Bioaccumulation and Biotransformation of Chlorinated Paraffins
Abstract
1. Introduction
2. Occurrence of CPs in Organisms
3. Tissue Distribution of CPs in Organisms
4. Bioaccumulation of CPs in Organisms
5. Biotransformation of CPs in Organisms
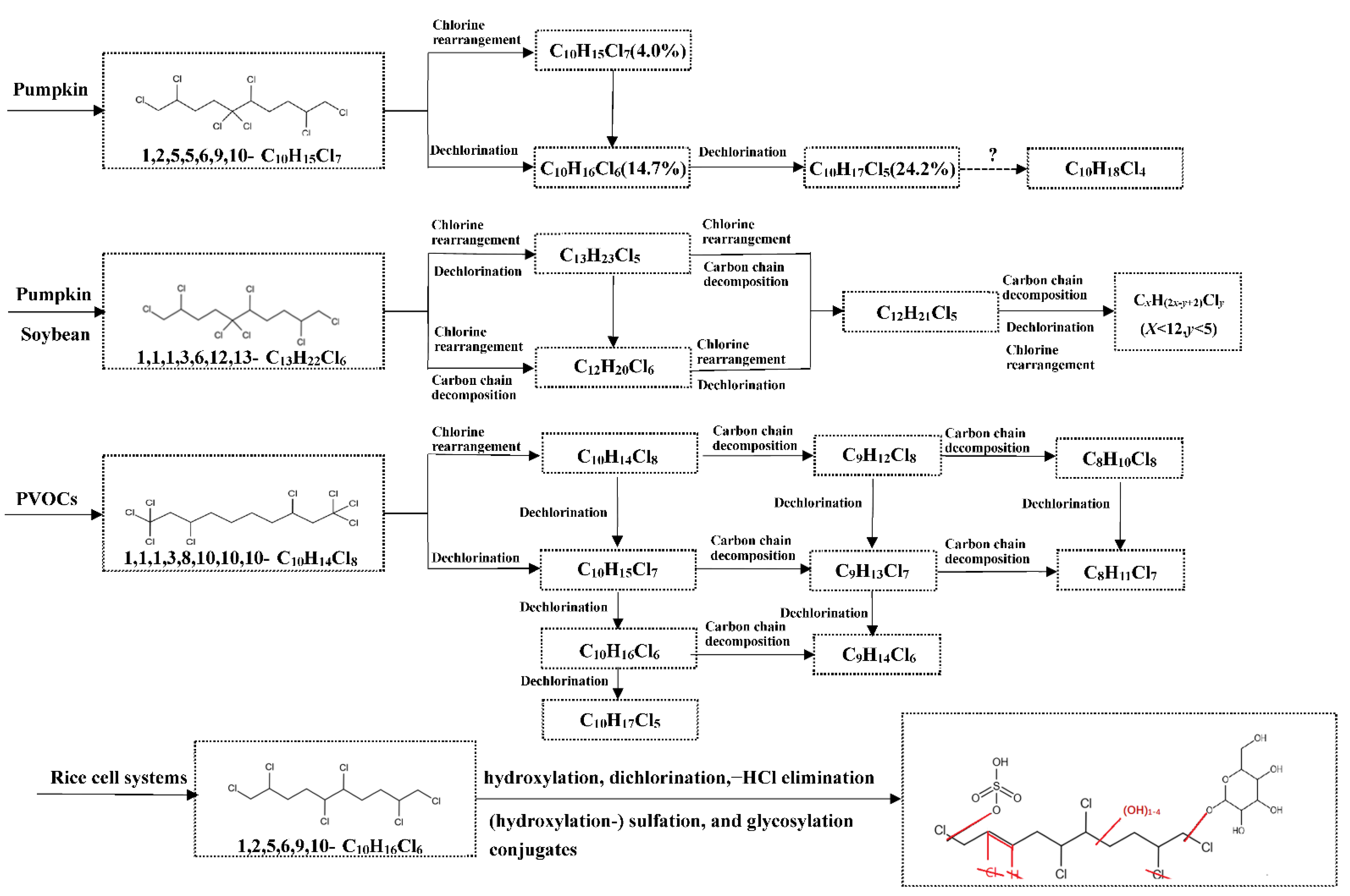
5.1. Biotransformation of CPs in Plants
5.2. Biotransformation of CPs in Invertebrates
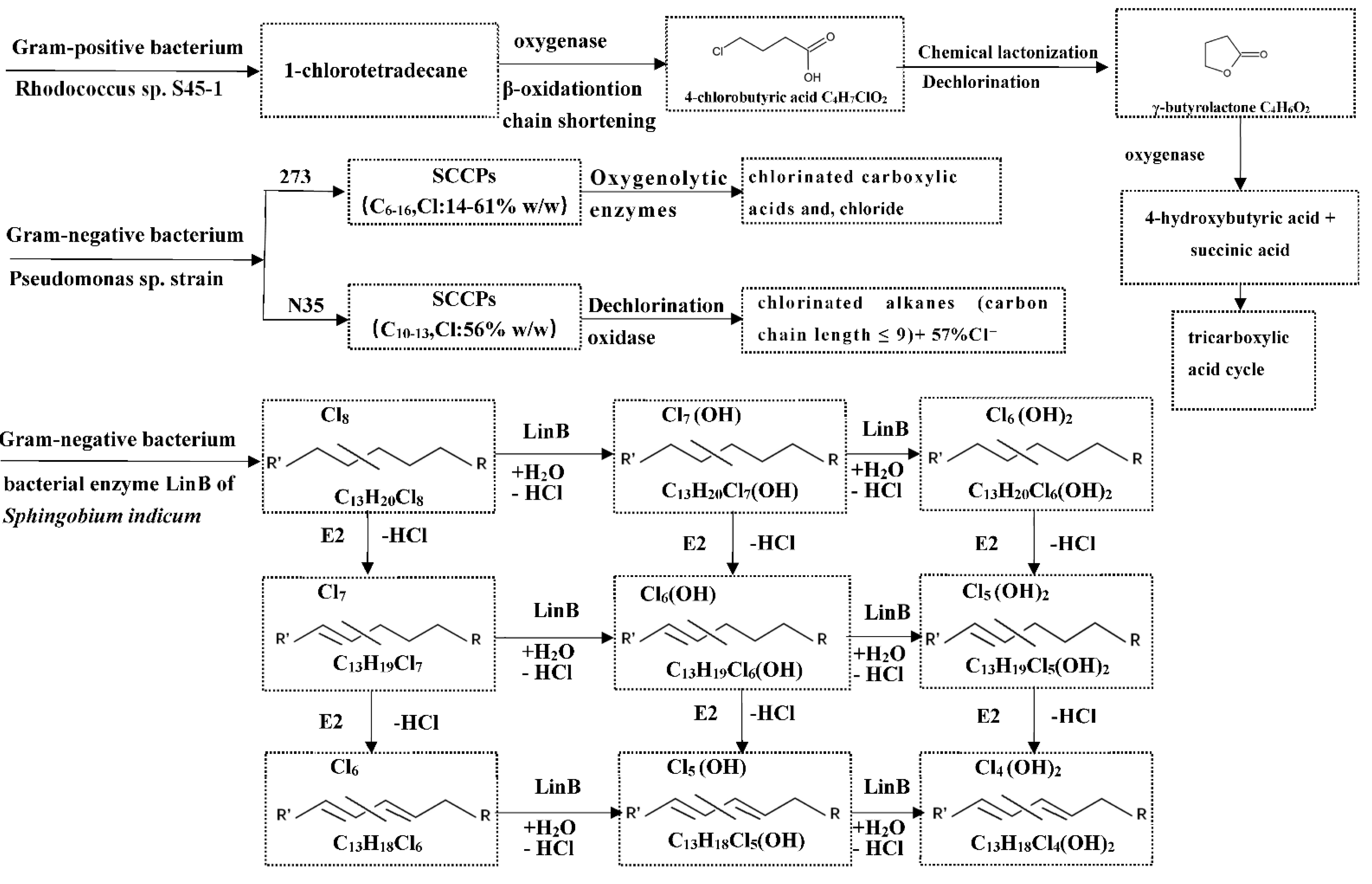
5.3. Biotransformation of CPs in Vertebrates
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, S.; Wang, M.; Lv, B.; Wang, J. Transformation pathways of chlorinated paraffins relevant for remediation: A mini-review. Environ. Sci. Pollut. Res. 2021, 28, 9020–9028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; de Wit, C.A.; Yin, G.; Du, X.; Yuan, B. Shorter than short-chain: Very short-chain chlorinated paraffins (vSCCPs) found in wildlife from the Yangtze River Delta. Environ. Int. 2019, 130, 104955. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, S.H.; Brits, M.; de Boer, J.; Leonards, P.E. Chlorinated paraffins and tris (1-chloro-2-propyl) phosphate in spray polyurethane foams—A source for indoor exposure? J. Hazard. Mater. 2021, 416, 125758. [Google Scholar] [CrossRef] [PubMed]
- Glüge, J.; Wang, Z.; Bogdal, C.; Scheringer, M.; Hungerbühler, K. Global production, use, and emission volumes of short-chain chlorinated paraffins—A minimum scenario. Sci. Total. Environ. 2016, 573, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, H.; Su, F.; Tian, Y.; Chen, J. Environmental occurrence and distribution of short chain chlorinated paraffins in sediments and soils from the Liaohe River Basin, P.R. China. Environ. Sci. Technol. 2012, 46, 3771–3778. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, T.; Han, W.; Yuan, B.; Liu, Q.; Wang, Y.; Jiang, G. Spatial and vertical distribution of short chain chlorinated paraffins in soils from wastewater irrigated farmlands. Environ. Sci. Technol. 2011, 45, 2100–2106. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Wang, Y.; Xu, Y.; Pan, X.; Zhang, G.; Luo, C.; Kobara, Y.; Nam, J.-J.; Jones, K.C. Atmospheric short-chain chlorinated paraffins in China, Japan, and South Korea. Environ. Sci. Technol. 2012, 46, 11948–11954. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, T.; Zhu, N.; Zhang, K.; Zeng, L.; Fu, J.; Wang, Y.; Jiang, G. Short chain chlorinated paraffins in mollusks from coastal waters in the chinese Bohai Sea. Environ. Sci. Technol. 2012, 46, 6489–6496. [Google Scholar] [CrossRef]
- Wang, X.T.; Xu, S.Y.; Wang, X.K.; Hu, B.P.; Jia, H.H. Occurrence, homologue patterns and source apportionment of short- and medium-chain chlorinated paraffins in suburban soils of Shanghai, China. Chemosphere 2017, 180, 302–311. [Google Scholar] [CrossRef]
- Vorkamp, K.; Rigét, F.F.; Bossi, R.; Sonne, C.; Dietz, R. Endosulfan, Short-chain chlorinated paraffins (sccps) and octachlorostyrene in wildlife from greenland: Levels, trends and methodological challenges. Arch Environ Contam Toxicol 2017, 73, 542–551. [Google Scholar] [CrossRef]
- Wang, X.; Jia, H.; Hu, B.; Cheng, H.; Zhou, Y.; Fu, R. Occurrence, sources, partitioning and ecological risk of short- and medium-chain chlorinated paraffins in river water and sediments in Shanghai. Sci. Total. Environ. 2019, 653, 475–484. [Google Scholar] [CrossRef] [PubMed]
- South, L.; Saini, A.; Harner, T.; Niu, S.; Parnis, J.M.; Mastin, J. Medium- and long-chain chlorinated paraffins in air: A review of levels, physicochemical properties, and analytical considerations. Sci. Total Environ. 2022, 843, 157094. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Luo, X.; Tang, B.; Chen, L.; Liu, Y.; Mai, B. Bioaccumulation of short chain chlorinated paraffins in a typical freshwater food web contaminated by e-waste in south China: Bioaccumulation factors, tissue distribution, and trophic transfer. Environ. Pollut. 2017, 222, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bu, D.; Fu, J.; Gao, Y.; Cong, Z.; Zhang, G.; Wang, Y.; Chen, X.; Zhang, A.; Jiang, G. Trophic dilution of short-chain chlorinated paraffins in a plant–plateau pika–eagle food chain from the Tibetan Plateau. Environ. Sci. Technol. 2019, 53, 9472–9480. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Luo, X.; Zhang, X.; He, M.; Chen, S.; Mai, B. Chlorinated paraffins in sediments from the Pearl River Delta, South China: Spatial and temporal distributions and implication for processes. Environ. Sci. Technol. 2011, 45, 9936–9943. [Google Scholar] [CrossRef]
