Toxicity Effects of Combined Mixtures of BDE-47 and Nickel on the Microalgae Phaeodactylum tricornutum (Bacillariophyceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Chemical Treatments
2.2. Physiological Parameter Analysis
2.3. Median Effective Concentrations (EC50)
2.4. Statistical Analyses
2.5. Transcriptomic Response Analysis
3. Results
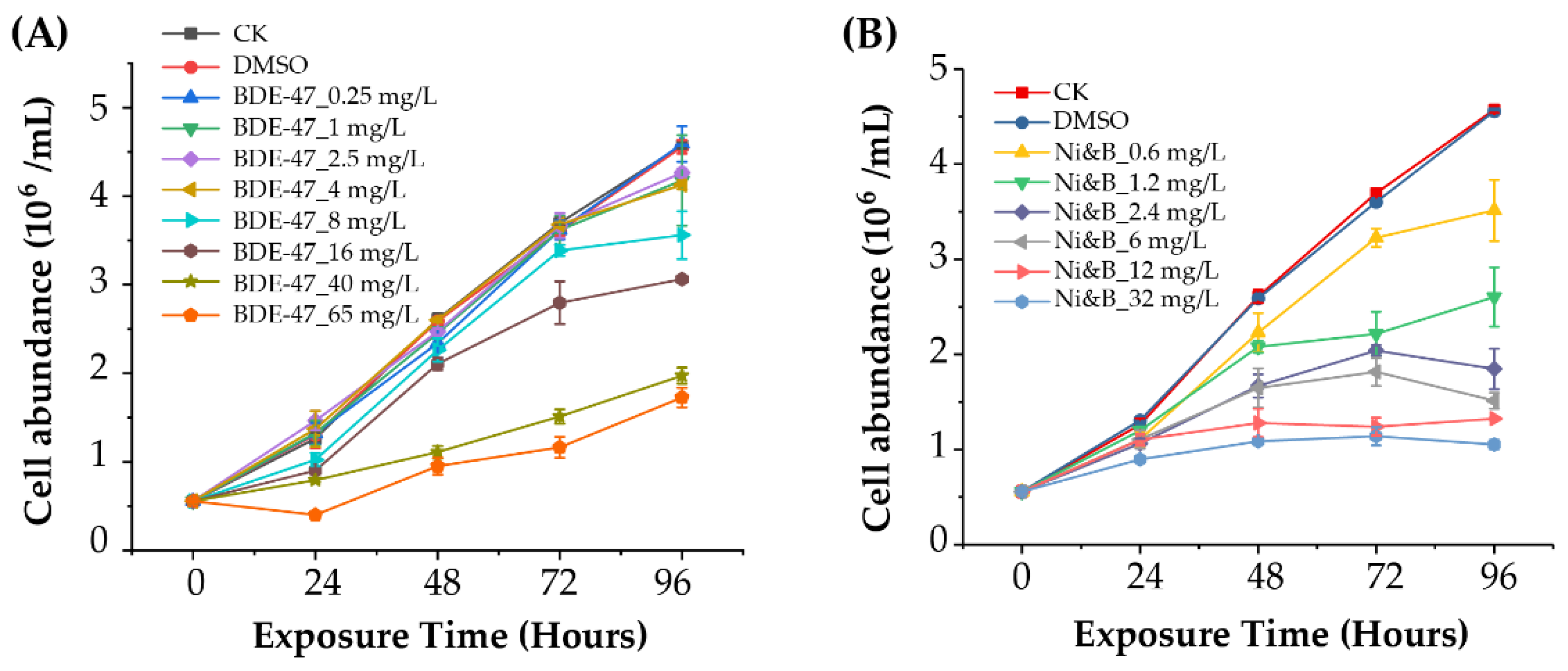
3.1. P. tricornutum Cell Abundance in Response to Toxicity Tests
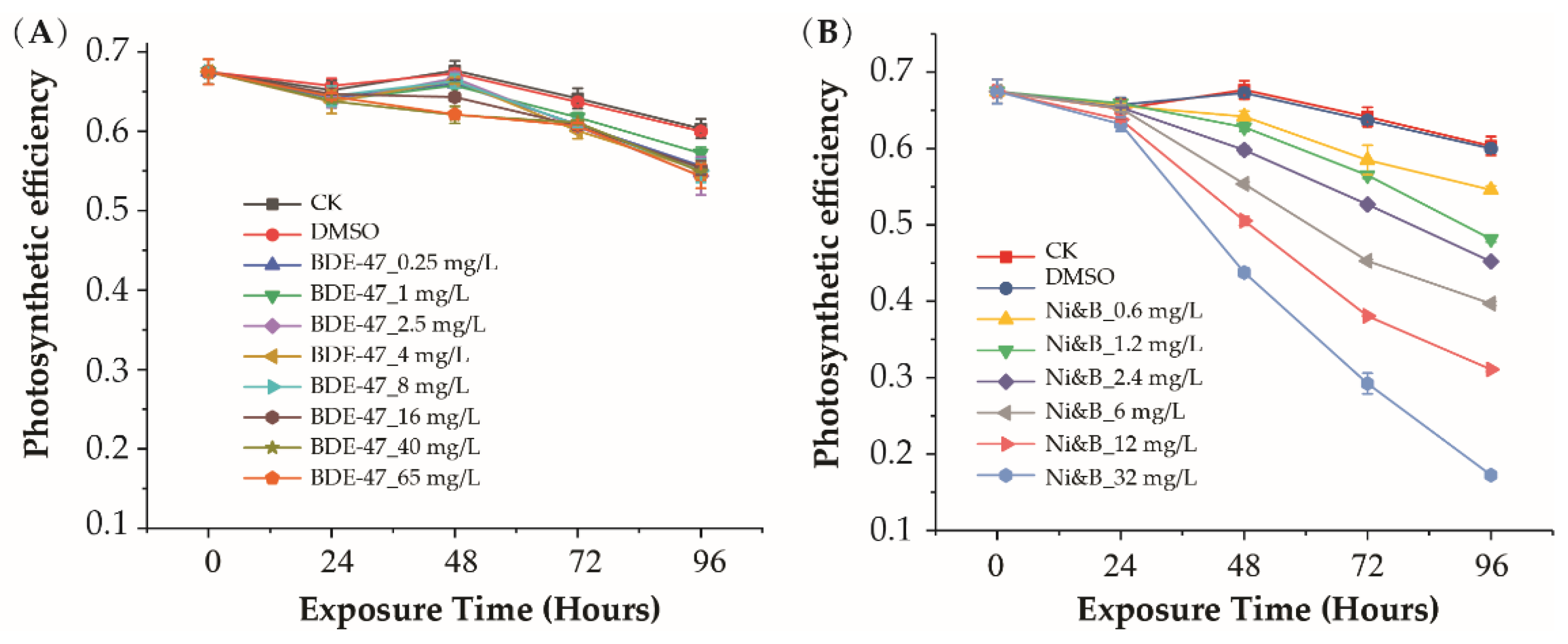
3.2. Photosynthetic Efficiency Response
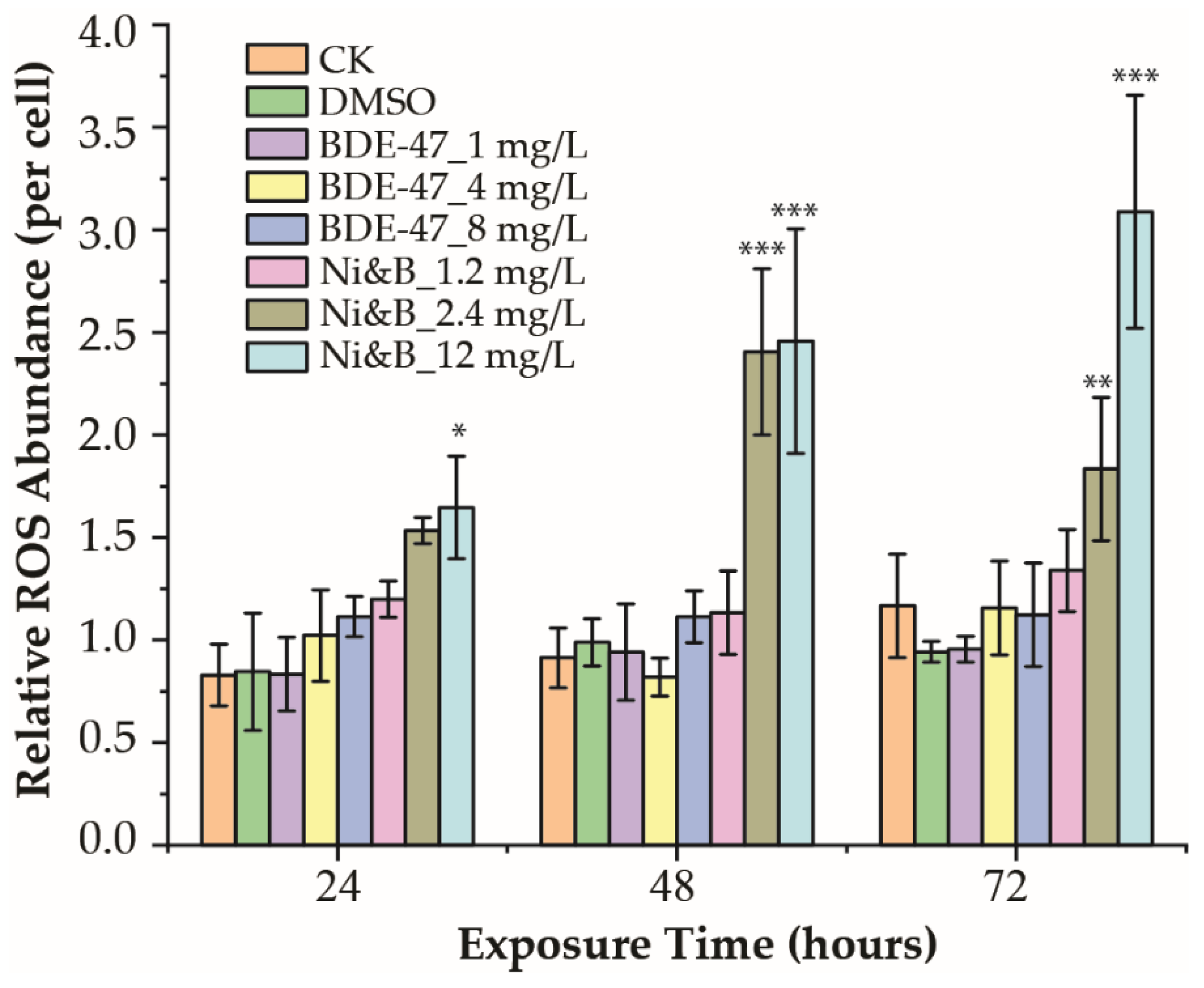
3.3. Reactive Oxidative Species (ROS) Production
3.4. Interactions of BDE-47 and Nickel in P. tricornutum
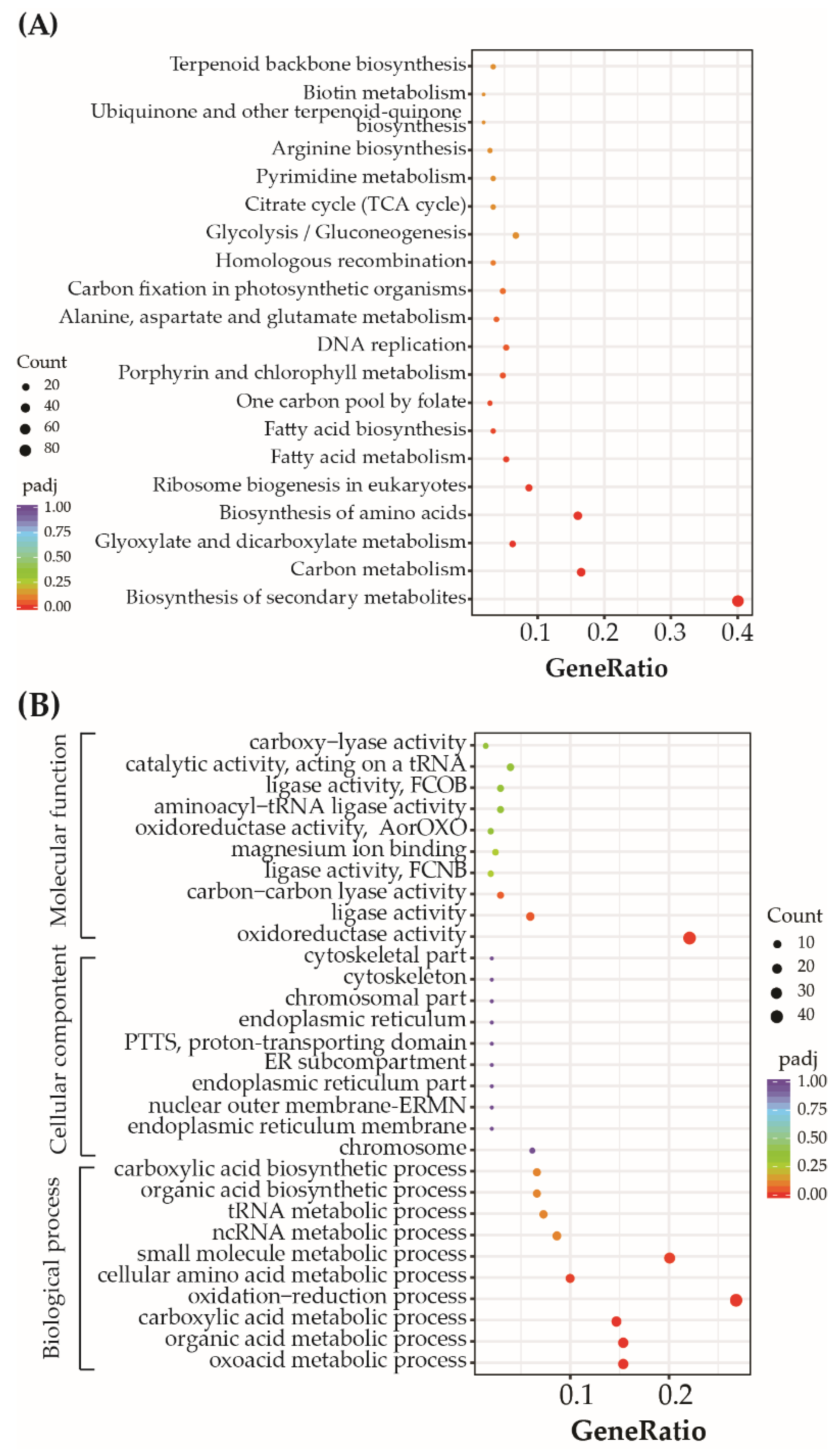
3.5. P. tricornutum Genes Differentially Expressed after Exposure to Mixtures of Nickel and BDE-47
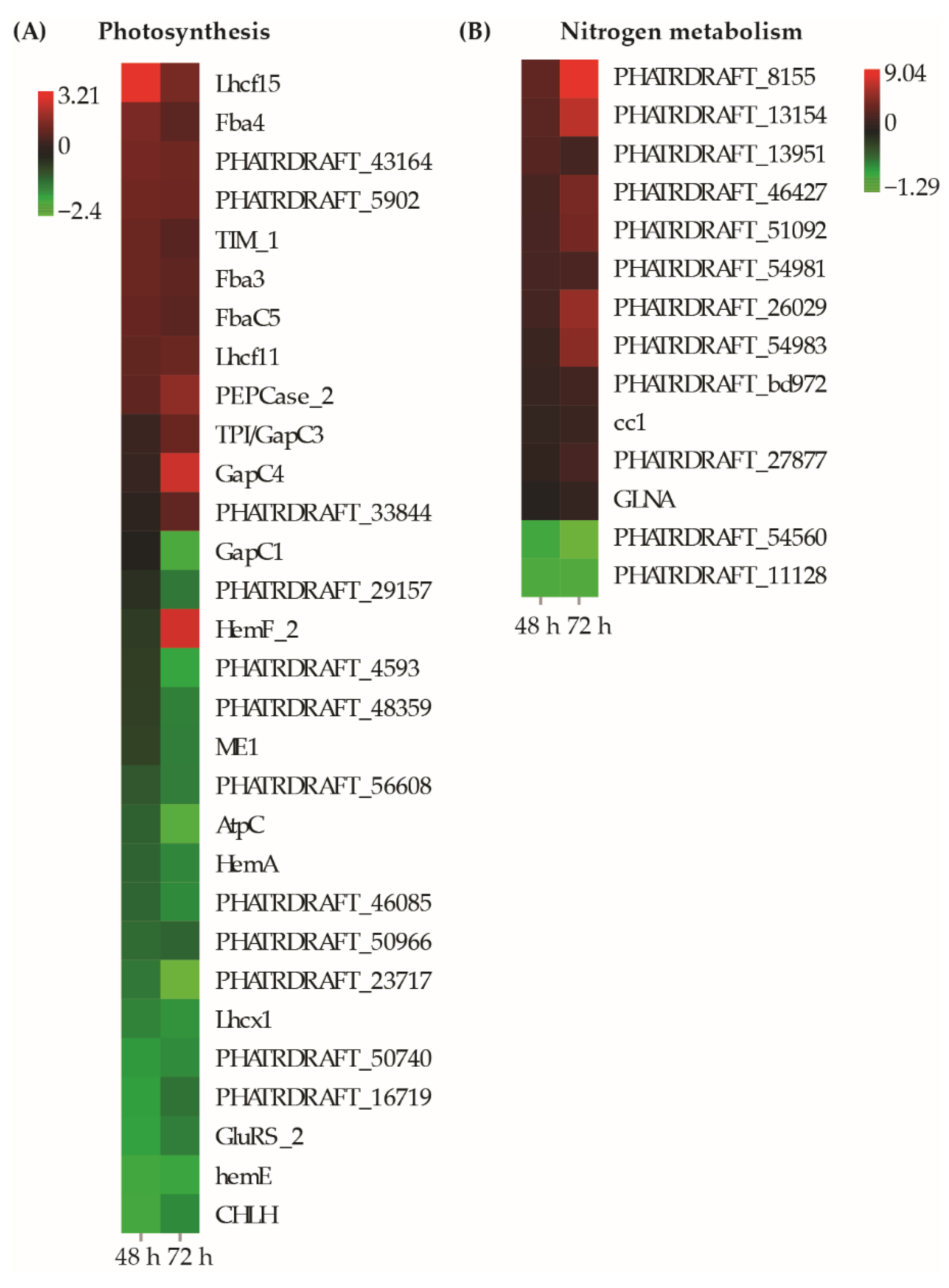
3.6. DEGs Related to Photosynthesis and Nitrogen Metabolism
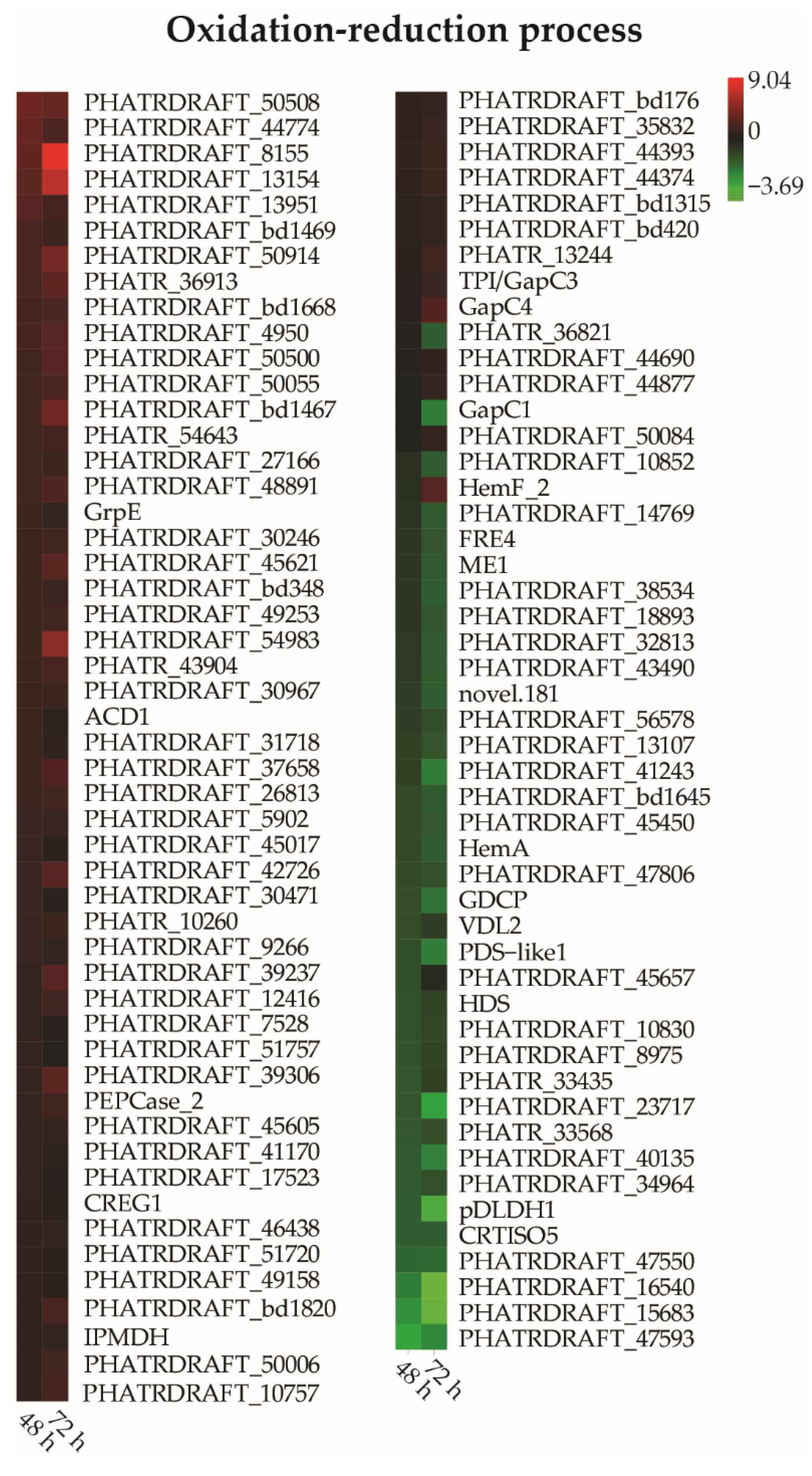
3.7. DEGs Related to Oxidation-Reduction Processes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Sun, X.; Xu, J. Heavy metal pollution in the East China Sea: A review. Mar. Pollut. Bull. 2020, 159, 111473. [Google Scholar] [CrossRef] [PubMed]
