Prioritizing the Effects of Emerging Contaminants on Estuarine Production under Global Warming Scenarios
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Development
2.2. ECs Brief Description and Selection
2.3. Including ECs, LC50 and EC50 in the Minho Ecosystem Model
2.4. Single- and Multiple-Stressors Simulated Scenarios
2.5. Global Sensitivity Analysis
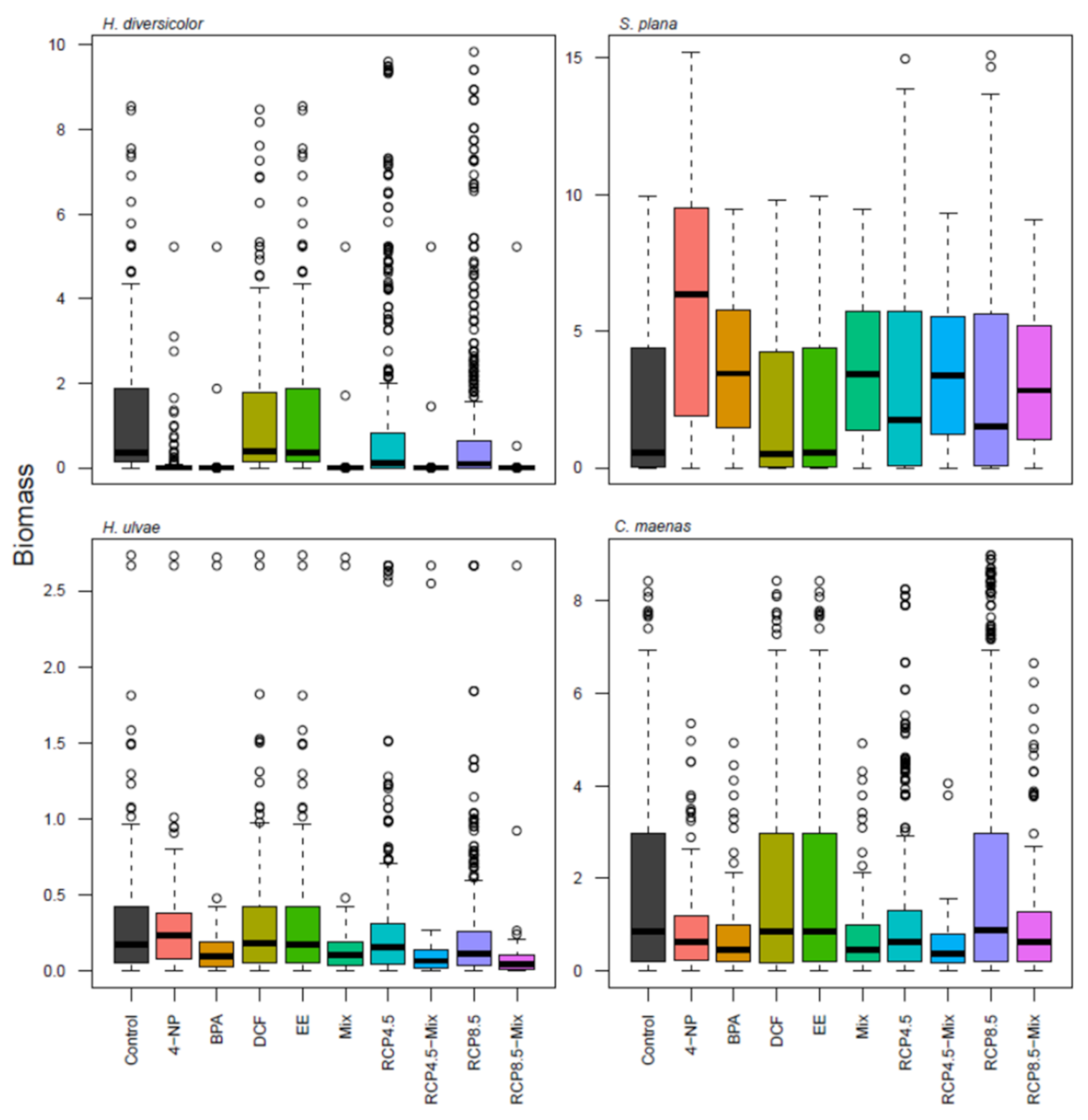
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strokal, M.; Spanier, J.E.; Kroeze, C.; Koelmans, A.A.; Flörke, M.; Franssen, W.; Hofstra, N.; Langan, S.; Tang, T.; van Vliet, M.T.; et al. Global multi-pollutant modelling of water quality: Scientific challenges and future directions. Curr. Opin. Environ. Sustain. 2018, 36, 116–125. [Google Scholar] [CrossRef]
- Capela, R.; Raimundo, J.; Santos, M.M.; Caetano, M.; Micaelo, C.; Vale, C.; Guimarães, L.; Reis-Henriques, M.A. The use of biomarkers as integrative tools for transitional water bodies monitoring in the Water Framework Directive context—A holistic approach in Minho river transitional waters. Sci. Total Environ. 2016, 539, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Warren, R.; He, Y.; Ye, J.; Li, Q.; Wang, G. Impacts of climate change on TN load and its control in a River Basin with complex pollution sources. Sci. Total Environ. 2018, 615, 1155–1163. [Google Scholar] [CrossRef]
- Andričević, R.; Galešić, M. Contaminant dilution measure for the solute transport in an estuary. Adv. Water Resour. 2018, 117, 65–74. [Google Scholar] [CrossRef]
- Romo, J.; Chaudhary, M.; Walker, T.R. Baseline assessment of contaminants in marine biota prior to remediation of industrial effluent impacted sediments in a former tidal estuary in Nova Scotia, Canada. Mar. Pollut. Bull. 2019, 145, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Yeh, A.; Young, G.; Gallagher, E.P. Contaminants of emerging concern in a large temperate estuary. Environ. Pollut. 2016, 213, 254–267. [Google Scholar] [CrossRef]
- Rimayi, C.; Odusanya, D.; Weiss, J.M.; de Boer, J.; Chimuka, L. Contaminants of emerging concern in the Hartbeespoort Dam catchment and the uMngeni River estuary 2016 pollution incident, South Africa. Sci. Total Environ. 2018, 627, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Letsinger, S.; Kay, P.; Rodríguez-Mozaz, S.; Villagrassa, M.; Barceló, D.; Rotchell, J.M. Spatial and temporal occurrence of pharmaceuticals in UK estuaries. Sci. Total Environ. 2019, 678, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.W.; Castro, V.; Chaves, R.; Espinosa, M.; Rodil, R.; Quintana, J.B.; Vieira, M.N.; Santos, M.M. Using zebrafish embryo bioassays combined with high-resolution mass spectrometry screening to assess ecotoxicological water bodies quality status: A case study in Panama rivers. Chemosphere 2021, 272, 129823. [Google Scholar] [CrossRef]
