Pollution Characteristics and Risk Prediction of Endocrine Disruptors in Lakes of Wuhan
Abstract
1. Introduction
2. Materials and Methods
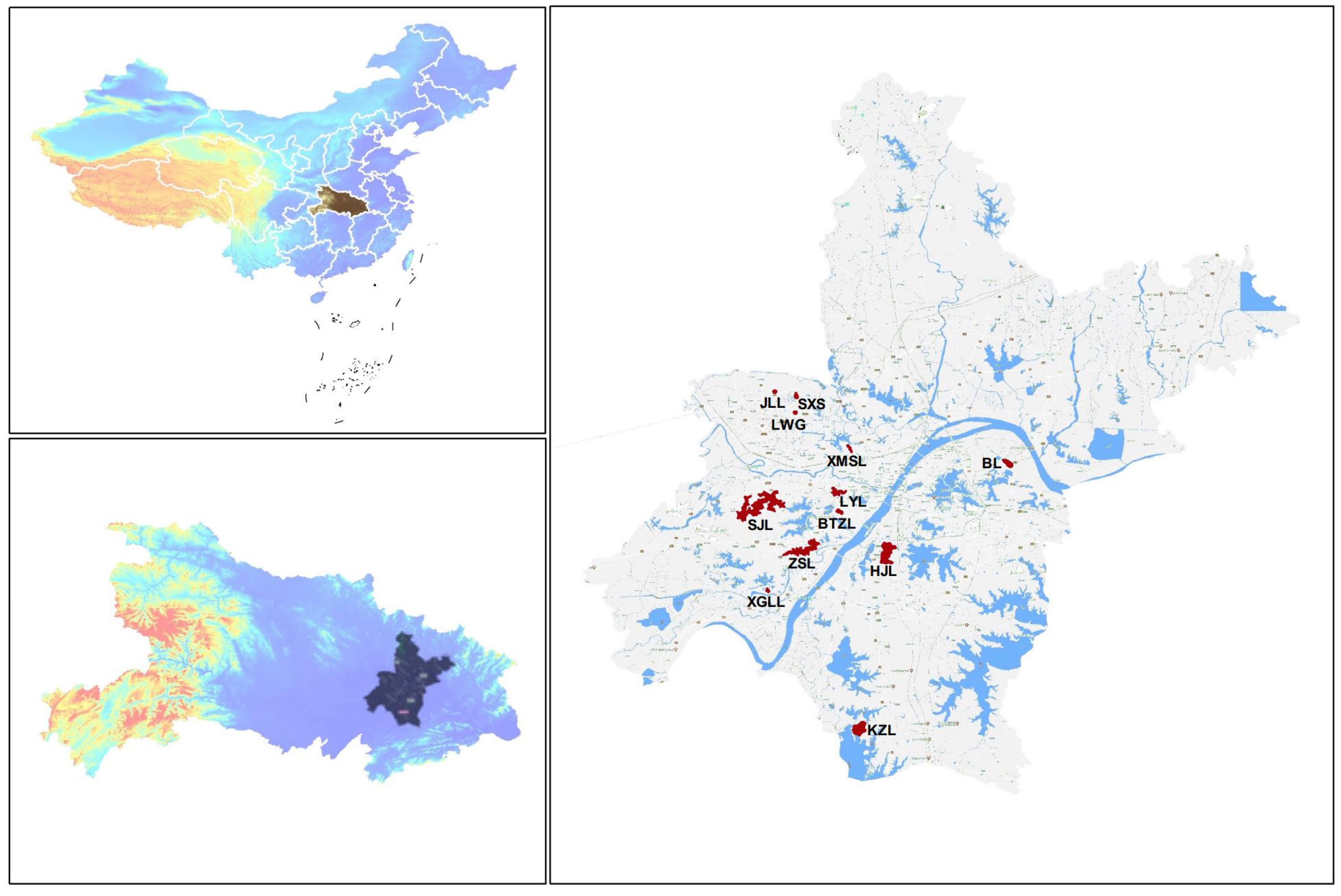
2.1. Study Area and Sample Collection
2.2. Sample Preparation
2.3. Analysis of EDCs by HPLC
2.4. Measurement of Water Quality Parameters
2.5. Ecological Risk Assessment
2.6. Statistical Analysis
3. Results and Discussion
3.1. Variance of EDC Content in 12 Lakes
3.2. Spatial Distribution of EDCs in 12 Lakes of Wuhan
3.3. Correlations between EDCs and Water Quality Parameters
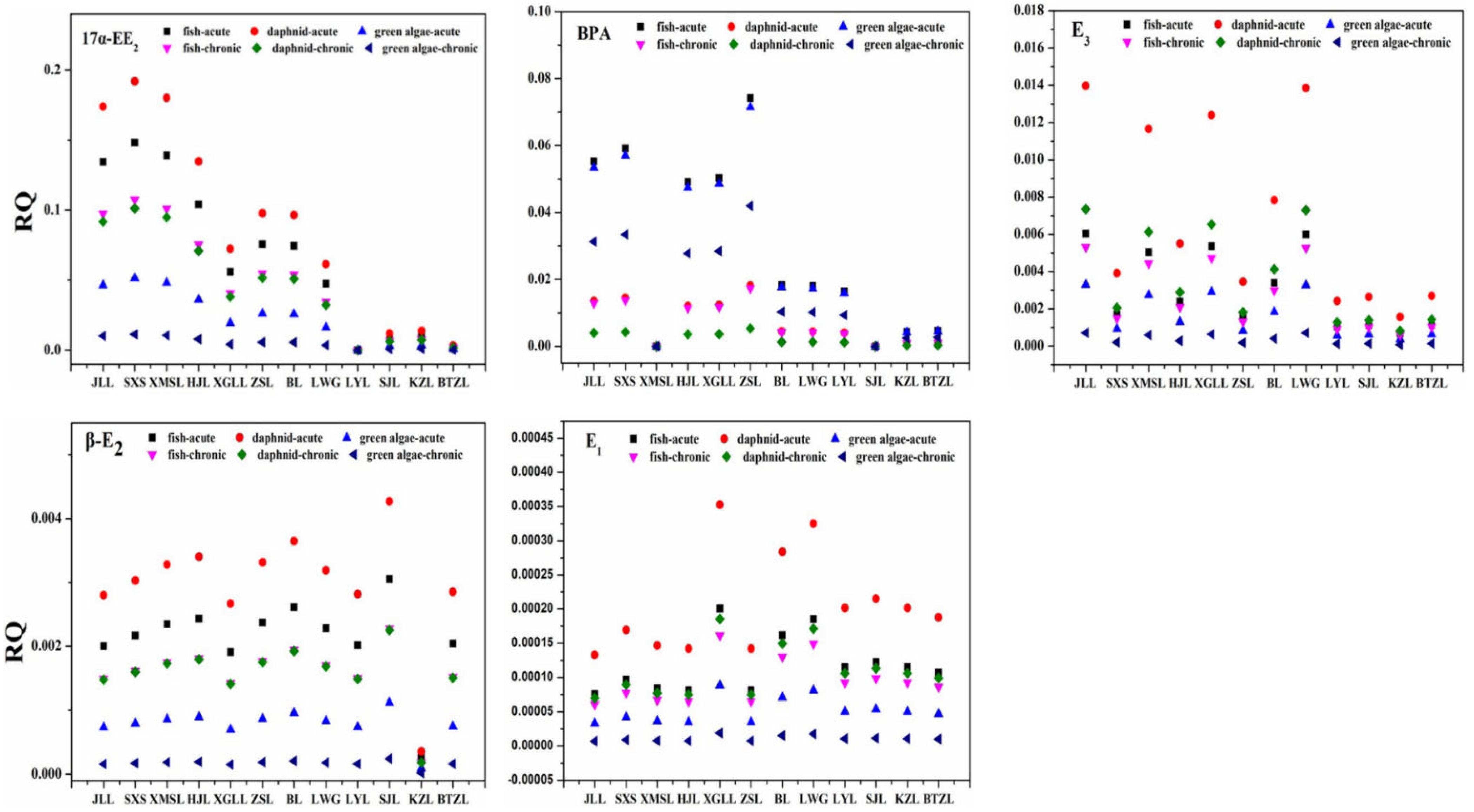
3.4. Ecological Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernando, M.D.; Mezcua, M.; Alba, A.R.F.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Ballesteros, E. Trace analysis of endocrine disrupting compounds in environmental water samples by use of solid-phase extraction and gas chromatography with mass spectrometry detection. J. Chromatogr. A 2014, 1360, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, B.; Ma, W.; Zhou, K.; Fan, H.; Wang, H. Discussion on Current Pollution Status and Legislation of Environmental Hormone in China. Procedia Environ. Sci. 2011, 11, 1267–1277. [Google Scholar] [CrossRef][Green Version]
- Asakura, H.; Matsuto, T.; Tanaka, N. Behavior of endocrine-disrupting chemicals in leachate from MSW landfill sites in Japan. Waste Manag. 2004, 24, 613–622. [Google Scholar] [CrossRef]
- European Commission. European Commission Implementation Decision 2018/840 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495. Off. J. Eur. Union 2018, 61, 9–12. [Google Scholar]
- Gusmaroli, L.; Buttiglieri, G.; Petrovic, M. The EU watch list compounds in the Ebro delta region: Assessment of sources, river transport and seasonal variations. Environ. Pollut. 2019, 253, 606–615. [Google Scholar] [CrossRef]
- Frédéric, O.; Yves, P. Pharmaceuticals in hospital wastewater: Their ecotoxicity and contribution to the environmental hazard of the effluent. Chemosphere 2014, 115, 31–39. [Google Scholar] [CrossRef]
- Combalbert, S.; Hernandez-Raquet, G. Occurrence, fate and biodegradation of estrogens in sewage and manure. Appl. Microbiol. Biotechnol. 2010, 86, 1671–1692. [Google Scholar] [CrossRef]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E.; Bisphenol, A. Nonylphenols, benzophenones and benzotriazoles in soils, groundwater, surface water, sediments and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef]
- Hansen, P.-D.; Dizer, H.; Hock, B.; Marx, A.; Sherry, J.; McMaster, M.; Blaise, C. Vitellogenin—A Biomarker for Endocrine Disruptors. Biosens. Environ. Diagn. 1998, 367, 253–261. [Google Scholar] [CrossRef]