- Yogui, G.T.; Sericano, J.L. Polybrominated diphenyl ether flame retardants in the U.S. marine environment: A review. Environ. Int. 2009, 35, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Mearns, A.J.; Morrison, A.M.; Arthur, C.; Rutherford, N.; Bissell, M.; Rempel-Hester, M.A. Effects of pollution on marine organisms. Water Environ. Res. 2020, 92, 1510–1532. [Google Scholar] [CrossRef]
- O’Brien, A.L.; Keough, M.J. Ecological responses to contamination: A meta-analysis of experimental marine studies. Environ. Pollut. 2014, 195, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Schuijt, L.M.; Peng, F.J.; Van den Berg, S.J.P.; Dingemans, M.M.L.; Van den Brink, P.J. (Eco)toxicological tests for assessing impacts of chemical stress to aquatic ecosystems: Facts, challenges, and future. Sci. Total Environ. 2021, 795, 148776. [Google Scholar] [CrossRef]
- Aronzon, C.M.; Peluso, J.; Coll, C.P. Mixture toxicity of copper and nonylphenol on the embryo-larval development of Rhinella arenarum. Environ. Sci. Pollut. Res. Int. 2020, 27, 13985–13994. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Topaz, T.; Egozi, R.; Suari, Y.; Ben-Ari, J.; Sade, T.; Chefetz, B.; Yahel, G. Environmental risk dynamics of pesticides toxicity in a Mediterranean micro-estuary. Environ. Pollut. 2020, 265, 114941. [Google Scholar] [CrossRef]
- Torres, M.A.; Barros, M.P.; Campos, S.C.; Pinto, E.; Rajamani, S.; Sayre, R.T.; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: A review. Ecotoxicol. Environ. Saf. 2008, 71, 1–15. [Google Scholar] [CrossRef]
- Martínez-Ruiz, E.B.; Martínez-Jerónimo, F. Nickel has biochemical, physiological, and structural effects on the green microalga Ankistrodesmus falcatus: An integrative study. Aquat. Toxicol. 2015, 169, 27–36. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.Y.; Lee, Y.C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Saf. 2020, 201, 110781. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.W.; Hedlund, K.F.; Oliveira, M.A.; Carissimi, E. Ecotoxicological analysis of acrylamide using a microalga as an Indicator organism. Water Environ. Res. 2018, 90, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Yunus, K.; Zuraidah, M.A.; John, A. A review on the accumulation of heavy metals in coastal sediment of Peninsular Malaysia. Ecofeminism Clim. Change 2020, 1, 21–35. [Google Scholar] [CrossRef]