- Fonseca, V.F.; Vasconcelos, R.P.; França, S.; Serafim, A.; Lopes, B.; Company, R.; Bebiano, M.J.; Costa, M.J.; Cabral, H.N. Modeling fish biological responses to contaminants and natural variability in estuaries. Mar. Environ. Res. 2014, 96, 45–55. [Google Scholar] [CrossRef]
- Lencioni, V.; Bellamoli, F.; Paoli, F. Multi-level effects of emerging contaminants on macroinvertebrates in Alpine streams: From DNA to the ecosystem. Ecol. Indic. 2020, 117, 106660. [Google Scholar] [CrossRef]
- Thompson, B.; Adelsbach, T.; Brown, C.; Hunt, J.; Kuwabara, J.; Neale, J.; Ohlendorf, H.; Schwarzbach, S.; Spies, R.; Taberski, K. Biological effects of anthropogenic contaminants in the San Francisco Estuary. Environ. Res. 2007, 105, 156–174. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, J.; Greenway, G.M.; Munshi, M.; Gómez, J.C.; Mazik, K.; Knight, A.W.; Hardege, J.D.; Elliot, M. Biological responses to contaminants in the Humber Estuary: Disentangling complex relationships. Mar. Environ. Res. 2011, 71, 295–303. [Google Scholar] [CrossRef]
- Hylland, K.; Burgeot, T.; Martínez-Gómez, C.; Lang, T.; Robinson, C.D.; Svavarsson, J.; Thain, J.E.; Vethaak, A.D.; Gubbins, M.J. How can we quantify impacts of contaminants in marine ecosystems? The ICON project. Mar. Environ. Res. 2015, 124, 2–10. [Google Scholar] [CrossRef]
- Little, S.; Spencer, K.L.; Schuttelaars, H.M.; Millward, G.E.; Elliot, M. Unbounded boundaries and shifting baselines: Estuaries and coastal seas in a rapidly changing world. Estuar. Coast. Shelf Sci. 2017, 198, 311–319. [Google Scholar] [CrossRef]
- Smith, S.L.; Cunniff, S.E.; Peyronnin, N.S.; Kritzer, J.P. Prioritizing coastal ecosystem stressors in the Northeast United States under increasing climate change. Environ. Sci. Policy 2017, 78, 49–57. [Google Scholar] [CrossRef]
- Cardoso, P.G.; Rodrigues, D.; Madureira, T.V.; Oliveira, N.; Rocha, M.J.; Rocha, E. Warming modulates the effects of the endocrine disruptor progestin levonorgestrel on the zebrafish fitness, ovary maturation kinetics and reproduction success. Environ. Pollut. 2017, 229, 300–311. [Google Scholar] [CrossRef]
- Ihde, T.F.; Townsend, H.M. Accounting for multiple stressors influencing living marine resources in a complex estuarine ecosystem using an Atlantis model. Ecol. Model. 2017, 365, 1–9. [Google Scholar] [CrossRef]