- Clotfelter, E.D.; Bell, A.M.; Levering, K.R. The role of animal behaviour in the study of endocrine-disrupting chemicals. Anim. Behav. 2004, 68, 665–676. [Google Scholar] [CrossRef]
- De Castro-Català, N.; Muñoz, I.; Armendáriz, L.; Campos, B.; Barceló, D.; López-Doval, J.; Pérez, S.; Petrovic, M.; Picó, Y.; Riera, J. Invertebrate community responses to emerging water pollutants in Iberian River basins. Sci. Total Environ. 2015, 503–504, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R. Prenatal exposure to diethylstilbestrol (DES). Fertil. Steril. 2008, 89, e55–e56. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Giovannelli, P.; Cernera, G.; Di Santi, A.; Marino, I.; Bilancio, A.; Galasso, G.; Auricchio, F.; Migliaccio, A.; Castoria, G. Non-Genomic Androgen Action Regulates Proliferative/Migratory Signaling in Stromal Cells. Front. Endocrinol. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Song, N.; Guo, R.; Chen, M.; Mai, D.; Yan, Z.; Han, Z.; Chen, J. Occurrence, distribution and sources of bisphenol analogues in a shallow Chinese freshwater lake (Taihu Lake): Implications for ecological and human health risk. Sci. Total Environ. 2017, 599–600, 1090–1098. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Lu, G.; Jiang, R.; Yan, Z.; Li, Y. Occurrence, toxicity and ecological risk of Bisphenol A analogues in aquatic environment—A review. Ecotoxicol. Environ. Saf. 2021, 208, 111481. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Z.-H.; Wang, H.; Dang, Z.; Liu, Y. A review of 17α-ethynylestradiol (EE2) in surface water across 32 countries: Sources, concentrations and potential estrogenic effects. J. Environ. Manag. 2021, 292, 112804. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, W.; Tang, T.; Li, Z.; Dai, R. Environmental estrogens in surface water and their interaction with microalgae: A review. Sci. Total Environ. 2021, 807, 150637. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Wang, C.; Zhang, H.; Zhao, Y.; Zhang, L.; Liu, B.; Wu, Z.; Zhou, Q. Characteristic analysis of phospholipid fatty acids (PLFAs) in typical nutrient polluted lake sediment in Wuhan. Int. J. Sediment Res. 2021, 36, 221–228. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M. Occurrence and risk assessment of multiclass endocrine disrupting compounds in an urban tropical river and a proposed risk management and monitoring framework. Sci. Total Environ. 2019, 671, 431–442. [Google Scholar] [CrossRef]
- Castillo, M.; Barceló, D. Analysis of industrial effluents to determine endocrine-disrupting chemicals. TrAC Trends Anal. Chem. 1997, 16, 574–583. [Google Scholar] [CrossRef]