- Ren, Z.; Zeng, Y.; Tang, B.; Luo, X.; Huang, C.; Mai, B. Bioaccumulative characteristics of halogenated flame retardants in aquatic and terrestrial biotas a case study of catfish and pigeons. Asian J. Ecotoxicol. 2018, 13, 163–168. [Google Scholar]
- Guan, K.; Liu, Y.; Luo, X.; Zeng, Y.; Mai, B. Short- and medium-chain chlorinated paraffins in aquatic organisms from an e-waste site: Biomagnification and maternal transfer. Sci. Total Environ. 2019, 708, 134840. [Google Scholar] [CrossRef]
- Chen, C.; Ma, X.; Guo, W.; Zhao, Y.; Lu, J.; Wang, Z.; Yao, Z. Congener specific distribution and bioaccumu-lation of short-chain chlorinated paraffins in Liao Estuary. Chin. Sci. Bull. 2014, 59, 578–585. [Google Scholar]
- Ma, X.; Zhang, H.; Wang, Z.; Yao, Z.; Chen, J.; Chen, J. Bioaccumulation and trophic transfer of short chain chlorinated paraffins in a marine food web from Liaodong Bay, north China. Environ. Sci. Technol. 2014, 48, 5964–5971. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Wu, Y.; Yang, C.; Luo, X.; Mai, B. Bioaccumulation of short-and medium-chain chlorinated paraffins in aquatic insects from an e-waste recycling site. Environ. Sci. Technol. 2021, 40, 3037–3045. [Google Scholar]
- Zeng, L.; Lam, J.C.W.; Chen, H.; Du, B.; Leung, K.M.Y.; Lam, P.K.S. Tracking dietary sources of short—and medium-chain chlorinated paraffins in marine mammals through a subtropical marine food web. Environ. Sci. Technol. 2017, 51, 9543–9552. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Gao, L.; Zheng, M.; Sun, Y.; Qiao, L.; Huang, H.; Zhang, H.; Fu, J.; Wu, Y.; Li, J.; et al. Identification and evaluation of chlorinated nonane paraffins in the environment: A persistent organic pollutant candidate for the Stockholm Convention? J. Hazard. Mater. 2019, 371, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gao, L.; Xia, D.; Qiao, L. Bioaccumulation and biomagnification of short and medium chain polychlorinated paraffins in different species of fish from Liaodong Bay, north China. Sci. Rep. 2017, 7, 10749. [Google Scholar] [CrossRef]
- Ma, X.; Chen, C.; Zhang, H.; Gao, Y.; Wang, Z.; Yao, Z.; Chen, J.; Chen, J. Congener-specific distribution and bioaccumulation of short-chain chlorinated paraffins in sediments and bivalves of the Bohai Sea, China. Mar. Pollut. Bull. 2014, 79, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, L.; Jiang, G.; He, Q.; Ren, L.; Gao, B.; Cai, L. Bioaccumulation and biomagnification of short-chain chlorinated paraffins in marine organisms from the Pearl River Estuary, South China. Sci. Total. Environ. 2019, 671, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Luo, X.; Tang, B.; Li, Z.; Huang, L.; Wang, T.; Mai, B. Short-chain chlorinated paraffins in marine organisms from the Pearl River Estuary in south China: Residue levels and interspecies differences. Sci. Total Environ. 2016, 553, 196–203. [Google Scholar] [CrossRef]
- Hu, H.; Qu, J.; Zhao, M.; Wu, P.; Zhu, W.; Zhou, Y.; Jin, H. Bioaccumulation and trophic magnification of short chain chlorinated paraffins in marine organisms from East China Sea. Mar. Pollut. Bull. 2021, 173, 113049. [Google Scholar] [CrossRef]
- Zeng, L.; Lam, J.C.W.; Wang, Y.; Jiang, G.; Lam, P.K.S. Temporal trends and pattern changes of short- and medium-chain chlorinated paraffins in marine mammals from the south china sea over the past decade. Environ. Sci. Technol. 2015, 49, 11348–11355. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, S.; Zou, Y.; Fan, M.; Wang, Y.; Cheng, J.; Wang, R.; Li, T.; Li, X.; Wang, P. Concentrations and sources of short- and medium-chain chlorinated paraffins in farmed chinese mitten crabs in China. J. Hazard. Mater. 2021, 411, 125076. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, G.; Du, X.; Xu, M.; Qiu, Y.; Ahlqvist, P.; Chen, Q.; Zhao, J. Short-chain chlorinated paraffins (SCCPs) in a freshwater food web from Dianshan Lake: Occurrence level, congener pattern and trophic transfer. Sci. Total Environ. 2018, 615, 1010–1018. [Google Scholar] [CrossRef]
- Lee, C.; Wu, Y.; Chen, C.; Tien, C. Spatiotemporal distribution and risk assessment of short-chain chlorinated paraffins in 30 major rivers in Taiwan. Sci. Total Environ. 2022, 806, 150969. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, L.; Zhou, W.; Han, X.; Zeng, L. Occurrence, distribution and seasonal variation of chlorinated paraffins in coral communities from South China Sea. J. Hazard. Mater. 2021, 402, 123529. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qing, X.; Jiang, G.; Chen, L.; He, Q.; Meng, X.-Z.; Gao, B. Short-chain chlorinated paraffins in fish from two developed regions of China: Occurrence, influencing factors and implication for human exposure via consumption. Chemosphere 2019, 236, 124317. [Google Scholar] [CrossRef] [PubMed]