- Holan, J.R.; King, C.K.; Proctor, A.H.; Davis, A.R. Increased sensitivity of subantarctic marine invertebrates to copper under a changing climate—Effects of salinity and temperature. Environ. Pollut. 2019, 249, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Fulton, E.A.; Link, J.S.; Kaplan, I.C.; Savina-Rolland, M.; Johnson, P.; Ainsworth, C.; Horne, P.; Gorton, R.; Gamble, R.J.; Smith, A.D.M.; et al. Lessons in modelling and management of marine ecosystems: The Atlantis experience. Fish Fish. 2011, 12, 171–188. [Google Scholar] [CrossRef]
- Baeta, A.; Niquil, N.; Marques, J.C.; Patrício, J. Modelling the effects of eutrophication, mitigation measures and an extreme flood event on estuarine benthic food webs. Ecol. Model. 2011, 222, 1209–1221. [Google Scholar] [CrossRef]
- Martins, I.; Dias, E.; Ilarri, M.I.; Campuzano, F.J.; Pinto, L.; Santos, M.M.; Antunes, C. Antagonistic effects of multiple stressors on macroinvertebrate biomass from a temperate estuary (Minho estuary, NW Iberian Peninsula). Ecol. Indic. 2019, 101, 792–803. [Google Scholar] [CrossRef]
- DeLorenzo, M.E. Impacts of climate change on the ecotoxicology of chemical contaminants in estuarine organisms. Curr. Zool. 2015, 61, 641–652. [Google Scholar] [CrossRef]
- European Commission. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC as Regards Priority Substances in the Field of Water Policy. Off. J. Eur. Union 2013, L 126, 1–17. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Lee, J.-S.; Filatova, T.; Ligmann-Zielinska, A.; Hassani-Mahmooei, B.; Stonedahl, F.; Lorscheid, I.; Voinov, A.; Polhill, G.; Sun, Z.; Parker, D.C. The Complexities of Agent-Based Modeling Output Analysis. J. Artif. Soc. Soc. Simul. 2015, 18, 4. [Google Scholar] [CrossRef]
- Bastos, R.; Martins, B.; Cabral, J.A.; Ceia, F.R.; Ramos, J.A.; Paiva, V.H.; Luís, A.; Santos, M. Oceans of stimuli: An individual-based model to assess the role of olfactory cues and local enhancement in seabirds’ foraging behaviour. Anim. Cogn. 2020, 23, 629–642. [Google Scholar] [CrossRef]
- Clough, J.S. Aquatox (RELEASE 3.1 Plus) Modeling Environmental Fate and Ecological Effects in Aquatic Ecosystems Volume 1: User’s Manual; US-EPA: Washington, DC, USA, 2014. [Google Scholar]
- Park, R.A.; Clough, J.S. Aquatox (Release 3.1 Plus) Modeling Environmental Fate and Ecological Effects in Aquatic Ecosystems Volume 2: Technical Documentation; US-EPA: Washington, DC, USA, 2014. [Google Scholar]
- Weber, M. Zur Okosystemstruktur des Minho Astuars (Iberische Westkuste) in German. Ph.D. Thesis, University of Kiel, Kiel, Germany, 1985. [Google Scholar]
