Vanadium Toxicity Monitored by Fertilization Outcomes and Metal Related Proteolytic Activities in Paracentrotus lividus Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Gamete Collection
2.2. Sodium Orthovanadate Stock Solution Preparation
2.3. Fertilization Test
2.4. Embryo Cultures and V Treatments
2.5. Gelatin Zymography by Polyacrylamide Gel Electrophoresis
2.6. Statistical Analysis
3. Results
3.1. V Reduces the Fertilization Rate and Increases Fertilized Egg Anomalies
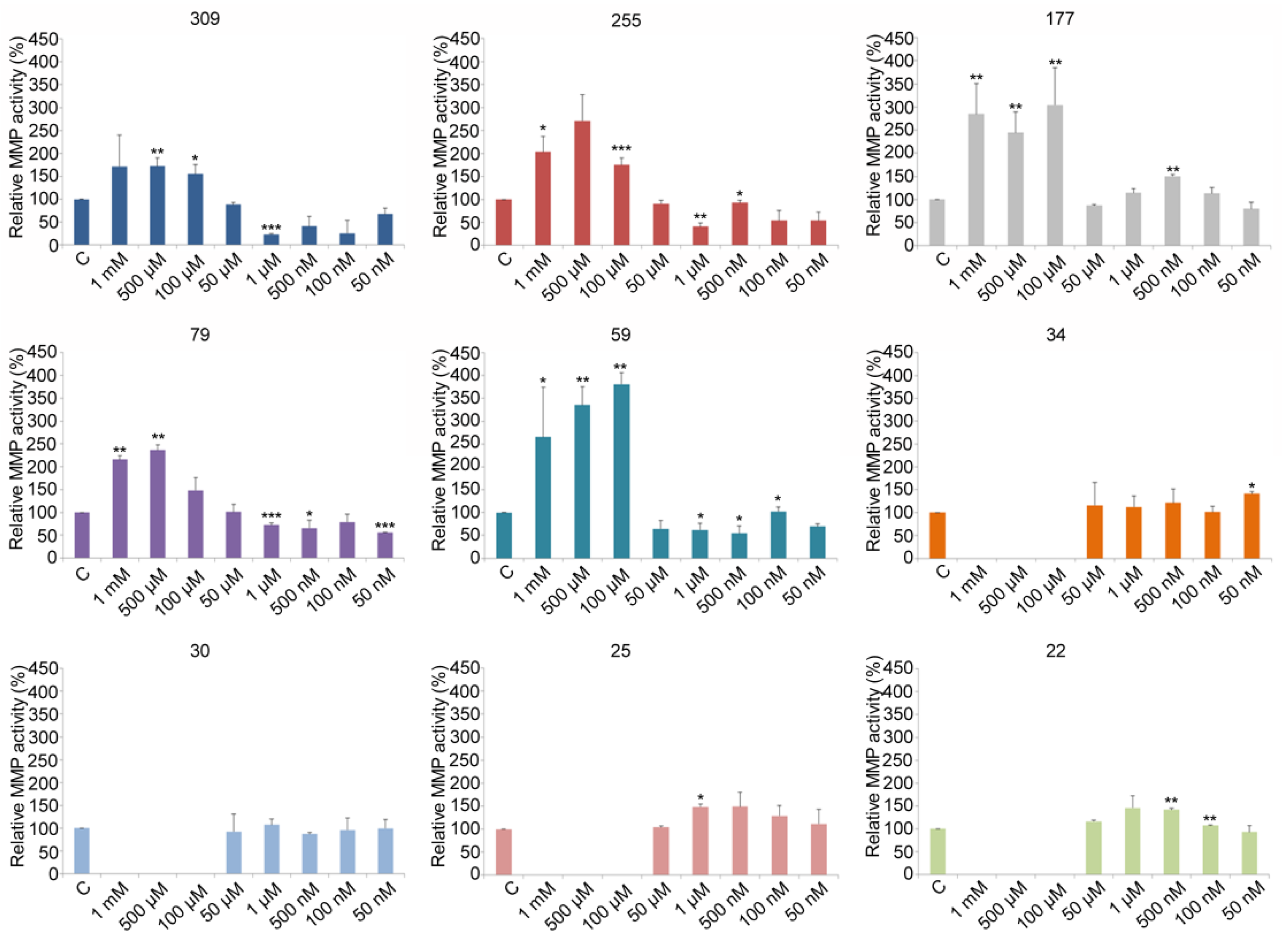
3.2. V Perturbs the Proteolytic Activities of Gelatinases
3.3. Relative Gelatinase Activities
3.4. Gelatinase Characterization Using Chemical Inhibitors
4. Discussion
4.1. V Effects on Fertilization Outcomes in a Dose Dependent Manner
4.2. Modulation of Gelatinase Activity Induced by V in Sea Urchin Embryos
4.3. V Induces the Activation of Two MMP-like Gelatinases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiarelli, R.; Roccheri, M.C. Heavy metals and metalloids as autophagy inducing agents: Focus on cadmium and arsenic. Cells 2012, 1, 597–616. [Google Scholar] [CrossRef] [PubMed]
- Casado-Martinez, M.C.; Smith, B.D.; Luoma, S.N.; Rainbow, P.S. Metal toxicity in a sediment-dwelling polychaete: Threshold body concentrations or overwhelming accumulation rates? Environ. Pollut. 2010, 158, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Koivula, M.J.; Eeva, T. Metal-related oxidative stress in birds. Environ. Pollut. 2010, 158, 2359–2370. [Google Scholar] [CrossRef] [PubMed]
- Soldevila-Barreda, J.J.; Sadler, P.J. Approaches to the design of catalytic metallodrugs. Curr. Opin. Chem. Biol. 2015, 25, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Byrne, M.; Roccheri, M.C.; Chiarelli, R. Interactive effects of increased temperature and gadolinium pollution in Paracentrotus lividus sea urchin embryos: A climate change perspective. Aquat. Toxicol. 2021, 232, 105750. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H. Overview and frontier for the development of metallopharmaceutics. J. Health Sci. 2012, 56, 129–143. [Google Scholar] [CrossRef]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef]
- Venkataraman, B.V.; Sudha, S. Vanadium toxicity. Asian J. Exp. Sci. 2005, 19, 127–134. [Google Scholar]
- Mukherjee, B.; Patra, B.; Mahapatra, S.; Banerjee, P.; Tiwari, A.; Chatterjee, M. Vanadium--an element of atypical biological significance. Toxicol. Lett. 2004, 150, 135–143. [Google Scholar] [CrossRef]