- Bekheet, T. The role of nickel in urea assimilation by algae. Planta 1982, 156, 385–387. [Google Scholar]
- Agawany, N.E.; Kaamoush, M.; El-Zeiny, A.; Ahmed, M. Effect of heavy metals on protein content of marine unicellular green alga Dunaliella tertiolecta. Environ. Monit. Assess. 2021, 193, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Lu, D.; Liu, C.; Hu, J.; Wang, P.; Dai, X. Toxic effect of nickel on microalgae Phaeodactylum tricornutum (Bacillariophyceae). Ecotoxicology 2022. [Google Scholar] [CrossRef]
- Vonderheide, A.P.; Mueller, K.E.; Meija, J.; Welsh, G.L. Polybrominated diphenyl ethers: Causes for concern and knowledge gaps regarding environmental distribution, fate and toxicity. Sci. Total Environ. 2008, 400, 425–436. [Google Scholar] [CrossRef]
- Kelly, B.C.; Ikonomou, M.G.; Blair, J.D.; Gobas, F.A. Hydroxylated and methoxylated polybrominated diphenyl ethers in a Canadian Arctic marine food web. Environ. Sci. Technol. 2008, 42, 7069–7077. [Google Scholar] [CrossRef]
- Salvadó, J.A.; Sobek, A.; Carrizo, D.; Gustafsson, Ö. Observation-based assessment of PBDE loads in Arctic Ocean Waters. Environ. Sci. Technol. 2016, 50, 2236–2245. [Google Scholar] [CrossRef]
- Xie, Z.; Möller, A.; Ahrens, L.; Sturm, R.; Ebinghaus, R. Brominated flame retardants in seawater and atmosphere of the Atlantic and the Southern Ocean. Environ. Sci. Technol. 2011, 45, 1820–1826. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Zhao, H.; Xie, Q.; Hou, M.; Zhang, Q.; Du, J.; Chen, J. Characterization of PBDEs and novel brominated flame retardants in seawater near a coastal mariculture area of the Bohai Sea, China. Sci. Total Environ. 2017, 580, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Tang, Z.; Meng, T.; Zhang, M. Concentration profile, spatial distributions and temporal trends of polybrominated diphenyl ethers in sediments across China: Implications for risk assessment. Ecotoxicol. Environ. Saf. 2020, 206, 111205. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fenghua, J.; Xiangfeng, K.; Yang, W.; Jingru, W.; Tianpeng, Z.; Zhaoyu, W.; Yingying, Z. Toxic effect of BDE-47 on the marine alga Skeletonema costatum: Population dynamics, photosynthesis, antioxidation and morphological changes. Chemosphere 2022, 286, 131674. