Polyacrylamide Functionalized Graphene Oxide/Alginate Beads for Removing Ciprofloxacin Antibiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GO/Ca-Alg2–PAM Beads
2.3. Characterization of the GO/Ca-Alg2–PAM Beads
2.4. Adsorption Experiments
3. Results
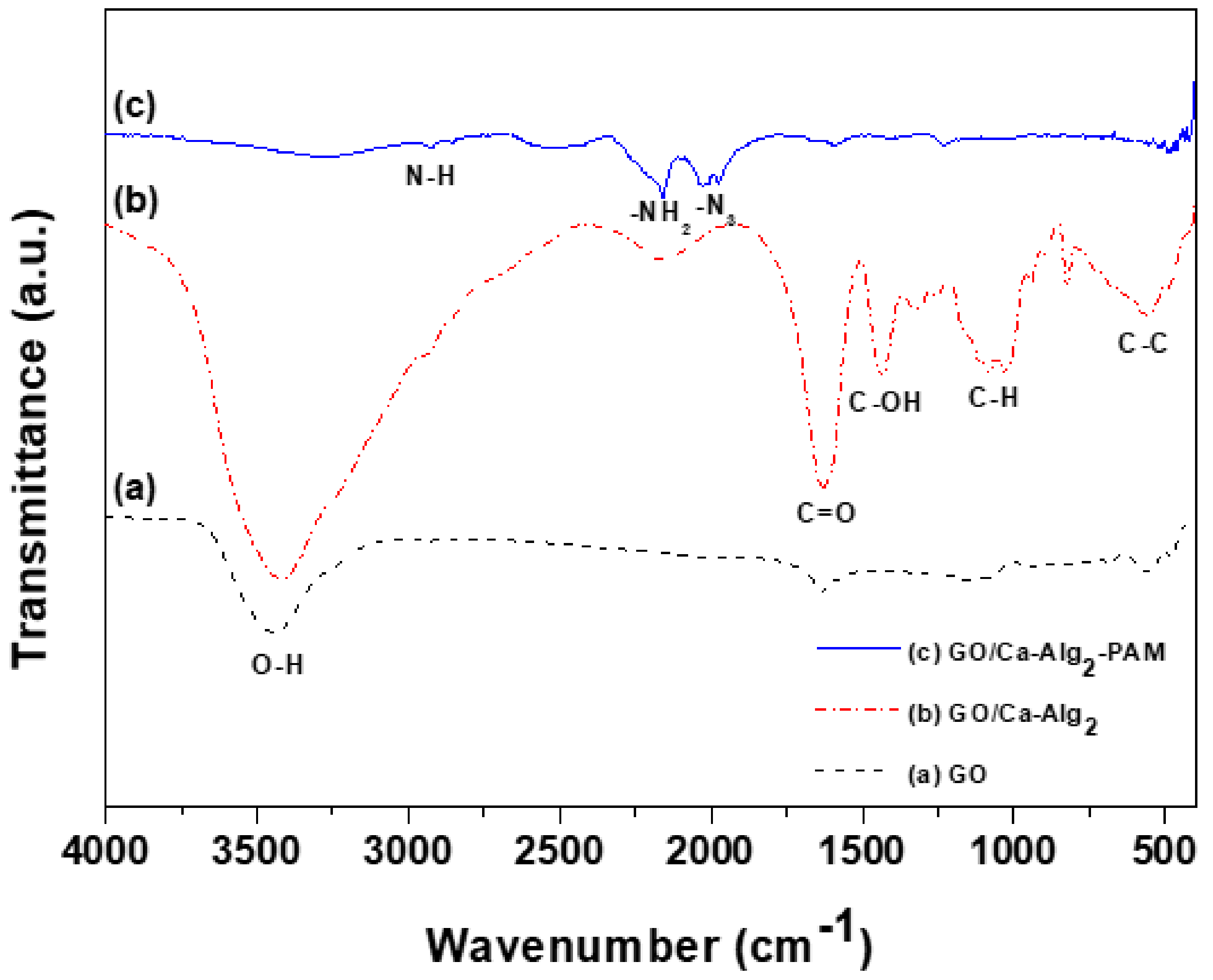
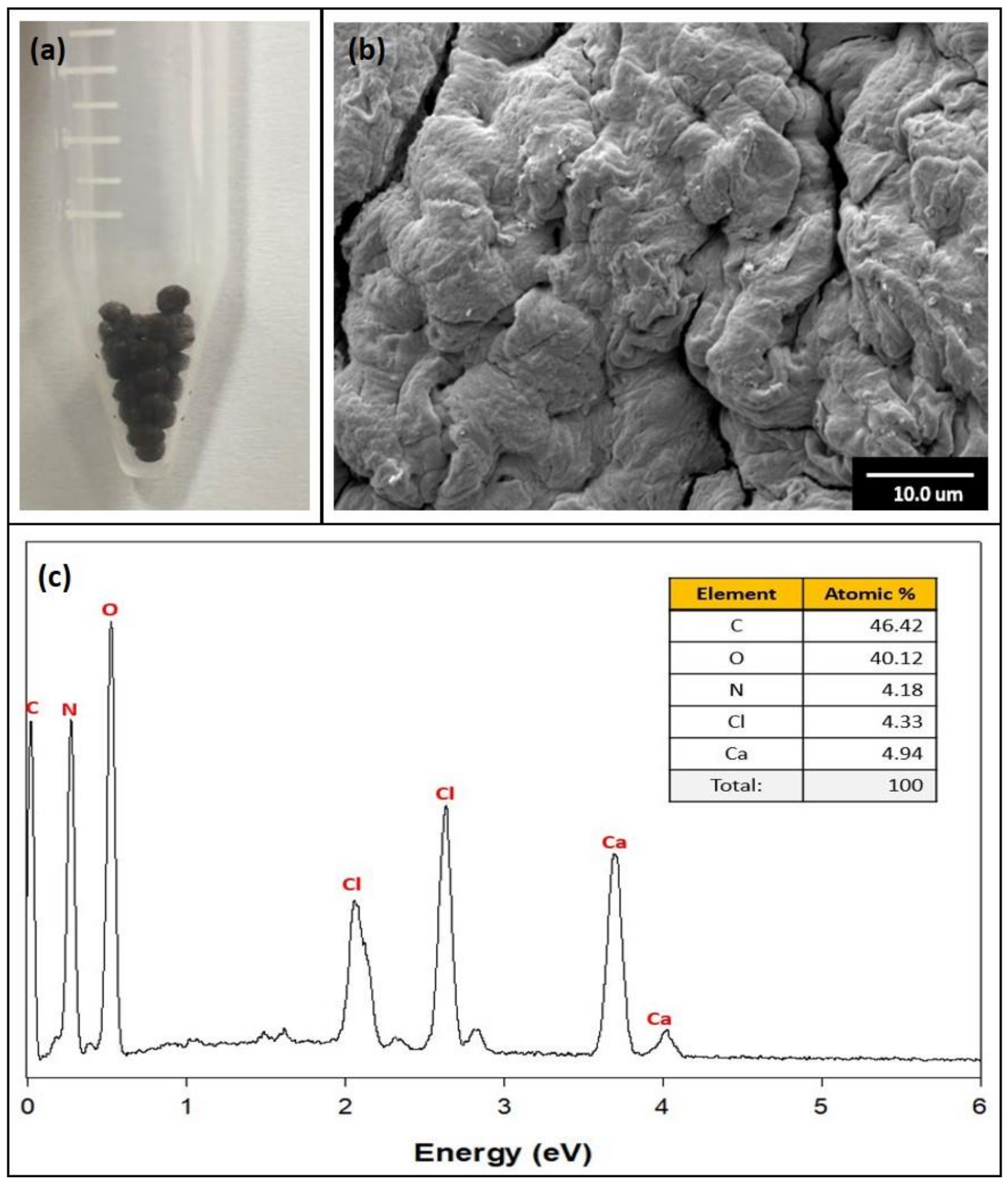
3.1. Characterization of GO/Ca-Alg2–PAM Beads
3.2. Adsorption Equilibrium Isotherms
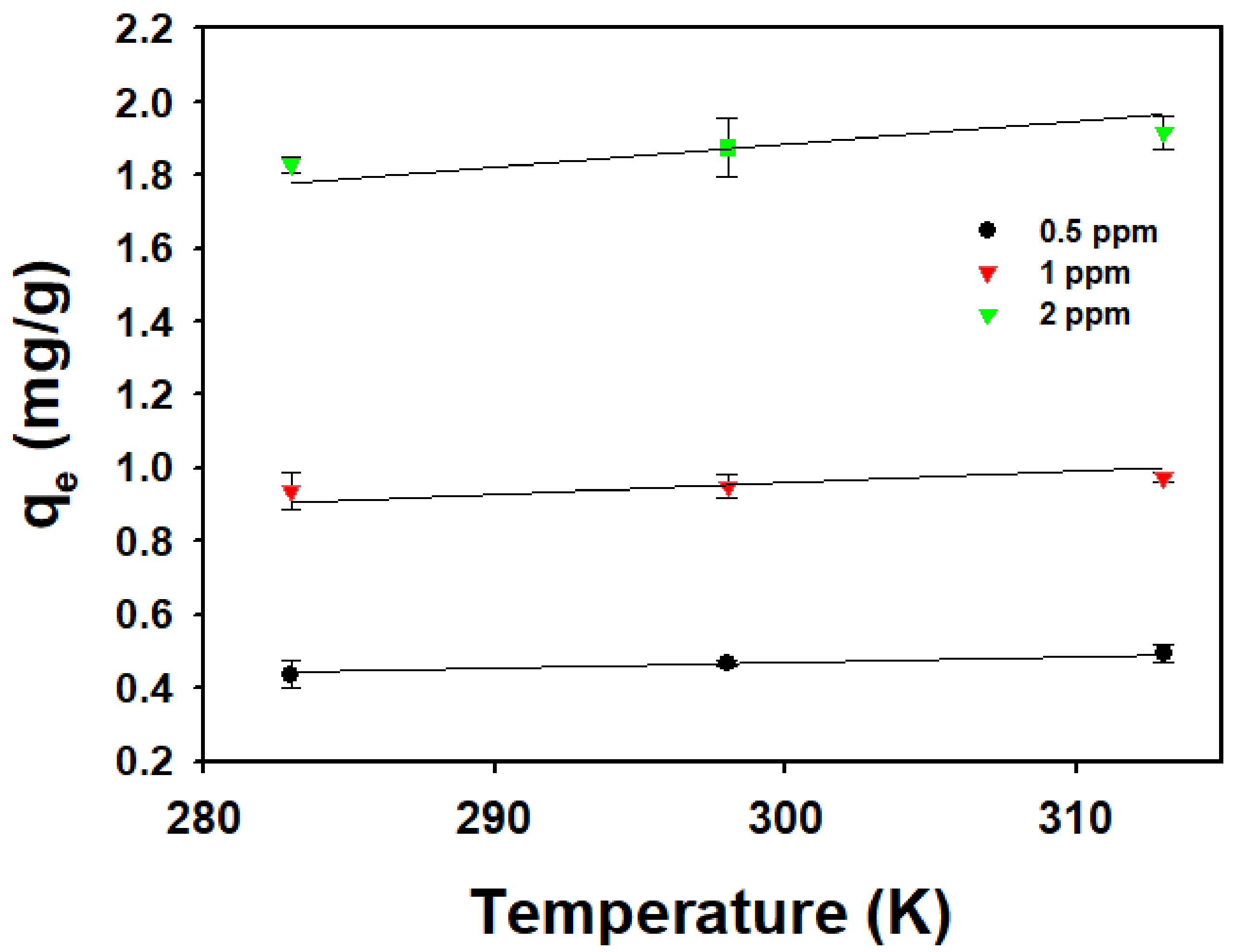
3.3. Effect of Temperature and Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kosutic, K.; Dolar, D.; Asperger, D.; Kunst, B. Removal of antibiotics from a model wastewater by RO/NF membranes. Sep. Purif. Technol. 2007, 53, 244–249. [Google Scholar] [CrossRef]
- Campoli-Richards, D.M.; Monk, J.P.; Price, A.; Benfield, P.; Todd, P.A.; Ward, A. Ciprofloxacin. Drugs 1988, 35, 373–447. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Markham, A.; Balfour, J.A. Ciprofloxacin. Drugs 1996, 51, 1019–1074. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Ke, X.X.; Shao, Z.D.; Zhong, L.B.; Zhao, Q.B.; Zheng, Y.M. Fish scale-based biochar with defined pore size and ultrahigh specific surface area for highly efficient adsorption of ciprofloxacin. Chemosphere 2022, 287, 131962. [Google Scholar]