- Kamika, I.; Momba, M.N. Effect of vanadium toxicity at its different oxidation states on selected bacterial and protozoan isolates in wastewater systems. Environ. Technol. 2014, 35, 2075–2085. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Kumar, A. Is there a role for sodium orthovanadate in the treatment of diabetes? Curr. Diabetes Rev. 2019, 15, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Gumerova, N.; Sciortino, G.; Garribba, E.; Rompel, A.; Debbie, C. Polyoxovanadates with emerging biomedical activities. Coord Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Biochemical and medical importance of vanadium compounds. Acta Biochim. Pol. 2012, 59, 195–200. [Google Scholar] [CrossRef]
- Chiarelli, R.; Martino, C.; Roccheri, M.C.; Cancemi, P. Toxic effects induced by vanadium on sea urchin embryos. Chemosphere 2021, 274, 129843. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Luz, A.L.; Wu, X.; Tokar, E.J. Toxicology of Inorganic Carcinogens. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J.M., Eds.; Elsevier: London, UK, 2018; pp. 1–46. [Google Scholar] [CrossRef]
- Desaulniers, D.; Cummings-Lorbetskie, C.; Leingartner, K.; Xiao, G.H.; Zhou, G.; Parfett, C. Effects of vanadium (sodium metavanadate) and aflatoxin-B1 on cytochrome p450 activities, DNA damage and DNA methylation in human liver cell lines. Toxicol. Vitr. 2021, 70, 105036. [Google Scholar] [CrossRef]
- Hussain Shah, S.Z.; Rashid, A.; Naveed, A.K.; Khan, S.A.; Jahan, S. Genotoxic And Cytotoxic Effects Of Oral Vanadyl Sulphate. J. Ayub. Med. Coll Abbottabad 2019, 31522–31526. [Google Scholar]
- Gustafsson, J.P. Vanadium geochemistry in the biogeosphere–speciation, solid-solution interactions, and ecotoxicity. Appl. Geochem. 2019, 102, 1–25. [Google Scholar] [CrossRef]
- Martino, C.; Bonaventura, R.; Byrne, M.; Roccheri, M.; Matranga, V. Effects of exposure to gadolinium on the development of geographically and phylogenetically distant sea urchins species. Mar. Environ. Res. 2017, 128, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Geraci, F.; Pinsino, A.; Turturici, G.; Savona, R.; Giudice, G.; Sconzo, G. Nickel, lead, and cadmium induce differential cellular responses in sea urchin embryos by activating the synthesis of different HSP70s. Biochem. Biophys. Res. Commun. 2004, 322, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, R.; Agnello, M.; Roccheri, M.C. Sea urchin embryos as a model system for studying autophagy induced by cadmium stress. Autophagy 2011, 7, 1028–1034. [Google Scholar] [CrossRef]
- Pinsino, A.; Turturici, G.; Sconzo, G.; Geraci, F. Rapid changes in heat-shock cognate 70 levels, heat-shock cognate phosphorylation state, heat-shock transcription factor, and metal transcription factor activity levels in response to heavy metal exposure during sea urchin embryonic development. Ecotoxicology 2011, 20, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Maisano, M.; Cappello, T.; Catanese, E.; Vitale, V.; Natalotto, A.; Giannetto, A.; Barreca, D.; Brunelli, E.; Mauceri, A.; Fasulo, S. Developmental abnormalities and neurotoxicological effects of CuO NPs on the black sea urchin Arbacia lixula by embryotoxicity assay. Mar. Environ. Res. 2015, 111, 121–127. [Google Scholar] [CrossRef]