- Sousa, R.; Dias, S.; Freitas, V.; Antunes, C. Subtidal macrozoobenthic assemblages along the River Minho estuarine gradient (north-west Iberian Peninsula). Aquat. Conserv. Mar. Freshw. Ecosyst. 2007, 18, 1063–1077. [Google Scholar] [CrossRef]
- Lillebø, A.I.; Pardal, M.A.; Marques, J.C. Population structure, dynamics and production of Hydrobia ulvae (Pennant) (Mollusca: Prosobranchia) along an eutrophication gradient in the Mondego estuary (Portugal). Acta Oecologica 1999, 20, 289–304. [Google Scholar] [CrossRef]
- Klassen, G.; Locke, A. A biological synopsis of the European green crab, Carcinus maenas. In Canadian Manuscript Report of Fisheries and Aquatic Sciences; Fisheries and Ocean Canada, Gulf Fisheries Centre: Moncton, NB, Canada, 2007; Volume 2818, p. vii+75pp. [Google Scholar]
- Verdelhos, T.; Marques, J.C.; Anastácio, P. Behavioral and mortality responses of the bivalves Scrobicularia plana and Cerastoderma edule to temperature, as indicator of climate change’s potential impacts. Ecol. Indic. 2015, 58, 95–103. [Google Scholar] [CrossRef]
- Bhuiyan, K.A.; Rodríguez, B.M.; Pires, A.; Riba, I.; Dellvals, A.; Freitas, R.; Conradi, M. Experimental evidence of uncertain future of the keystone ragworm Hediste diversicolor (O.F. Müller, 1776) under climate change conditions. Sci. Total Environ. 2020, 750, 142031. [Google Scholar] [CrossRef] [PubMed]
- García-Arberas, L.; Rallo, A. Demography and Secondary Production of the Polychaete Hediste diversicolor in a Non-Polluted Estuary in the Bay of Biscay. Mar. Ecol. 2002, 23, 237–251. [Google Scholar] [CrossRef]
- Riera, P.; Stal, L.J.; Nieuwenhuise, J.; Richard, P.; Blanchard, G.; Gentil, F. Determination of food sources for benthic invertebrates in a saltmarsh (Aiguillion Bay, France) by carbon and nitrogen stable isotopes: Importance of locally produced sources. Mar. Ecol. Prog. Ser. 1999, 187, 301–307. [Google Scholar] [CrossRef]
- Morelle, J.; Maire, O.; Richard, A.; Slimani, A.; Orvain, F. Contrasted impact of two macrofaunal species (Hediste diversicolor and Scrobicularia plana) on microphytobenthos spatial distribution and photosynthetic activity at microscale. Mar. Environ. Res. 2020, 163, 105228. [Google Scholar] [CrossRef]
- Directive 2000/60/EC Establishment and Framework for Community Action in the Field of Water Policy; European Parliament and the Council of the European Union: Luxembourg, 2000.
- Directive 2003/53/EC Amending for the 26th Time the Council Directive 76/769/EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Nonylphenol, Nonylphenol Ethoxylate and Cement); European Parliament and the Council of the European Union: Luxembourg, 2003.