- Chen, Q.; Zhu, L.; Zhao, C.; Wang, Q.; Zheng, J. A Robust, One-Pot Synthesis of Highly Mechanical and Recoverable Double Network Hydrogels Using Thermoreversible Sol-Gel Polysaccharide. Adv. Mater. 2013, 25, 4171–4176. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, K.; Liu, Z.; Xu, F. Double network hydrogel with high mechanical strength: Performance, progress and future perspective. Sci. China Technol. Sci. 2012, 55, 2241–2254. [Google Scholar] [CrossRef]
- Wang, X.; Deng, W.; Xie, Y.; Wang, C. Selective removal of mercury ions using a chitosan–poly(vinyl alcohol) hydrogel adsorbent with three-dimensional network structure. Chem. Eng. J. 2013, 228, 232–242. [Google Scholar] [CrossRef]
- Tian, J.; Yang, Y.; Xue, T.; Chao, G.; Fan, W.; Liu, T. Highly flexible and compressible polyimide/silica aerogels with integrated double network for thermal insulation and fire-retardancy. J. Mater. Sci. Technol. 2022, 105, 194–202. [Google Scholar] [CrossRef]
- Karimzadeh, Z.; Namazi, H. Nontoxic double-network polymeric hybrid aerogel functionalized with reduced graphene oxide: Preparation, characterization, and evaluation as drug delivery agent. J. Polym. Res. 2022, 29, 1–14. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Graphene oxide and its application as an adsorbent for wastewater treatment. J. Chem. Technol. Biotechnol. 2013, 89, 196–205. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Yu, F.; Chen, J.; Ma, J. Batch and column adsorption of methylene blue by graphene/alginate nanocomposite: Comparison of single-network and double-network hydrogels. J. Environ. Chem. Eng. 2016, 4, 147–156. [Google Scholar] [CrossRef]
- Sikorski, P.; Mo, F.; Skjåk-Bræk, G.; Stokke, B.T. Evidence for Egg-Box-Compatible Interactions in Calcium–Alginate Gels from Fiber X-ray Diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef]
- Risbud, M.V.; Bhonde, R.R. Polyacrylamide-chitosan hydrogels: In vitro biocompatibility and sustained antibiotic release studies. Drug Deliv. 2000, 7, 69–75. [Google Scholar]
- Fei, Y.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. J. Colloid Interface Sci. 2016, 484, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.M.; Huang, L.J.; Wang, Y.X.; Zhao, Y.C.; Tang, J.G.; Wang, Y.; Zhang, Y.; Hedayati, M.; Kipper, M.J.; Wickramasinghe, S.R. Synthesis