- Wang, J.; Cai, X.; Chen, F.; Zhang, Z.; Zhang, Y.; Sun, K.; Zhang, T.; Chen, X. Hundred-year spatial trajectory of lake coverage changes in response to human activities over Wuhan. Environ. Res. Lett. 2020, 15, 094022. [Google Scholar] [CrossRef]
- Liu, S.; Xu, X.-R.; Qi, Z.-H.; Chen, H.; Hao, Q.-W.; Hu, Y.-X.; Zhao, J.-L.; Ying, G.-G. Steroid bioaccumulation profiles in typical freshwater aquaculture environments of South China and their human health risks via fish consumption. Environ. Pollut. 2017, 228, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, X.; Zhou, L.J.; Ji, X.; Gao, B.; Xu, G.Z.; Li, A. Multi-phase distribution and risk assessment of endocrine disrupting chemicals in the surface water of the Shaying River, Huai River Basin, China. Ecotoxicol. Environ. Saf. 2019, 173, 45–53. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. American Public Health Association, American Water Works Asso Ciation, Water Environment Federation (APHA-AWWA-WEF). 2001. Standard Methods for the Examination of Water and Wastewater, 22nd Edn, Washington, DC. An. Hidrol. Med. 2001, 5, 186. [Google Scholar]
- Sanderson, H.; Johnson, D.J.; Wilson, C.J.; Brain, R.A.; Solomon, K.R. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003, 144, 383–395. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Nie, M.; Yan, C.; Dong, W.; Liu, M.; Zhou, J.; Yang, Y. Occurrence, distribution and risk assessment of estrogens in surface water, suspended particulate matter and sediments of the Yangtze Estuary. Chemosphere 2015, 127, 109–116. [Google Scholar] [CrossRef]
- Omar, T.F.T.; Ahmad, A.; Aris, A.Z.; Yusoff, F.M. Endocrine disrupting compounds (EDCs) in environmental matrices: Review of analytical strategies for pharmaceuticals, estrogenic hormones and alkylphenol compounds. TrAC Trends Anal. Chem. 2016, 85, 241–259. [Google Scholar] [CrossRef]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Endocrine-disrupting compounds: Occurrence, detection methods, effects and promising treatment pathways—A critical review. J. Environ. Chem. Eng. 2021, 9, 104558. [Google Scholar] [CrossRef]
- Oehlmann, J.; Oetken, M.; Schulte-Oehlmann, U. A critical evaluation of the environmental risk assessment for plasticizers in the freshwater environment in Europe, with special emphasis on bisphenol A and endocrine disruption. Environ. Res. 2008, 108, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Le Thi Minh, T.; Nguyen Phuoc, D.; Dinh Quoc, T.; Ngo, H.H.; Do Hong Lan, C. Presence of e-EDCs in surface water and effluents of pollution sources in Sai Gon and Dong Nai river basin. Sustain. Environ. Res. 2016, 26, 20–27. [Google Scholar] [CrossRef]