- European Commission. Commission Implementing Decision (EU) 2018/840 of 5 June 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495. Off. J. Eur. Union 2018, L141, 9. [Google Scholar]
- Jalal, N.; Surendranath, A.R.; Pathak, J.R.; Yu, S.; Chung, C.Y. Bisphenol A (BPA) the mighty and the mutagenic. Toxicol. Rep. 2017, 5, 76–84. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Siracusa, J.S.; Yin, L.; Measel, E.; Liang, S.; Yu, X. Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod. Toxicol. 2018, 79, 96–123. [Google Scholar] [CrossRef]
- Torres, T.; Ruivo, R.; Santos, M.M. Epigenetic biomarkers as tools for chemical hazard assessment: Gene expression profiling using the model Danio rerio. Sci. Total Environ. 2021, 773, 144830. [Google Scholar] [CrossRef]
- Vought, V.; Wang, H.-S. Impact of common environmental chemicals bisphenol A and bisphenol S on the physiology of Lumbriculus variegatus. Environ. Toxicol. Pharmacol. 2018, 60, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Guiyesse, B.; Jefferson, B.; Cartmell, E.; Lester, J.N. (Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef]
- Salgueiro-González, N.; Concha-Graña, E.; Turnes-Carou, I.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Determination of alkylphenols and bisphenol A in seawater samples by dispersive liquid–liquid microextraction and liquid chromatography tandem mass spectrometry for compliance with environmental quality standards (Directive 2008/105/EC). J. Chromatogr. A 2012, 1223, 1–8. [Google Scholar] [CrossRef]
- Salgueiro-González, N.; Turnes-Carou, I.; Viñas-Diéguez, L.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Occurrence, distribution and bioaccumulation of endocrine disrupting compounds in water, sediment and biota samples from a European river basin. Chemosphere 2015, 131, 241–247. [Google Scholar] [CrossRef]
- Tabata, A.; Kashiwada, S.; Ohnishi, Y.; Ishikawa, H.; Miyamoto, N.; Itoh, M.; Magara, Y. Estrogenic influences of estradiol-17 beta, p-nonylphenol and bisphenol-A on Japanese Medaka (Oryzias latipes) at detected environmental concentrations. Water Sci. Technol. 2001, 43, 109–116. [Google Scholar] [CrossRef]
- Lavado, R.; Thibaut, R.; Raldua, D.; Martin, R.; Porte, C. First evidence of endocrine disruption in feral carp from the Ebro River. Toxicol. Appl. Pharmacol. 2004, 196, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Jie, X.; Jianmei, L.; Zheng, F.; Lei, G.; Biao, Z.; Jie, Y. Neurotoxic effects of nonylphenol: A review. Wien. Klin. Wochenschr. 2013, 125, 61–70. [Google Scholar] [CrossRef]
- HSDB. Ethinylestradiol. Hazardous Substances Data Bank. Available online: https://www.nlm.nih.gov/databases/download/hsdb.html (accessed on 22 November 2021).
- Al-Ansari, A.M.; Atkinson, S.K.; Doyle, J.R.; Trudeau, V.L.; Blais, J.M. Dynamics of uptake and elimination of 17α-ethinylestradiol in male goldfish (Carassius auratus). Aquat. Toxicol. 2013, 132–133, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Maes, H.M.; Maletz, S.X.; Ratte, H.T.; Hollender, J.; Schaeffer, A. Elimination, and Biotransformation of 17α-Ethinylestradiol by the Freshwater Alga Desmodesmus subspicatus. Environ. Sci. Technol. 2014, 48, 12354–12361. [Google Scholar] [CrossRef] [PubMed]
- Vandenbergh, G.F.; Adriaens, D.; Verslycke, T.; Janssen, C.R. Effects of 17α-ethinylestradiol on sexual development of the amphipod Hyalella azteca. Ecotoxicol. Environ. Saf. 2003, 54, 216–222. [Google Scholar] [CrossRef]
- Schramm, K.-W.; Jaser, W.; Welzl, G.; Pfister, G.; Wöhler-Moorhof, G.F.; Hense, B.A. Impact of 17α-ethinylestradiol on the plankton in freshwater microcosms—I: Response of zooplankton and abiotic variables. Ecotoxicol. Environ. Saf. 2008, 69, 437–452. [Google Scholar] [CrossRef]