- De Wit, C.A.; Bossi, R.; Dietz, R.; Dreyer, A.; Faxneld, S.; Garbus, S.E.; Hellström, P.; Koschorreck, J.; Lohmann, N.; Roos, A.; et al. Organohalogen compounds of emerging concern in Baltic Sea biota: Levels, biomagnification potential and comparisons with legacy contaminants. Environ. Int. 2020, 144, 106037. [Google Scholar] [CrossRef]
- Li, H.; Fu, J.; Zhang, A.; Zhang, Q.; Wang, Y. Occurrence, bioaccumulation and long-range transport of short-chain chlorinated paraffins on the Fildes Peninsula at King George Island, Antarctica. Environ. Int. 2016, 94, 408–414. [Google Scholar] [CrossRef]
- Li, H.; Fu, J.; Pan, W.; Wang, P.; Li, Y.; Zhang, Q.; Wang, Y.; Zhang, A.; Liang, Y.; Jiang, G. Environmental behaviour of short-chain chlorinated paraffins in aquatic and terrestrial ecosystems of Ny-Ålesund and London Island, Svalbard, in the Arctic. Sci. Total Environ. 2017, 590-591, 163–170. [Google Scholar] [CrossRef]
- Jafarabadi, A.R.; Dashtbozorg, M.; Raudonytė-Svirbutavičienė, E.; Bakhtiari, A.R. Chlorinated paraffins (SCCPs and MCCPs) in corals and water-SPM-sediment system in the Persian Gulf, Iran: A potential global threat for coral reefs. Environ. Pollut. 2021, 275, 116531. [Google Scholar] [CrossRef]
- Labadie, P.; Blasi, C.; Le Menach, K.; Geneste, E.; Babut, M.; Perceval, O.; Budzinski, H. Evidence for the widespread occurrence of short- and medium-chain chlorinated paraffins in fish collected from the Rhône River basin (France). Chemosphere 2019, 223, 232–239. [Google Scholar] [CrossRef]
- Casà, M.V.; van Mourik, L.M.; Weijs, L.; Mueller, J.; Nash, S.B. First detection of short-chain chlorinated paraffins (SCCPs) in humpback whales (Megaptera novaeangliae) foraging in Antarctic waters. Environ. Pollut. 2019, 250, 953–959. [Google Scholar] [CrossRef]
- Reth, M.; Ciric, A.; Christensen, G.N.; Heimstad, E.S.; Oehme, M. Short- and medium-chain chlorinated paraffins in biota from the European Arctic—differences in homologue group patterns. Sci. Total. Environ. 2006, 367, 252–260. [Google Scholar] [CrossRef]
- Reth, M.; Zencak, Z.; Oehme, M. First study of congener group patterns and concentrations of short- and medium-chain chlorinated paraffins in fish from the North and Baltic Sea. Chemosphere 2005, 58, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; McLachlan, M.S.; Roos, A.M.; Simon, M.; Strid, A.; de Wit, C.A. Long-chain chlorinated paraffins have reached the Arctic. Environ. Sci. Technol. Lett. 2021, 8, 753–759. [Google Scholar] [CrossRef]
- Knudtzon, N.C.; Thorstensen, H.; Ruus, A.; Helberg, M.; Bæk, K.; Enge, E.K.; Borgå, K. Maternal transfer and occurrence of siloxanes, chlorinated paraffins, metals, PFAS and legacy POPs in herring gulls (Larus argentatus) of different urban influence. Environ. Int. 2021, 152, 106478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Rüdel, H.; de Wit, C.A.; Koschorreck, J. Identifying emerging environmental concerns from long-chain chlorinated paraffins towards German ecosystems. J. Hazard. Mater. 2021, 424, 127607. [Google Scholar] [CrossRef] [PubMed]
- Choo, G.; Ekpe, O.D.; Park, K.W.; Chung, D.; Lee, J.; Oh, J.-E. Temporal and spatial trends of chlorinated paraffins and organophosphate flame retardants in black-tailed gull (Larus crassirostris) eggs. Sci. Total Environ. 2022, 803, 150137. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Fu, J.; Wang, Y.; Jiang, G. Short-chain chlorinated paraffins in soil, paddy seeds (Oryza sativa) and snails (Ampullariidae) in an e-waste dismantling area in China: Homologue group pattern, spatial distribution and risk assessment. Environ. Pollut. 2017, 220, 608–615. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, X.; Tang, B.; Mai, B. Habitat- and species-dependent accumulation of organohalogen pollutants in home-produced eggs from an electronic waste recycling site in south China: Levels, profiles, and human dietary exposure. Environ. Pollut. 2016, 216, 64–70. [Google Scholar] [CrossRef]
- Luo, X.; Sun, Y.; Wu, J.; Chen, S.; Mai, B. Short-chain chlorinated paraffins in terrestrial bird species inhabiting an e-waste recycling site in south China. Environ. Pollut. 2015, 198, 41–46. [Google Scholar] [CrossRef]