- Pawlowski, S.; Ternes, T.; Bonerz, M.; Kluczka, T.; Van Der Burg, B.; Nau, H.; Erdinger, L.; Braunbeck, T. Combined in Situ and in Vitro Assessment of the Estrogenic Activity of Sewage and Surface Water Samples. Toxicol. Sci. 2003, 75, 57–65. [Google Scholar] [CrossRef]
- Mnguni, S.; Schoeman, C.; Marais, S.; Cukrowska, E.; Chimuka, L. Determination of oestrogen hormones in raw and treated water samples by reverse phase ultra-fast liquid chromatography mass spectrometry—A case study in Johannesburg South, South Africa. Water SA 2018, 44, 111–117. [Google Scholar] [CrossRef]
- Chafi, S.; Azzouz, A.; Ballesteros, E. Occurrence and distribution of endocrine disrupting chemicals and pharmaceuticals in the river Bouregreg (Rabat, Morocco). Chemosphere 2021, 287, 132202. [Google Scholar] [CrossRef]
- Pessoa, G.P.; de Souza, N.C.; Vidal, C.; Alves, J.A.; Firmino, P.I.M.; Nascimento, R.; dos Santos, A.B. Occurrence and removal of estrogens in Brazilian wastewater treatment plants. Sci. Total Environ. 2014, 490, 288–295. [Google Scholar] [CrossRef]
- Peteffi, G.P.; Fleck, J.D.; Kael, I.M.; Rosa, D.C.; Antunes, M.V.; Linden, R. Ecotoxicological risk assessment due to the presence of bisphenol A and caffeine in surface waters in the Sinos River Basin—Rio Grande do Sul—Brazil. Braz. J. Biol. 2019, 79, 712. [Google Scholar] [CrossRef]
- Liu, Y.; Su, W.; Zhu, Y.; Xiao, L.; Hu, T. Endocrine disrupting compounds in the middle and lower reaches of the Lhasa River Basin: Occurrence, distribution and risk assessment. Sci. Total Environ. 2020, 727, 138694. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, X.; Zhang, M.; Wang, J. Occurrence and distribution of endocrine-disrupting compounds in the Honghu Lake and East Dongting Lake along the Central Yangtze River, China. Environ. Sci. Pollut. Res. 2015, 22, 17644–17652. [Google Scholar] [CrossRef]
- Jiang, R.; Liu, J.; Huang, B.; Wang, X.; Luan, T.; Yuan, K. Assessment of the potential ecological risk of residual endocrine-disrupting chemicals from wastewater treatment plants. Sci. Total Environ. 2020, 714, 136689. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Rene, E.R.; Yan, Y.; Ma, W.; Xiang, Y. Spatiotemporal evolvement and factors influencing natural and synthetic EDCs and the microbial community at different groundwater depths in the Chaobai watershed: A long-term field study on a river receiving reclaimed water. J. Environ. Manag. 2019, 246, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Sun, Q.; Ma, C.; Rashid, A.; Li, Y.; Mulla, S.I.; Yu, C.