of graphene oxide/polyacrylamide composite membranes for organic dyes/water separation in water purification. J. Mater. Sci. 2019, 54, 252–264. [Google Scholar] [CrossRef]
- Shen, J.; Yan, B.; Li, T.; Long, Y.; Li, N.; Ye, M. Study on graphene-oxide-based polyacrylamide composite hydrogels. Compos. A Appl. Sci. Manuf. 2012, 43, 1476–1481. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Chiu, W.-T.; Wang, C.-C. Regression analysis for the sorption isotherms of basic dyes on sugarcane dust. Bioresour. Technol. 2005, 96, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Qiao, G.-L.; Zhao, F.; Wang, Y. Thermodynamic and kinetic parameters of ciprofloxacin adsorption onto modified coal fly ash from aqueous solution. J. Mol. Liq. 2011, 163, 53–56. [Google Scholar] [CrossRef]
- Zhu, X.; Tsang, D.C.; Chen, F.; Li, S.; Yang, X. Ciprofloxacin adsorption on graphene and granular activated carbon: Kinetics, isotherms, and effects of solution chemistry. Environ. Technol. 2015, 36, 3094–3102. [Google Scholar] [CrossRef] [PubMed]
- MacKay, A.A.; Seremet, D.E. Probe Compounds to Quantify Cation Exchange and Complexation Interactions of Ciprofloxacin with Soils. Environ. Sci. Technol. 2008, 42, 8270–8276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, C.-H. Adsorption and oxidation of fluoroquinolone antibacterial agents and structurally related amines with goethite. Chemosphere 2007, 66, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhu, Y.; Zhang, Y.; Lin, T.; Zeng, G.; Zhang, S.; Wang, Y.; He, W.; Zhang, M.; Long, H. An efficient adsorbent: Simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption. Bioresour. Technol. 2019, 288, 121511. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef]

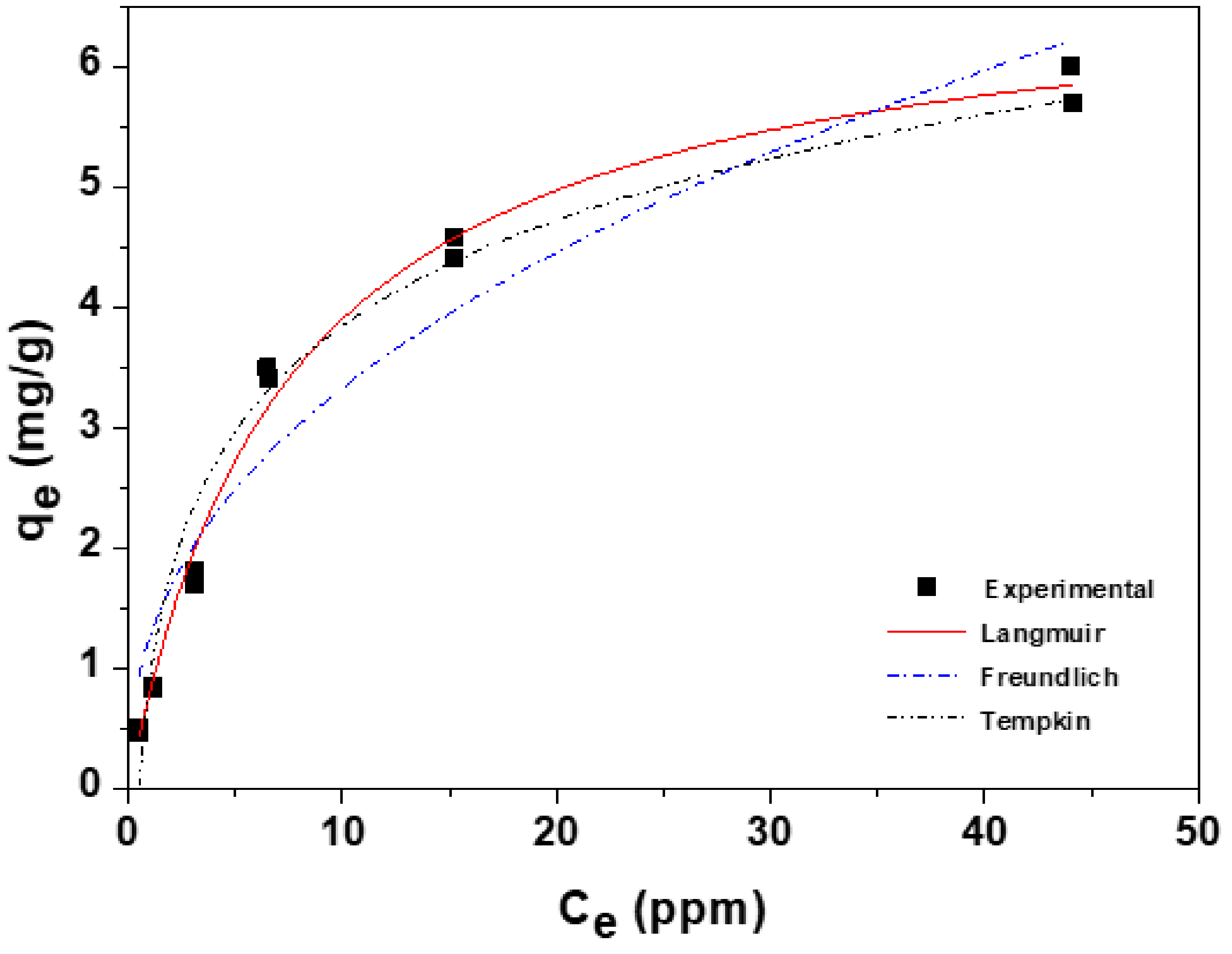

| Isotherm Model | Parameters | r2 | χ2 |
|---|---|---|---|
| Langmuir | qm = 6.846 mg/g b = 0.132 L/mg | 0.991 | 0.037 |
| Freundlich | KF = 1.260 (mg/g)(L/mg)1/n n = 2.372 | 0.932 | 0.282 |
| Temkin | B = 1954.69 L/mg KT = 2.074 L/g | 0.966 | 0.142 |
| Adsorbents | qm (mg/g) | Temperature (K) | References |
|---|---|---|---|
| GO/Ca-Alg2–PAM | 6.846 | 298 | This study |
| MCFA | 1.547 | 313 | [20] |
| GAC | 217.4 | 298 | [21] |
| Kaolinite | 7.952 | 308 | [22] |