- Sun, R.; Chen, J.; Shao, H.; Tang, L.; Zheng, X.; Li, Q.X.; Wang, Y.; Luo, X.; Mai, B. Bioaccumulation of short-chain chlorinated paraffins in chicken (Gallus domesticus): Comparison to fish. J. Hazard. Mater. 2020, 396, 122590. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Zeng, Y.; Wang, Q.; Tu, W.; Yang, C.; Mai, B. Trophic magnification of short- and medium-chain chlorinated paraffins in terrestrial food webs and their bioamplification in insects and amphibians during metamorphosis. Environ. Sci. Technol. 2020, 54, 11282–11291. [Google Scholar] [CrossRef]
- Wu, J.; Gao, W.; Liang, Y.; Fu, J.; Shi, J.; Lu, Y.; Wang, Y.; Jiang, G. Short- and medium-chain chlorinated paraffins in multi-environmental matrices in the Tibetan Plateau environment of China: A regional scale study. Environ. Int. 2020, 140, 105767. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bu, D.; Gao, Y.; Zhu, N.; Wu, J.; Chen, X.; Fu, J.; Wang, Y.; Zhang, A.; Jiang, G. Long-range atmospheric transport and alpine condensation of short-chain chlorinated paraffins on the southeastern Tibetan Plateau. J. Environ. Sci. 2020, 99, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hou, X.; Liu, Y.; Hu, X.; Liu, J.; Schnoor, J.L.; Jiang, G. Medium- and short-chain chlorinated paraffins in mature maize plants and corresponding agricultural soils. Environ. Sci. Technol. 2021, 55, 4669–4678. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-P.; Chang, H.; Zhang, C.-X.; Feng, C.-L.; Wu, F.-C. Occurrence of chlorinated paraffins in a wetland ecosystem: Removal and distribution in plants and sediments. Environ. Sci. Technol. 2021, 55, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Gao, L.; Zheng, M.; Xia, D.; Li, J.; Zhang, L.; Wu, Y.; Wang, R.; Cui, L.; Xu, C. Mass fractions, congener group patterns, and placental transfer of short- and medium-chain chlorinated paraffins in paired maternal and cord serum. Environ. Sci. Technol. 2018, 52, 10097–10103. [Google Scholar] [CrossRef]
- Xu, C.; Wang, K.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Qiao, L.; Huang, D.; Wang, S.; et al. Highly elevated levels, infant dietary exposure and health risks of medium-chain chlorinated paraffins in breast milk from China: Comparison with short-chain chlorinated paraffins. Environ. Pollut. 2021, 279, 116922. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Wang, Y.; Jiang, G. Distribution and pattern profiles of chlorinated paraffins in human placenta of Henan province, China. Environ. Sci. Technol. Lett. 2018, 5, 9–13. [Google Scholar] [CrossRef]
- Han, X.; Chen, H.; Shen, M.; Deng, M.; Du, B.; Zeng, L. Hair and nails as noninvasive bioindicators of human exposure to chlorinated paraffins: Contamination patterns and potential influencing factors. Sci. Total Environ. 2021, 798, 149257. [Google Scholar] [CrossRef]
- Wang, R.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Wang, G.; Xiong, L.; Ding, D.; Lu, D.; et al. Characterization of short- and medium-chain chlorinated paraffins in cereals and legumes from 19 Chinese provinces. Chemosphere 2019, 226, 282–289. [Google Scholar] [CrossRef]
- Ding, L.; Luo, N.; Liu, Y.; Fang, X.; Zhang, S.; Li, S.; Jiang, W.; Zhao, N. Short and medium-chain chlorinated paraffins in serum from residents aged from 50 to 84 in Jinan, China: Occurrence, composition and association with hematologic parameters. Sci. Total Environ. 2020, 728, 137998. [Google Scholar] [CrossRef]
- Li, T.; Wan, Y.; Gao, S.; Wang, B.; Hu, J. High-throughput determination and characterization of short-, medium-, and long-Chain chlorinated paraffins in human blood. Environ. Sci. Technol. 2017, 51, 3346–3354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Aamir, M.; Li, M.; Liu, K.; Hu, Y.; Liu, N.; Xu, Y.; Du, J.; Xu, J.; Liu, W. Prenatal and postnatal exposure risk assessment of chlorinated paraffins in mothers and neonates: Occurrence, congener profile, and transfer behavior. J. Hazard. Mater. 2020, 395, 122660. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Qiao, L.; Tian, Q.; Huang, H.; Liu, W.; et al. Health risks posed to infants in rural China by exposure to short- and medium-chain chlorinated paraffins in breast milk. Environ. Int. 2017, 103, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Harada, K.H.; Hitomi, T.; Niisoe, T.; Wang, P.; Shi, Y.; Yang, H.-R.; Takasuga, T.; Koizumi, A. Lactational exposure to short-chain chlorinated paraffins in China, Korea, and Japan. Chemosphere 2017, 173, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, W.; Lam, J.C.; Ge, J.; Li, J.; Zeng, L. Blood partitioning and whole-blood-based maternal transfer assessment of chlorinated paraffins in mother-infant pairs from South China. Environ. Int. 2020, 142, 105871. [Google Scholar] [CrossRef]