-P. Occurrence, seasonal variation and risk evaluation of selected endocrine disrupting compounds and their transformation products in Jiulong River and estuary, China. Mar. Pollut. Bull. 2019, 145, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wen, T.; Wang, G.-S.; Cheng, H.; Lin, Y.; Lien, G. Determining estrogenic steroids in Taipei waters and removal in drinking water treatment using high-flow solid-phase extraction and liquid chromatography/tandem mass spectrometry. Sci. Total Environ. 2007, 378, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.; Lei, B.; Li, N.; Ma, M.; Wang, Z. Determination of estrogens and estrogenic activities in water from three rivers in Tianjin, China. J. Environ. Sci. (China) 2013, 25, 1164–1171. [Google Scholar] [CrossRef]
- Liu, R.; Luo, X.; Shu, S.; Ding, J.; Zhang, G.; Wang, Z.; Zou, H.; Zhang, Y. Impact of rainfall on the occurrence, spatiotemporal distribution and partition trend of micropollutants in Taihu Lake, China: Bisphenol A and 4-nonylphenol as examples. Ecotoxicol. Environ. Saf. 2020, 204, 111064. [Google Scholar] [CrossRef]
- Huang, G.-Y.; Liu, Y.-S.; Liang, Y.-Q.; Shi, W.-J.; Yang, Y.-Y.; Liu, S.-S.; Hu, L.-X.; Chen, H.-X.; Xie, L.; Ying, G.-G. Endocrine disrupting effects in western mosquitofish Gambusia affinis in two rivers impacted by untreated rural domestic wastewaters. Sci. Total Environ. 2019, 683, 61–70. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Zhang, S.-H.; Ji, G.-X.; Wu, S.-M.; Guo, R.-X.; Cheng, J.; Yan, Z.-Y.; Chen, J.-Q. Occurrence, distribution and risk assessment of suspected endocrine-disrupting chemicals in surface water and suspended particulate matter of Yangtze River (Nanjing section). Ecotoxicol. Environ. Saf. 2017, 135, 90–97. [Google Scholar] [CrossRef]
- Van Zijl, M.C.; Aneck-Hahn, N.H.; Swart, P.; Hayward, S.; Genthe, B.; De Jager, C. Estrogenic activity, chemical levels and health risk assessment of municipal distribution point water from Pretoria and Cape Town, South Africa. Chemosphere 2017, 186, 305–313. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef]
- Ismail, N.A.H.; Aris, A.Z.; Wee, S.Y.; Nasir, H.M.; Razak, M.R.; Kamarulzaman, N.H.; Omar, T.F.T. Occurrence and distribution of endocrine-disrupting chemicals in mariculture fish and the human health implications. Food Chem. 2021, 345, 128806. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, S.; Xu, H.; Zhang, Q.; Zhang, S.; Shi, L.; Yao, C.; Liu, Y.; Cheng, J. Distribution and bioaccumulation of endocrine disrupting chemicals in water, sediment and fishes in a shallow Chinese freshwater lake: Implications for ecological and human health risks. Ecotoxicol. Environ. Saf. 2017, 140, 222–229. [Google Scholar] [CrossRef]
