risk Assessment and Decision Making" Toxics 10, no. 5: 232. https://doi.org/10.3390/toxics10050232
APA StyleChang, X., Tan, Y.-M., Allen, D. G., Bell, S., Brown, P. C., Browning, L., Ceger, P., Gearhart, J., Hakkinen, P. J., Kabadi, S. V., Kleinstreuer, N. C., Lumen, A., Matheson, J., Paini, A., Pangburn, H. A., Petersen, E. J., Reinke, E. N., Ribeiro, A. J. S., Sipes, N., ... Mumtaz, M. (2022). IVIVE: Facilitating the Use of In Vitro Toxicity Data in Risk Assessment and Decision Making. Toxics, 10(5), 232. https://doi.org/10.3390/toxics10050232