- Li, H.; Gao, S.; Yang, M.; Zhang, F.; Cao, L.; Xie, H.; Chen, X.; Cai, Z. Dietary exposure and risk assessment of short-chain chlorinated paraffins in supermarket fresh products in Jinan, China. Chemosphere 2020, 244, 125393. [Google Scholar] [CrossRef]
- Wang, R.; Gao, L.; Zheng, M.; Tian, Y.; Li, J.; Zhang, L.; Wu, Y.; Huang, H.; Qiao, L.; Liu, W.; et al. Short- and medium-chain chlorinated paraffins in aquatic foods from 18 chinese provinces: Occurrence, spatial distributions, and risk assessment. Sci. Total Environ. 2018, 615, 1199–1206. [Google Scholar] [CrossRef]
- Cao, Y.; Harada, K.H.; Liu, W.; Yan, J.; Zhao, C.; Niisoe, T.; Adachi, A.; Fujii, Y.; Nouda, C.; Takasuga, T.; et al. Short-chain chlorinated paraffins in cooking oil and related products from China. Chemosphere 2015, 138, 104–111. [Google Scholar] [CrossRef]
- Gao, W.; Bai, L.; Ke, R.; Cui, Y.; Yang, C.; Wang, Y.; Jiang, G. Distributions and congener group profiles of short-chain and medium-chain chlorinated paraffins in cooking oils in chinese markets. J. Agric. Food Chem. 2020, 68, 7601–7608. [Google Scholar] [CrossRef]
- Huang, H.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Wang, R.; Xia, D.; Qiao, L.; Cui, L.; et al. Dietary exposure to short- and medium-chain chlorinated paraffins in meat and meat products from 20 provinces of China. Environ. Pollut. 2018, 233, 439–445. [Google Scholar] [CrossRef]
- Han, X.; Chen, H.; Deng, M.; Du, B.; Zeng, L. Chlorinated paraffins in infant foods from the Chinese market and estimated dietary intake by infants. J. Hazard. Mater. 2021, 411, 125073. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Qiao, L.; Xu, C.; Wang, K.; Huang, D. Short- and medium-chain chlorinated paraffins in foods from the sixth chinese total diet study: Occurrences and estimates of dietary intakes in south China. J. Agric. Food Chem. 2020, 68, 9043–9051. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Wang, Y.; Zhang, S.; Dong, S.; Zhou, W. Short- and medium-chain chlorinated paraffins in green tea from 11 Chinese provinces and their migration from packaging. J. Hazard. Mater. 2022, 427, 128192. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, L.; Zheng, M.; Qiao, L.; Xu, C.; Wang, K.; Huang, D. Occurrences, congener group profiles, and risk assessment of short- and medium-chain chlorinated paraffins in cup instant noodles from China. Chemosphere 2021, 279, 130503. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, S.; Li, X.; Wei, S.; Li, T.; Zou, Y.; Zhang, W.; Cheng, J.; Wang, R.; Wang, P.; et al. Occurrence of short- and medium-chain chlorinated paraffins in raw dairy cow milk from five chinese provinces. Environ. Int. 2020, 136, 105466. [Google Scholar] [CrossRef]
- Krätschmer, K.; Schächtele, A.; Vetter, W. Chlorinated paraffins in baby food from the German market. Food Control 2021, 123, 107689. [Google Scholar] [CrossRef]
- Perkons, I.; Abdulajeva, E.; Bartkiene, E.; Zacs, D. Short- and medium-chain chlorinated paraffins in commercial complementary baby food produced in different European countries: Occurrence, congener group profiles, portion-based dietary intake, and risk assessment. Sci. Total. Environ. 2022, 814, 152733. [Google Scholar] [CrossRef]
- Sprengel, J.; Wieselmann, S.; Kröpfl, A.; Vetter, W. High amounts of chlorinated paraffins in oil-based vitamin E dietary supplements on the German market. Environ. Int. 2019, 128, 438–445. [Google Scholar] [CrossRef]
- Thomas, G.O.; Farrar, D.; Braekevelt, E.; Stern, G.; Kalantzi, O.I.; Martin, F.L.; Jones, K.C. Short and medium chain length chlorinated paraffins in UK human milk fat. Environ. Int. 2006, 32, 34–40. [Google Scholar] [CrossRef]
- Tomasko, J.; Stupak, M.; Parizkova, D.; Polachova, A.; Sram, R.J.; Topinka, J.; Pulkrabova, J. Short- and medium-chain chlorinated paraffins in human blood serum of Czech population. Sci. Total Environ. 2021, 797, 149126. [Google Scholar] [CrossRef]
- Ueberschar, K.; Matthes, S. Dose–response feeding study of chlorinated paraffins in broiler chickens: Effects on growth rate and tissue distribution. Food Addit. Contam. 2004, 21, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Ueberschär, K.-H.; Dänicke, S.; Matthes, S. Dose–response feeding study of short chain chlorinated paraffins (SCCPs) in laying hens:effects on laying performance and tissue distribution, accumulation and elimination kinetics. Mol. Nutr. Food Res. 2007, 51, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Li, T.; Wan, Y.; Sun, Y.; Hu, J. Physiologically based pharmacokinetic modeling for chlorinated paraffins in rats and humans: Importance of biliary excretion. Environ. Sci. Technol. 2020, 54, 938–946. [Google Scholar] [CrossRef]