- Nagpal, N.; Meays, C. Water Quality Guidelines for Pharmaceutically-Active-Compounds (PhACs): 17α-Ethinylestradiol (EE2)—Technical Appendix; Ministry of the Environment, Province of British Columbia, Science and Information Branch, Water Stewardship Division: Victoria, BC, Canada, 2009. [Google Scholar]
- Soares, J.; Coimbra, A.M.; Reis-Henriques, M.A.; Monteiro, N.M.; Vieira, M.N.; Oliveira, J.M.A.; Guedes-Dias, P.; Fontaínhas-Fernandes, A.; Sila Parra, S.; Carvalho, A.P.; et al. Disruption of zebrafish (Danio rerio) embryonic development after full life-cycle parental exposure to low levels of ethinylestradiol. Aquat. Toxicol. 2009, 95, 330–338. [Google Scholar] [CrossRef]
- Silva, P.; Rocha, M.J.; Cruzeiro, C.; Malhão, F.; Reis, B.; Urbatzka, R.; Monteiro, R.A.F.; Rocha, E. Testing the effects of ethinylestradiol and of an environmentally relevant mixture of xenoestrogens as found in the Douro River (Portugal) on the maturation of fish gonads—A stereological study using the zebrafish (Danio rerio) as model. Aquat. Toxicol. 2012, 124–125, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Neuparth, T.; Lyssimachou, A.; Lima, D.; André, A.I.D.; Reis-Henriques, M.A.; Castro, L.F.C.; Carvalho, A.P.; Monteiro, N.M.; Santos, M.M. 17α-ethynilestradiol and tributyltin mixtures modulates the expression of NER and p53 DNA repair pathways in male zebrafish gonads and disrupt offspring embryonic development. Ecol. Indic. 2018, 95, 1008–1018. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.; Khan, A.A. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef]
- Meteyer, C.U.; Rideout, B.A.; Gilbert, M.; Shivaprasad, H.L.; Oaks, J.L. Pathology and proposed pathophysiology of Diclofenac poisoning in free-living and experimentally exposed Oriental White-Backed Vultures (Gyps bengalensis). J. Wildl. Dis. 2005, 41, 707–716. [Google Scholar] [CrossRef]
- Ribeiro, S.; Torres, T.; Martins, R.; Santos, M.M. Toxicity screening of Diclofenac, Propranolol, Sertraline and Simvastatin using Danio rerio and Paracentrotus lividus embryo bioassays. Ecotoxicol. Environ. Saf. 2015, 114, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Balbi, T.; Montagna, M.; Fabbri, R.; Carbone, C.; Franzellitti, S.; Fabbri, E.; Canesi, L. Diclofenac affects early embryo development in the marine bivalve Mytilus galloprovincialis. Sci. Total Environ. 2018, 642, 601–609. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakamura, K.; Yokomizo, H. Relative robustness of NOEC and ECx against large uncertainties in data. PLoS ONE 2018, 13, e0206901. [Google Scholar] [CrossRef]
- SCHER (Scientific Committee on Health and Environmental Risks). Opinion on Draft Environmental Quality Standards under the Water Framework Directive—Diclofenac; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Napierska, D.; Sanseverino, I.; Loos, R.; Cortés, L.G.; Niegowska, M.; Lettieri, T. Modes of Action of the Current Priority Substances List under the Water Framework Directive and other Substances of Interest; EUR 29008 EN; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-77301-3. JRC110117. [Google Scholar] [CrossRef]
- Morris, D.J.; Speirs, D.C.; Cameron, A.I.; Heath, M.R. Global sensitivity analysis of an end-to-end marine ecosystem model of the North Sea: Factors affecting the biomass of fish and benthos. Ecol. Model. 2014, 273, 251–263. [Google Scholar] [CrossRef]
- Saltelli, A.; Aleksankina, K.; Becker, W.; Fennell, P.; Ferretti, F.; Holst, N.; Li, S.; Wu, Q. Why so many published sensitivity analyses are false: A systematic review of sensitivity analysis practices. Environ. Model. Softw. 2019, 114, 29–39. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. Available online: https://www.R-project.org/ (accessed on 22 November 2021).