- Chiarelli, R.; Martino, C.; Agnello, M.; Bosco, L.; Roccheri, M.C. Autophagy as a defense strategy against stress: Focus on Paracentrotus lividus sea urchin embryos exposed to cadmium. Cell Stress Chaperones 2016, 21, 19–27. [Google Scholar] [CrossRef]
- Cappello, T.; Vitale, V.; Oliva, S.; Villari, V.; Mauceri, A.; Fasulo, S.; Maisano, M. Alteration of neurotransmission and skeletogenesis in sea urchin Arbacia lixula embryos exposed to copper oxide nanoparticles. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 199, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Chiarelli, R.; Bosco, L.; Roccheri, M.C. Induction of skeletal abnormalities and autophagy in Paracentrotus lividus sea urchin embryos exposed to gadolinium. Mar. Environ. Res. 2017, 130, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, R.; Zito, F.; Chiaramonte, M.; Costa, C.; Russo, R. Nickel toxicity in P. lividus embryos: Dose dependent effects and gene expression analysis. Mar. Environ. Res. 2018, 139, 113–121. [Google Scholar] [CrossRef]
- Giannetto, A.; Cappello, T.; Oliva, S.; Parrino, V.; De Marco, G.; Fasulo, S.; Mauceri, A.; Maisano, M. Copper oxide nanoparticles induce the transcriptional modulation of oxidative stress-related genes in Arbacia lixula embryos. Aquat. Toxicol. 2018, 201, 187–197. [Google Scholar] [CrossRef]
- Martino, C.; Costa, C.; Roccheri, M.C.; Koop, D.; Scudiero, R.; Byrne, M. Gadolinium perturbs expression of skeletogenic genes, calcium uptake and larval development in phylogenetically distant sea urchin species. Aquat. Toxicol. 2018, 194, 57–66. [Google Scholar] [CrossRef]
- Chiarelli, R.; Martino, C.; Roccheri, M.C. Cadmium stress effects indicating marine pollution in different species of sea urchin employed as environmental bioindicators. Cell Stress Chaperones 2019, 24, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.D.; Nogueira, L.S.; Domingos-Moreira, F.X.V.; Gomes Costa, P.; Bianchini, A.; Wood, C.M. Effects of sublethal Cd, Zn, and mixture exposures on antioxidant defense and oxidative stress parameters in early life stages of the purple sea urchin Strongylocentrotus purpuratus. Aquat. Toxicol. 2019, 217, 105338. [Google Scholar] [CrossRef] [PubMed]
- Matranga, V.; Pinsino, A.; Bonaventura, R.; Costa, C.; Karakostis, K.; Martino, C.; Russo, R.; Zito, F. Cellular and molecular bases of biomineralization in sea urchin embryos. Cah. Biol. Mar. 2013, 54, 467–468. [Google Scholar]