- Mézière, M.; Marchand, P.; Hutinet, S.; Larvor, F.; Baéza, E.; Le Bizec, B.; Dervilly, G.; Cariou, R. Transfer of short-, medium-, and long-chain chlorinated paraffins to eggs of laying hens after dietary exposure. Food Chem. 2021, 343, 128491. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yuan, B.; Zhou, Y.; De Wit, C.A.; Zheng, Z.; Yin, G. Chlorinated paraffins in two snake species from the Yangtze River Delta: Tissue distribution and biomagnification. Environ. Sci. Technol. 2020, 54, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Takai, J.I.; Takahasi, K.; Endo, Y.; Fujimoto, K.; Koike, S.; Matsumoto, W. Effect of dietary tung oil on the growth and lipid metabolism of laying hens. J. Nutr. Sci. Vitaminol. 2002, 48, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.; Arnot, J.A.; Gobas, F.A.; Powell, D.E. Mathematical relationships between metrics of chemical bioaccumulation in fish. Environ. Toxicol. Chem. 2013, 32, 1459–1466. [Google Scholar] [CrossRef]
- Weisbrod, A.V.; Woodburn, K.B.; Koelmans, A.A.; Parkerton, T.F.; McElroy, A.E.; Borgå, K. Evaluation of bioaccumulation using in vivo laboratory and field studies. Integr. Environ. Assess. Manag. 2009, 5, 598–623. [Google Scholar] [CrossRef]
- Renberg, L.; Tarkpea, M.; Sundström, G. The use of the bivalve Mytilus edulis as a test organism for bioconcentration studies: II. the bioconcentration of two 14C-labeled chlorinated paraffins. Ecotoxicol. Environ. Saf. 1986, 11, 361–372. [Google Scholar] [CrossRef]
- Castro, M.; Sobek, A.; Yuan, B.; Breitholtz, M. Bioaccumulation potential of cps in aquatic organisms: Uptake and depuration in daphnia magna. Environ. Sci. Technol. 2019, 53, 9533–9541. [Google Scholar] [CrossRef]
- Fisk, A.T.; Tomy, G.T.; Cymbalisty, C.D.; Muir, D.C.G. Dietary accumulation and quantitative structure-activity relationships for depuration and biotransformation of short (C10), medium (C14), and long (C18) carbon-chain polychlorinated alkanes by juvenile rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2000, 19, 1508–1516. [Google Scholar] [CrossRef]
- Houde, M.; Muir, D.; Tomy, G.T.; Whittle, D.M.; Teixeira, C.; Moore, S. Bioaccumulation and trophic magnification of short- and medium-chain chlorinated paraffins in food webs from Lake Ontario and Lake Michigan. Environ. Sci. Technol. 2008, 42, 3893–3899. [Google Scholar] [CrossRef] [PubMed]
- Goutte, A.; Alliot, F.; Budzinski, H.; Simonnet-Laprade, C.; Santos, R.; Lachaux, V.; Maciejewski, K.; Le Menach, K.; Labadie, P. Trophic transfer of micropollutants and their metabolites in an urban riverine food web. Environ. Sci. Technol. 2020, 54, 8043–8050. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wang, T.; Wang, P.; Liu, Q.; Han, S.; Yuan, B.; Zhu, N.; Wang, Y.; Jiang, G. Distribution and trophic transfer of short-chain chlorinated paraffins in an aquatic ecosystem receiving effluents from a sewage treatment plant. Environ. Sci. Technol. 2011, 45, 5529–5535. [Google Scholar] [CrossRef]
- Muijs, B.; Jonker, M.T.O. Temperature-dependent bioaccumulation of polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2009, 43, 4517–4523. [Google Scholar] [CrossRef]
- Bettina, H.; Hermann, F.; Wolfgang, V.; Mehmet, C. Effects of chain length, chlorination degree, and structure on the octanol−water partition coefficients of polychlorinated n-alkanes. Environ. Sci. Technol. 2011, 45, 2842–2849. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Chen, W.; Liu, J.; Zhou, Q.; Schnoor, J.L.; Jiang, G. Carbon chain decomposition of short chain chlorinated paraffins mediated by pumpkin and soybean seedlings. Environ. Sci. Technol. 2019, 53, 6765–6772. [Google Scholar] [CrossRef]
- Darnerud, P.O.; Biessmann, A.; Brandt, I. Metabolic fate of chlorinated paraffins: Degree of chlorination of [1–14C]-chlorododecanes in relation to degradation and excretion in mice. Arch. Toxicol. 1982, 50, 217–226. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Yu, M.; Zhou, Q.; Liu, J.; Schnoor, J.L.; Jiang, G. Dechlorination and chlorine rearrangement of 1,2,5,5,6,9,10-heptachlorodecane mediated by the whole pumpkin seedlings. Environ. Pollut. 2017, 224, 524–531. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Kong, W.; Liu, J.; Schnoor, J.L.; Jiang, G. Transformation of 1,1,1,3,8,10,10,10-octachlorodecane in air phase increased by phytogenic volatile organic compounds of pumpkin seedlings. Sci. Total Environ. 2020, 704, 135455. [Google Scholar] [CrossRef]