- Moreira, S.M.; Lima, I.; Ribeiro, R.; Guilhermino, L. Effects of estuarine sediment contamination on feeding and on Effects of estuarine sediment contamination on feeding and on key physiological functions of the polychaete Hediste diversicolor: Laboratory and in situ assays. Aquat. Toxicol. 2006, 78, 186–201. [Google Scholar] [CrossRef]
- Nesto, N.; Simonini, R.; Prevedelli, D.; Da Ros, L. Effects of diet and density on growth, survival and gametogenesis of Hediste diversicolor (O.F. Müller, 1776) (Nereididae, Polychaeta). Aquaculture 2012, 362–363, 1–9. [Google Scholar] [CrossRef]
- Bossier, S.; Nielsen, J.R.; Neuenfeldt, S. Exploring trophic interactions and cascades in the Baltic Sea using a complex end-to-end ecosystem model with extensive food web integration. Ecol. Model. 2020, 436, 109281. [Google Scholar] [CrossRef]
- Grechi, L.; Franco, A.; Palmeri, L.; Pivato, A.; Barausse, A. An ecosystem model of the lower Po river for use in ecological risk assessment of xenobiotics. Ecol. Model. 2016, 332, 42–58. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J. AQUATOX coupled foodweb model for ecosystem risk assessment of polybrominated diphenyl ethers (PBDEs) in lake ecosystems. Environ. Pollut. 2014, 191, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, A.; Franco, A.; Pivato, A.; Barausse, A. Food web modeling of a river ecosystem for risk assessment of down-the-drain chemicals: A case study with AQUATOX. Sci. Total Environ. 2015, 508, 214–227. [Google Scholar] [CrossRef]
- James, C.A.; Lanksbury, J.; Khangaonkar, T.; West, J. Evaluating exposures of bay mussels (Mytilus trossulus) to contaminants of emerging concern through environmental sampling and hydrodynamic modeling. Sci. Total Environ. 2019, 709, 136098. [Google Scholar] [CrossRef]
- Vieira, R.; Martin, A.; Engelen, A.E.; Thomsen, M.S.; Arenas, F. Interactive effects of co-occurring anthropogenic stressors on the seagrass, Zostera noltei. Ecol. Indic. 2019, 109, 10578. [Google Scholar] [CrossRef]
- Tišler, T.; Krel, A.; Gerželj, U.; Erjavec, B.; Dolenc, M.S.; Pintar, A. Hazard identification and risk characterization of bisphenols A, F and AF to aquatic organisms. Environ. Pollut. 2016, 212, 472–479. [Google Scholar] [CrossRef]
- Alexander, H.C.; Dill, D.C.; Smith, L.W.; Guiney, P.D.; Dorn, P. Bisphenol-A: Acute aquatic toxicity. Environ. Toxicol. Chem. 1988, 7, 19–26. [Google Scholar] [CrossRef]
- Tato, T.; Salgueiro-González, N.; León, V.M.; González, S.; Beiras, R. Ecotoxicological evaluation of the risk posed by bisphenol A, triclosan, and 4-nonylphenol in coastal waters using early life stages of marine organisms (Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Acartia clausi). Environ. Pollut. 2018, 232, 173–182. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pid-cock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; p. 32. [Google Scholar]
- Dissanayake, A.; Galloway, T.S.; Jones, M.B. Seasonal differences in the physiology of Carcinus maenas (Crustacea: Decapoda) from estuaries with varying levels of anthropogenic contamination. Estuar. Coast. Shelf Sci. 2011, 93, 320–327. [Google Scholar] [CrossRef]
- Sanchez-Salazar, M.E.; Griffiths, C.L.; Seed, R. The effect of size and temperature on the predation of cockles Cerastoderma edule (L.) by the shore crab Carcinus maenas (L.). J. Exp. Mar. Biol. Ecol. 1987, 111, 181–193. [Google Scholar] [CrossRef]
- Segurado, P.; Almeida, C.; Neves, R.; Ferreira, M.T.; Branco, P. Understanding multiple stressors in a Mediterranean basin: Combined effects of land use, water scarcity and nutrient enrichment. Sci. Total Environ. 2018, 624, 1221–1233. [Google Scholar] [CrossRef]