- Marie, K.; Ana, S.; Espino, M.P. Occurrence and distribution of hormones and bisphenol A in Laguna. Chemosphere 2020, 256, 127122. [Google Scholar] [CrossRef]
- Luo, Z.; Tu, Y.; Li, H.; Qiu, B.; Liu, Y.; Yang, Z. Endocrine-disrupting compounds in the Xiang jiang River of China: Spatio-temporal distribution, source apportionment and risk assessment. Ecotoxicol. Environ. Saf. 2019, 167, 476–484. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. An Introduction to the Sources, Fate, Occurrence and Effects of Endocrine Disrupting Chemicals Released into the Environment. Environ. Res. 2022, 207, 112658. [Google Scholar] [CrossRef]
- Hoga, C.A.; Almeida, F.L.; Reyes, F.G.R. A review on the use of hormones in fish farming: Analytical methods to determine their residues. CyTA J. Food 2018, 16, 679–691. [Google Scholar] [CrossRef]
- Koumaki, E.; Mamais, D.; Noutsopoulos, C. Assessment of the environmental fate of endocrine disrupting chemicals in rivers. Sci. Total Environ. 2018, 628–629, 947–958. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, T.; Wang, C.; Zong, D.; Li, H.; Yang, Z. Occurrence and distribution of taste and odor compounds in subtropical water supply reservoirs and their fates in water treatment plants. Environ. Sci. Pollut. Res. 2017, 24, 2904–2913. [Google Scholar] [CrossRef]
- Liu, W.-R.; Zhao, J.-L.; Liu, Y.-S.; Chen, Z.-F.; Yang, Y.-Y.; Zhang, Q.-Q.; Ying, G.-G. Biocides in the Yangtze River of China: Spatiotemporal distribution, mass load and risk assessment. Environ. Pollut. 2015, 200, 53–63. [Google Scholar] [CrossRef]
- Romanucci, V.; Siciliano, A.; Guida, M.; Libralato, G.; Saviano, L.; Luongo, G.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Disinfection by-products and ecotoxic risk associated with hypochlorite treatment of irbesartan. Sci. Total Environ. 2020, 712, 135625. [Google Scholar] [CrossRef]
- Shekhar, S.; Sood, S.; Showkat, S.; Lite, C.; Chandrasekhar, A.; Vairamani, M.; Barathi, S.; Santosh, W. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen. Comp. Endocrinol. 2017, 241, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Sakamoto, H.; Nishikawa, K.; Sakuma, S.; Nakajima, A.; Fujimoto, Y.; Kamisaki, Y. Life Style-Related Diseases of the Digestive System: Endocrine Disruptors Stimulate Lipid Accumulation in Target Cells Related to Metabolic Syndrome. J. Pharmacol. Sci. 2007, 105, 133–137. [Google Scholar] [CrossRef] [PubMed]




| Area | EDCs | Concentration (ng/L) | References |
|---|---|---|---|
| Vietnam, Sai Gon and Dong Nai river basin | Nonylphenol | 5.9–235 | [33] |
| Southwest Germany, river Rhine | 17α-EE2 | <1.5 | [34] |
| South Africa, south of Johannesburg | E1 | 0.90–4.43 | [35] |