- Chen, W.; Yu, M.; Zhang, Q.; Hou, X.; Kong, W.; Wei, L.; Mao, X.; Liu, J.; Schnoor, J.L.; Jiang, G. Metabolism of sccps and mccps in suspension rice cells based on paired mass distance (PMD) analysis. Environ. Sci. Technol. 2020, 54, 9990–9999. [Google Scholar] [CrossRef] [PubMed]
- Omori, T.; Kimura, T.; Kodama, T. Bacterial cometabolic degradation of chlorinated paraffins. Appl. Microbiol. Biotechnol. 1987, 25, 553–557. [Google Scholar] [CrossRef]
- Allpress, J.D.; Gowland, P.C. Biodegradation of chlorinated paraffins and long-chain chloroalkanes by Rhodococcus sp. S45-1. Int. Biodeterior. 1999, 43, 173–179. [Google Scholar] [CrossRef]
- Heath, E.; Brown, W.A.; Jensen, S.R.; Bratty, M.P. Biodegradation of chlorinated alkanes and their commercial mixtures by Pseudomonas sp. strain 273. J. Ind. Microbiol. Biotechnol. 2006, 33, 197–207. [Google Scholar] [CrossRef]
- Lu, M. Degradation of short chain polychlorinated paraffins by a new isolate: Tests in pure culture and sewage sludge. J. Chem. Technol. Biotechnol. 2012, 88, 1273–1279. [Google Scholar] [CrossRef]
- Heeb, N.V.; Schalles, S.; Lehner, S.; Schinkel, L.; Schilling, I.; Lienemann, P.; Bogdal, C.; Kohler, H.-P.E. Biotransformation of short-chain chlorinated paraffins (SCCPs) with LinA2: A HCH and HBCD converting bacterial dehydrohalogenase. Chemosphere 2019, 226, 744–754. [Google Scholar] [CrossRef]
- Knobloch, M.C.; Schinkel, L.; Kohler, H.-P.E.; Mathis, F.; Kern, S.; Bleiner, D.; Heeb, N.V. Transformation of short-chain chlorinated paraffins and olefins with the bacterial dehalogenase LinB from Sphingobium Indicum—Kinetic models for the homologue-specific conversion of reactive and persistent material. Chemosphere 2021, 283, 131199. [Google Scholar] [CrossRef]
- Knobloch, M.C.; Mathis, F.; Fleischmann, T.; Kohler, H.-P.E.; Kern, S.; Bleiner, D.; Heeb, N.V. Enzymatic synthesis and formation kinetics of mono- and di-hydroxylated chlorinated paraffins with the bacterial dehalogenase LinB from Sphingobium indicum. Chemosphere 2022, 291, 132939. [Google Scholar] [CrossRef]
- Darnerud, P.O. Chlorinated Paraffins: Effect of some Microsomal Enzyme Inducers and inhibitors on the degradation of 1-14c-chlorododecanes to14co2in mice. Acta Pharmacol. et Toxicol. 1984, 55, 110–115. [Google Scholar] [CrossRef]
- Darnerud, P.O.; Bengtsson, B.-E.; Bergman, Å.; Brandt, I. Chlorinated paraffins: Disposition of a polychloro-[1–14c]-hexadecane in carp (Cyprinus carpio) and bleak (Alburnus alburnus). Toxicol. Lett. 1983, 19, 345–351. [Google Scholar] [CrossRef]
- Biessmann, A.; Brandt, I.; Darnerud, P.O. Comparative distribution and metabolism of two 14C-labelled chlorinated paraffins in Japanese quail coturnix coturnix japonica. Environ. Pollut. Ser. A Ecol. Biol. 1982, 28, 109–120. [Google Scholar] [CrossRef]
- Biessmann, A.; Darnerud, P.O.; Brandt, I. Chlorinated paraffins: Disposition of a highly chlorinated polychlorohexadecane in mice and quail. Arch. Toxicol. 1983, 53, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Åhlman, M.; Bergman, Å.; Darnerud, P.O.; Egestad, B.; Sjövall, J. Chlorinated paraffins: Formation of sulphur-containing metabolites of polychlorohexadecane in rats. Xenobiotica 1986, 16, 225–232. [Google Scholar] [CrossRef]
- He, C.; van Mourik, L.; Tang, S.; Thai, P.; Wang, X.; Brandsma, S.H.; Leonards, P.E.; Thomas, K.V.; Mueller, J.F. In vitro biotransformation and evaluation of potential transformation products of chlorinated paraffins by high resolution accurate mass spectrometry. J. Hazard. Mater. 2021, 405, 124245. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Abdallah, M.A.-E.; Chen, L.-J.; Luo, X.-J.; Mai, B.-X.; Harrad, S. Comparative in vitro metabolism of short chain chlorinated paraffins (SCCPs) by human and chicken liver microsomes: First insight into heptachlorodecanes. Sci. Total. Environ. 2022, 851, 158261. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Y.; Li, G.; An, T. Increased adverse effects during metabolic transformation of short-chain chlorinated paraffins by cytochrome P450: A theoretical insight into 1-chlorodecane. J. Hazard. Mater. 2021, 407, 124391. [Google Scholar] [CrossRef] [PubMed]
- UNEP. UNEP/POPS/COP.8/SC-8/11. Listing of Short-Chain Chlorinated Paraffins. 2017. Available online: http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.8-SC-8-11.English.pdf (accessed on 18 February 2019).