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, X.; Zhang, X.; Yang, Y.; Sun, Z.; Jian, X.; Zhao, Y.; Zhang, X. Evaluation of the toxic response induced by BDE-47 in a marine alga, Phaeodactylum tricornutum, based on photosynthesis-related parameters. Aquat. Toxicol. 2020, 227, 105588. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, X.; Lv, M.; Liu, Q.; Li, J.; Zhang, B.; Li, L.; Zhang, X.; Zhao, Y. The molecular response mechanisms of a diatom Thalassiosira pseudonana to the toxicity of BDE-47 based on whole transcriptome analysis. Aquat. Toxicol. 2020, 229, 105669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lin, X.; Qu, K.; Xia, B.; Sun, X.; Chen, B. Toxicity of BDE-47, BDE-99 and BDE-153 on swimming behavior of the unicellular marine microalgae Platymonas subcordiformis and implications for seawater quality assessment. Ecotoxicol. Environ. Saf. 2019, 174, 408–416. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, X.; Quigg, A.; Lv, M.; Zhao, Y. The toxic mechanisms of BDE-47 to the marine diatom Thalassiosira pseudonana-a study based on multiple physiological processes. Aquat. Toxicol. 2019, 212, 20–27. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Li, Y.; Santschi, P.H.; Quigg, A. Response of photosynthesis and the antioxidant defense system of two microalgal species (Alexandrium minutum and Dunaliella salina) to the toxicity of BDE-47. Mar. Pollut. Bull. 2017, 124, 459–469. [Google Scholar] [CrossRef]
- Boisvert, S.; Joly, D.; Leclerc, S.; Govindachary, S.; Harnois, J.; Carpentier, R. Inhibition of the oxygen-evolving complex of photosystem II and depletion of extrinsic polypeptides by nickel. Biometals Int. J. Role Metal Ions Biol. Biochem. Med. 2007, 20, 879–889. [Google Scholar] [CrossRef]
- Fastelli, P.; Renzi, M. Exposure of key marine species to sunscreens: Changing ecotoxicity as a possible indirect effect of global warming. Mar. Pollut. Bull. 2019, 149, 110517. [Google Scholar] [CrossRef]
- Bowler, C.; Allen, A.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Al, E. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; Organisation for Economic Cooperation and Development (OECD): Paris, France, 2011.