- Agnello, M.; Bosco, L.; Chiarelli, R.; Martino, C.; Roccheri, M.C. The role of autophagy and apoptosis during embryo development. In Cell Death-Autophagy, Apoptosis and Necrosis; Ntuli, T.M., Ed.; InTechOpen: London, UK, 2015; pp. 83–112. [Google Scholar] [CrossRef][Green Version]
- Agnello, M.; Chiarelli, R.; Martino, C.; Bosco, L.; Roccheri, M.C. Autophagy is required for sea urchin oogenesis and early development. Zygote 2016, 24, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Petanidis, S.; Kioseoglou, E.; Hadzopoulou-Cladaras, M.; Salifoglou, A. Novel ternary vanadium-betaine-peroxido species suppresses H-ras and matrix metalloproteinase-2 expression by increasing reactive oxygen species-mediated apoptosis in cancer cells. Cancer Lett. 2013, 335, 387–396. [Google Scholar] [CrossRef]
- Colín-Barenque, L.; Martínez-Hernández, M.G.; Baiza-Gutman, L.A.; Avila-Costa, M.R.; Ordóñez-Librado, J.L.; Bizarro-Nevares, P.; Rodriguez-Lara, V.; Piñón-Zarate, G.; Rojas-Lemus, M.; Mussali-Galante, P.; et al. Matrix metalloproteinases 2 and 9 in central nervous system and their modification after vanadium inhalation. J. Appl. Toxicol. 2008, 28, 718–723. [Google Scholar] [CrossRef]
- Oliver, S.J.; Firestein, G.S.; Arsenault, L.; Cruz, T.F.; Cheng, T.P.; Banquerigo, M.L.; Boyle, D.L.; Brahn, E. Vanadate, an inhibitor of stromelysin and collagenase expression, suppresses collagen induced arthritis. J. Rheumatol. 2007, 34, 1802–1809. [Google Scholar]
- Li, L.; Gao, L.; Liu, S.; Liu, Q.; Sun, S.; Huan, Y.; Li, C.; Peng, J.; Hou, G.; Liu, W.; et al. Bis(α-furancarboxylato)oxovanadium(IV) exerts durable antidiabetic effects and suppresses matrix metalloproteinase-2 activity in spontaneous type 2 diabetic KKAy mice. Biol. Trace Elem. Res. 2013, 153, 329–339. [Google Scholar] [CrossRef]
- Martino, C.; Chiarelli, R.; Roccheri, M.C.; Matranga, V.; Byrne, M. Effects of magnesium deprivation on development and biomineralization in the sea urchin Arbacia lixula. Invertebr. Reprod. Dev. 2019, 63, 165–176. [Google Scholar] [CrossRef]
- Roe, J.L.; Park, H.R.; Strittmatter, W.J.; Lennarz, W.J. Inhibitors of metalloendoproteases block spiculogenesis in sea urchin primary mesenchyme cells. Exp. Cell Res. 1989, 181, 542–550. [Google Scholar] [CrossRef]
- Ingersoll, E.P.; Wilt, F.H. Matrix metalloproteinase inhibitors disrupt spicule formation by primary mesenchyme cells in the sea urchin embryo. Dev. Biol. 1998, 196, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, E.P.; McDonald, K.L.; Wilt, F.H. Ultrastructural localization of spicule matrix proteins in normal and metalloproteinase inhibitor-treated sea urchin primary mesenchyme cells. J. Exp. Zool. A Comp. Exp. Biol. 2003, 300, 101–112. [Google Scholar] [CrossRef]