- Teichert, N.; Borja, A.; Chust, G.; Uriarte, A.; Lepage, M. Restoring fish ecological quality in estuaries: Implication of interactive and cumulative effects among anthropogenic stressors. Sci. Total Environ. 2016, 542, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Goussen, B.; Price, O.R.; Rendal, C.; Ashauer, R. Integrated presentation of ecological risk from multiple stressors. Sci. Rep. 2016, 6, 36004. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.M.; van der Grient, J.M.A. OSIRIS: A model for integrating the effects of multiple stressors on marine ecosystems. J. Theor. Biol. 2020, 493, 110211. [Google Scholar] [CrossRef]

| Scenario Name | Contaminant | Concentration (µgL−1) | Temperature Increase (°C) | Note | Reference |
|---|---|---|---|---|---|
| RCP4.5 | Without any EC | - | +1.8 | - | [25] |
| RCP8.5 | Without any EC | - | +3.7 | - | [25] |
| 4-NP | 4-Nonylphenol | 0.3 | - | AA-EQS | [39,40] |
| 4-NP-RCP4.5 | 4-Nonylphenol | 0.3 | +1.8 | AA-EQS | [39,40] |
| 4-NP-RCP8.5 | 4-Nonylphenol | 0.3 | +3.7 | AA-EQS | [39,40] |
| BPA | Bisphenol A | 0.2 | - | AA-EQS | [24] |
| BPA-RCP4.5 | Bisphenol A | 0.2 | +1.8 | AA-EQS | [24] |
| BPA-RCP8.5 | Bisphenol A | 0.2 | +3.7 | AA-EQS | [24] |
| DCF | Diclofenac | 0.1 | - | AA-EQS | [67,68] |
| DCF-RCP4.5 | Diclofenac | 0.1 | +1.8 | AA-EQS | [67,68] |
| DCF-RCP8.5 | Diclofenac | 0.1 | +3.7 | AA-EQS | [67,68] |
| EE2 | 17α-ethinylestradiol | 3.5 × 10−5 | - | AA-EQS | [24,68] |
| EE2-RCP4.5 | 17α-ethinylestradiol | 3.5 × 10−5 | +1.8 | AA-EQS | [24,68] |
| EE2-RCP8.5 | 17α-ethinylestradiol | 3.5 × 10−5 | +3.7 | AA-EQS | [24,68] |
| Multi-EC | 4-NP, BPA, DCF, EE2 | The same as above | - | AA-EQS | The same as above |
| Multi-EC-RCP4.5 | 4-NP, BPA, DCF, EE2 | The same as above | +1.8 | AA-EQS | The same as above |
| Multi-EC-RCP8.5 | 4-NP, BPA, DCF, EE2 | The same as above | +3.7 | AA-EQS | The same as above |
| Biomass Production | Control | BPA | 4NP | EE2 | DCF | RCP4.5 | RCP8.5 | Mixed ECs | Mixed ECs-RCP4.5 | Mixed ECs-RCP8.5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Benthic Invertebrates (gDWm−2) | 15.39 | 14.99 | 17.62 | 16.20 | 16.15 | 14.84 | 15.34 | 14.92 | 14.46 | 14.45 |
| Variable | Estimate | Std. Error | t Value | Pr (>|t|) |
|---|---|---|---|---|
| BPA | −2.72 | 0.15 | −18.35 | <0.001 |
| Control | 1.51 | 0.15 | 10.15 | <0.001 |
| DCF | 1.50 | 0.15 | 10.02 | <0.001 |
| EE2 | 1.51 | 0.15 | 10.12 | <0.001 |
| Mixed EC | −2.67 | 0.15 | −17.97 | <0.001 |
| H. diversicolor | −7.08 | 0.12 | −58.40 | <0.001 |
| H. ulvae | −1.93 | 0.12 | −15.90 | <0.001 |
| S. plana | 0.62 | 0.12 | 5.08 | 0.335 |
| Time | −3 × 10−4 | 4 × 10−5 | −8.70 | <0.001 |
| Variable Removed | df | Sum of Squares | Residual Sum of Squares | AIC |
|---|---|---|---|---|
| None | 173,404 | 28,027.59 | ||
| Temperature | 2 | 58 | 173,346 | 28,028.34 |
| Time | 1 | 1347 | 174,751 | 28,101 |
| Contaminant | 5 | 34,324 | 207,728 | 29,773 |
| Species | 3 | 88,959 | 262,363 | 32,047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, I.; Soares, J.; Neuparth, T.; Barreiro, A.F.; Xavier, C.; Antunes, C.; Santos, M.M. Prioritizing the Effects of Emerging Contaminants on Estuarine Production under Global Warming Scenarios. Toxics 2022, 10, 46. https://doi.org/10.3390/toxics10020046
Martins I, Soares J, Neuparth T, Barreiro AF, Xavier C, Antunes C, Santos MM. Prioritizing the Effects of Emerging Contaminants on Estuarine Production under Global Warming Scenarios. Toxics. 2022; 10(2):46. https://doi.org/10.3390/toxics10020046
Chicago/Turabian StyleMartins, Irene, Joana Soares, Teresa Neuparth, Aldo F. Barreiro, Cândido Xavier, Carlos Antunes, and Miguel M. Santos. 2022. "Prioritizing the Effects of Emerging Contaminants on Estuarine Production under Global Warming Scenarios" Toxics 10, no. 2: 46. https://doi.org/10.3390/toxics10020046
APA StyleMartins, I., Soares, J., Neuparth, T., Barreiro, A. F., Xavier, C., Antunes, C., & Santos, M. M. (2022). Prioritizing the Effects of Emerging Contaminants on Estuarine Production under Global Warming Scenarios. Toxics, 10(2), 46. https://doi.org/10.3390/toxics10020046