- Jonker, M.J.; Svendsen, C.; Bedaux, J.J.; Bongers, M.; Kammenga, J.E. Significance testing of synergistic/antagonistic, dose level-dependent, or dose ratio-dependent effects in mixture dose-response analysis. Environ. Toxicol. Chem. 2005, 24, 2701–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, W.; Unkelbach, H.D.; Pöch, G.; Sühnel, J.; Kundi, M.; Bödeker, W. Consensus on concepts and terminology for combined-action assessment: The Saariselkä agreement. Arch. Complex Environ. Stud. 1992, 4, 65–69. [Google Scholar]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar] [PubMed]
- Vieira Dos Santos, C.; Rey, P. Plant thioredoxins are key actors in the oxidative stress response. Trends Plant Sci. 2006, 11, 329–334. [Google Scholar] [CrossRef]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Patwari, P.; Lee, R.T. Thioredoxins, mitochondria, and hypertension. Am. J. Pathol. 2007, 170, 805–808. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Trivedi, P.K. Glutathione S-Transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef] [Green Version]
- Marchand, C.; Le Maréchal, P.; Meyer, Y.; Miginiac-Maslow, M.; Issakidis-Bourguet, E.; Decottignies, P. New targets of Arabidopsis thioredoxins revealed by proteomic analysis. Proteomics 2004, 4, 2696–2706. [Google Scholar] [CrossRef]
- Agrawal, B.; Czymmek, K.J.; Sparks, D.L.; Bais, H.P. Transient Influx of nickel in root mitochondria modulates organic acid and reactive oxygen species production in nickel hyperaccumulator Alyssum murale. J. Biol. Chem. 2013, 288, 7351–7362. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Le, X.C.; Zhu, L. Metabolomics and transcriptomics reveal defense mechanism of rice (Oryza sativa) grains under stress of 2,2’,4,4’-tetrabromodiphenyl ether. Environ. Int. 2019, 133, 105154. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, K.; Le, X.C.; Zhu, L. Metabolomic analysis of two rice (Oryza sativa) varieties exposed to 2, 2’, 4, 4’-tetrabromodiphenyl ether. Environ. Pollut. 2018, 237, 308–317. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhou, P.; Xiao, Q.; Shi, D. Effects of foliar application of organic acids on alleviation of aluminum toxicity in alfalfa. J. Plant Nutr. Soil Sci. 2014, 177, 421–430. [Google Scholar] [CrossRef]
- Song, J.; Zhang, H.; Duan, C.; Cui, X. Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress. J. For. Res. 2018, 29, 1497–1505. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, J.; Zhou, H.; Cheung, S.G.; Tam, N.F.Y. Degradation of BDE-47 in mangrove sediments with amendment of extra carbon sources. Mar. Pollut. Bull. 2020, 153, 110972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Rogers, M.J.; Ding, C.; He, J. Reductive Debromination of Polybrominated Diphenyl Ethers—Microbes, Processes and Dehalogenases. Front. Microbiol. 2018, 9, 1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, D.; Chen, H.X.; Wong, Y.S.; Tam, N. Physiological response and oxidative transformation of 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47) by a Chlorella isolate. Sci. Total Environ. 2020, 744, 140869. [Google Scholar] [CrossRef]
- Singh, S.K.; Barnaby, J.Y.; Reddy, V.R.; Sicher, R.C. Varying response of the concentration and yield of soybean seed mineral elements, carbohydrates, organic acids, amino acids, protein, and oil tophosphorus starvation and CO(2) enrichment. Front. Plant Sci. 2016, 7, 1967. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Guo, R.; Lu, D.; Wang, P.; Dai, X. Toxicity Effects of Combined Mixtures of BDE-47 and Nickel on the Microalgae Phaeodactylum tricornutum (Bacillariophyceae). Toxics 2022, 10, 211. https://doi.org/10.3390/toxics10050211
Shi X, Guo R, Lu D, Wang P, Dai X. Toxicity Effects of Combined Mixtures of BDE-47 and Nickel on the Microalgae Phaeodactylum tricornutum (Bacillariophyceae). Toxics. 2022; 10(5):211. https://doi.org/10.3390/toxics10050211
Chicago/Turabian StyleShi, Xiaolai, Ruoyu Guo, Douding Lu, Pengbin Wang, and Xinfeng Dai. 2022. "Toxicity Effects of Combined Mixtures of BDE-47 and Nickel on the Microalgae Phaeodactylum tricornutum (Bacillariophyceae)" Toxics 10, no. 5: 211. https://doi.org/10.3390/toxics10050211
APA StyleShi, X., Guo, R., Lu, D., Wang, P., & Dai, X. (2022). Toxicity Effects of Combined Mixtures of BDE-47 and Nickel on the Microalgae Phaeodactylum tricornutum (Bacillariophyceae). Toxics, 10(5), 211. https://doi.org/10.3390/toxics10050211