- Carballeira, C.; Martín-Díaz, L.; Delvalls, T.A. Influence of salinity on fertilization and larval development toxicity tests with two species of sea urchin. Mar. Environ. Res. 2011, 72, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Ramdial, K.S.; Abell, R.; Last, K.S. Elevated toxicity of resuspended mine tailings over time. Mar. Environ. Res. 2021, 171, 105471. [Google Scholar] [CrossRef]
- Pinsino, A.; Roccheri, M.C.; Matranga, V. Manganese overload affects p38 MAPK phosphorylation and metalloproteinase activity during sea urchin embryonic development. Mar. Environ. Res. 2014, 93, 64–69. [Google Scholar] [CrossRef]
- Farkas, A.; Salánki, J.; Varanka, I. Heavy metal concentrations in fish of Lake Balaton. Lake Reserv. Manag. 2000, 5, 271–279. [Google Scholar] [CrossRef]
- Sarras, M.P. BMP-1 and the astacin family of metalloproteinases: A potential link between the extracellular matrix, growth factors and pattern formation. Bioessays 1996, 18, 439–442. [Google Scholar] [CrossRef]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef]
- Blobel, C.P. ADAMs: Key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005, 6, 32–43. [Google Scholar] [CrossRef]
- Brenner, C.A.; Adler, R.R.; Rappolee, D.A.; Pedersen, R.A.; Werb, Z. Genes for extracellular-matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev. 1989, 3, 848–859. [Google Scholar] [CrossRef] [PubMed]



| V Concentrations | TI |
|---|---|
| 1 mM | 0.8 |
| 500 µM | 0.7 |
| 100 µM | 0.6 |
| 50 µM | 0.4 |
| 1 µM | 0.2 |
| 500 nM | 0.04 |
| 100 nM | 0.04 |
| 50 nM | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarelli, R.; Martino, C.; Roccheri, M.C.; Geraci, F. Vanadium Toxicity Monitored by Fertilization Outcomes and Metal Related Proteolytic Activities in Paracentrotus lividus Embryos. Toxics 2022, 10, 83. https://doi.org/10.3390/toxics10020083
Chiarelli R, Martino C, Roccheri MC, Geraci F. Vanadium Toxicity Monitored by Fertilization Outcomes and Metal Related Proteolytic Activities in Paracentrotus lividus Embryos. Toxics. 2022; 10(2):83. https://doi.org/10.3390/toxics10020083
Chicago/Turabian StyleChiarelli, Roberto, Chiara Martino, Maria Carmela Roccheri, and Fabiana Geraci. 2022. "Vanadium Toxicity Monitored by Fertilization Outcomes and Metal Related Proteolytic Activities in Paracentrotus lividus Embryos" Toxics 10, no. 2: 83. https://doi.org/10.3390/toxics10020083
APA StyleChiarelli, R., Martino, C., Roccheri, M. C., & Geraci, F. (2022). Vanadium Toxicity Monitored by Fertilization Outcomes and Metal Related Proteolytic Activities in Paracentrotus lividus Embryos. Toxics, 10(2), 83. https://doi.org/10.3390/toxics10020083