| Morocco, Bouregreg River | Nonylphenol | 11–200 | [36] |
| Morocco, Bouregreg river | E1 | 5–277 | [36] |
| Morocco, Bouregreg river | E2 | 21–200 | [36] |
| Brazil, five full-scale wastewater treatment plants | 17β-E2 | ND–776 | [37] |
| Brazil, Sinos River basin | BPA | ND–517 | [38] |
| China, Lhasa River Basin | E1 | ND–3.9 | [39] |
| China, Lhasa River Basin | BPA | ND–433 | [39] |
| China, Honghu Lake | 17α-EE2 | ND–33.28 | [40] |
| China, 38 wastewater treatment plants | β-E2 | ND–62.92 | [41] |
| China, Chaobai watershed | E3 | ND–23.51 | [42] |
| China, Jiulong river and estuary | E3 | ND–118 | [43] |
| Dan-shui River | E3 | ND–73.5 | [44] |
| China, three rivers in Tianjin | 17α-EE2 | 1.55–24.40 | [45] |
| China, Taihu Lake | BPA | ND–112 | [46] |
| China, Taihu Lake | 4-nonylphenol | ND–324 | [46] |
| China, Xiangshui River and Heng River | 17β-boldenone | ND–0.91 | [47] |
| China, Yangtze River (Nanjing section) | BPA | 1.7–563 | [48] |
| JLL | HJL | ZSL | ||||
|---|---|---|---|---|---|---|
| Terrain Category | Class Size | Proportion | Class Size | Proportion | Class Size | Proportion |
| road | 0.18 | 4.45 | 7.67 | 6.17 | 3.58 | 5.67 |
| farmland | 1.77 | 44.86 | 9.32 | 7.50 | 9.76 | 15.44 |
| river | 0.05 | 1.23 | 1.04 | 0.84 | 2.09 | 3.30 |
| lake | 0.36 | 9.14 | 38.26 | 30.80 | 7.36 | 11.65 |
| built-up area | 0.12 | 3.06 | 46.23 | 37.21 | 19.96 | 31.58 |
| pit-pond | 1.26 | 32.01 | 1.10 | 0.89 | 1.93 | 3.05 |
| woodland | 0.19 | 4.78 | 10.63 | 8.56 | 7.92 | 12.52 |
| paddy field | 0.02 | 0.40 | 3.38 | 2.72 | 4.59 | 7.26 |
| garden plot | 0.00 | 0.06 | 6.57 | 5.29 | 6.03 | 9.53 |
| population | 2019 | 106,087 | 29,824 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cao, J.; Ke, T.; Tao, Y.; Wu, W.; Wang, P.; Zhou, M.; Chen, L. Pollution Characteristics and Risk Prediction of Endocrine Disruptors in Lakes of Wuhan. Toxics 2022, 10, 93. https://doi.org/10.3390/toxics10020093
Zhang Y, Cao J, Ke T, Tao Y, Wu W, Wang P, Zhou M, Chen L. Pollution Characteristics and Risk Prediction of Endocrine Disruptors in Lakes of Wuhan. Toxics. 2022; 10(2):93. https://doi.org/10.3390/toxics10020093
Chicago/Turabian StyleZhang, Yurui, Jun Cao, Tan Ke, Yue Tao, Wanyin Wu, Panpan Wang, Min Zhou, and Lanzhou Chen. 2022. "Pollution Characteristics and Risk Prediction of Endocrine Disruptors in Lakes of Wuhan" Toxics 10, no. 2: 93. https://doi.org/10.3390/toxics10020093
APA StyleZhang, Y., Cao, J., Ke, T., Tao, Y., Wu, W., Wang, P., Zhou, M., & Chen, L. (2022). Pollution Characteristics and Risk Prediction of Endocrine Disruptors in Lakes of Wuhan. Toxics, 10(2), 93. https://doi.org/10.3390/toxics10020093