| Goethite | 19.88 | 295 | [23] |
| ZnO-BC-2-650 | 0.449 | 298 | [24] |
| CPX (ppm) | ΔH (kJ/mol) | ΔS (kJ/mol∙K) | ΔG (kJ/mol) | ||
|---|---|---|---|---|---|
| 283 K | 298 K | 313 K | |||
| 0.5 | 10.7 | 0.056 | −5.29 | −6.13 | −6.98 |
| 1 | 4.4 | 0.035 | −5.57 | −6.10 | −6.63 |
| 2 | 7.2 | 0.044 | −5.25 | −5.91 | −6.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-W.; Choi, S.-J. Polyacrylamide Functionalized Graphene Oxide/Alginate Beads for Removing Ciprofloxacin Antibiotics. Toxics 2022, 10, 77. https://doi.org/10.3390/toxics10020077
Choi J-W, Choi S-J. Polyacrylamide Functionalized Graphene Oxide/Alginate Beads for Removing Ciprofloxacin Antibiotics. Toxics. 2022; 10(2):77. https://doi.org/10.3390/toxics10020077
Chicago/Turabian StyleChoi, Jung-Weon, and Sang-June Choi. 2022. "Polyacrylamide Functionalized Graphene Oxide/Alginate Beads for Removing Ciprofloxacin Antibiotics" Toxics 10, no. 2: 77. https://doi.org/10.3390/toxics10020077
APA StyleChoi, J.-W., & Choi, S.-J. (2022). Polyacrylamide Functionalized Graphene Oxide/Alginate Beads for Removing Ciprofloxacin Antibiotics. Toxics, 10(2), 77. https://doi.org/10.3390/toxics10020077