| Site | Organisms | SCCPs | MCCPs | Reference | ||
|---|---|---|---|---|---|---|
| Mean/Median | Range | Mean/Median | Range | |||
| Aquatic biota | ||||||
| Qingyuan, China | Fish and invertebrates | 1700–95,000 a | [13] | |||
| Qingyuan, China | Fish, snake, bird eggs | 1200–250,000 a | 2300–200,000 a | [17] | ||
| Qingyuan, China | Insects | 52–410 c | 40–740 c | [20] | ||
| Guiyu, China | Catfish | 30,800 a | 11,400–70,400 a | [16] | ||
| Hong Kong, China | Fishes | 801 ± 253 a | 280–1940 a | 1820 ± 934 a | 502–4770 a | [21] |
| Crustaceans | 422 ± 162 a | 202–694 a | 593 ± 306 a | 205–1190 a | ||
| Mollusks | 328 ± 79 a | 259–506 a | 603 ± 132 a | 464–874 a | ||
| Liao Estuary, China | Mollusks | 66,500 a | 28,100–120,400 a | [18] | ||
| Liaodong Bay, China | Fish | 968 b | 53.3–2907 b | [22] | ||
| Liaodong Bay, China | Invertebrates and fish | 20,100 a | 2300–76,500 a | [19] | ||
| Liaodong Bay, China | Fish | 376.3–8596 a | 22.37–5097 a | [23] | ||
| Bohai Sea, China | Bivalves | 1710.5 b | 476.4–3269.5 b | [24] | ||
| Pearl River Estuary, China | Marine organism | 870–36,000 a | [25] | |||
| Pearl River Estuary, China | Marine organism | 210–21,000 a | [26] | |||
| East China Sea | Marine organism | 155 ± 215 c | 12.8–1819 c | [27] | ||
| South China Sea | Finless porpoises | 2800 a | 570–5800 a | 5100 a | 670–11,000 a | [28] |
| Humpback dolphins | 5500 a | 920–24,000 a | 13,000 a | 1400–56,000 a | ||
| Bohai Sea, China | Mollusk | 1410 b | 64.9–5510 b | [8] | ||
| China | Farmed crabs | 543 a | 82–1760 a | nd–680 a | [29] | |
| Dianshan Lake, China | Wild aquatic organisms | 10,000–1,300,000 a | [30] | |||
| Taiwan, China | Freshwater Fish | nd–2,320,000 a | [31] | |||
| Hainan, China | Coral | /800 a | 184–7410 a | /1490 a | 305–14,800 a | [32] |
| China | Fish of the Pearl Real Delta | 16,000 ± 12,000 a | 3000–41,000 a | [33] | ||
| Farmed freshwater fish | 5900 ± 8100 a | 220–51,000 a | ||||
| Fish of the Yangtze River Delta | 3000 ± 1600 a | 900–7300 a | ||||
| Baltic Sea | Marine organisms | nd–220 a | nd–390 a | [34] | ||
| Antarctic | Fish and mosses | 262 b | 69.0–504 b | [22] | ||
| Antarctic | Fish | 1500 ± 500 a | [35] | |||
| Ny-Ålesund and London Island, Svalbard, Arctic | Algae, gammarids, and cod | 178.9 b | [36] | |||
| Persian Gulf, Iranian | Larak coral tissue | 112 b | 28.5–200 b | 65.9 b | 15.5–136 b | [37] |
| France | Fish | /728 a | 63–1492 a | /4430 a | 99–11,300 a | [38] |
| Antarctic | Humpback whales | nd–46 a | [39] | |||
| Northern Europe | Fish and seabird | 28–880 a | 14–3700 a | [40] | ||
| North Baltic Sea | Liver of fish | 19–286 c | 25–260 c | [41] | ||
| Greenland and Iceland and Sweden | Bivalves marine mammals | <5.2–570 a | <8.6–270 a | [42] | ||
| Norway | Herring gull blood and eggs | 5–200 c | 3–630 c | [43] | ||
| German | Aquatic and terrestrial organisms | nd–350 a | nd–1800 a | [44] | ||
| Greenland, Denmark | Bird and marine mammals | 220–2200 c | [10] | |||
| South Korea | Black-tailed gull eggs | 1180–2931 a | 694–2023 a | [45] | ||
| Terrestrial biota | ||||||
| Taizhou, China | Apple snail | 314 b | 137–821 b | [46] | ||
| Guiyu, China | Chicken eggs | 2300–6800 a | [47] | |||
| Goose eggs | nd–150,000 a | |||||
| Guiyu, China | Pigeon | 7600 a | 4700–11,000 a | [16] | ||
| Qingyuan, China | Bird species | 620–17,000 a | [48] | |||
| Qingyuan, China | Chicken | 460–13,000 a | [49] | |||
| Qingyuan, China | Insect | 2200–9000 a | 990–11,000 a | [50] | ||
| Birds | 6100–48,000 a | 2000–33,000 a | ||||
| Frogs and toads | 8100–24,000 a | 4600–17,000 a | ||||
| Tibetan, China | tree bark | 2900–7000 a | 1800–5700 a | [51] | ||
| needle | 2400–6400 a | 1600–5000 a | ||||
| lichen | 1400–6100 a | 700–4000 a | ||||
| moss samples | 1500–5300 a | 900–4000 a | ||||
| Tibetan, China | Plants | 4300 ± 2830 a | [14] | |||
| Plateau pika | 1870 ± 1090 a | |||||
| Eagle | 723 ± 536 a | |||||
| Tibetan, China | Chicken and goose eggs | 3098–6999 a | [52] | |||
| China | Mature maize plant | 381 b | 119–61,999 b | 551 b | 77.6–52,930 b | [53] |
| Beijing, China | Plants | 127 ± 116 b | 13–593 b | 289 ± 148 b | 21–785 b | [54] |
| German | Fauna sample (mussels, fish, birds, earthworms, and roe deer) Flora sample (tree shoots and leaves) | nd–350 a | nd–1800 a | [44] | ||
| Greenland, Denmark | Black guillemot eggs Glaucous gull liver Ringed seal blubber Polar bear adipose tissue | 220–2200 c | [10] | |||
| South Korea | Black-tailed gull (Larus crassirostris) eggs | 1180–2931 a | 694–2023 a | [45] | ||
| Humans and human foodstuffs | ||||||
| Beijing, China | Maternal serum | 21.7–373 c | 3.76–31.8 c | [55] | ||
| Cord serum | 8.51–107 c | 1.33–12.9 c | ||||
| China | Breast milk in urban and rural areas | /393 a /525 a | 131–808 a 139–1543 a | /472 a /576 a | 94–1714 a 211–1089 a | [56] |
| Henan, China | Human placentas | 593 a | 98.5–3771 a | 316 a | 80.8–954 a | [57] |
| China | Hair | /239 b | 19.2–877 b | /325 b | 16.9–893 b | [58] |
| Nails | /154 b | 57.7–355 b | /233 b | 61.0–476 b | ||
| China | Cereal samples | 343 c | 51.6–981 c | 213 c | [59] | |
| Legume samples | 328 c | 47.1–801 c | 184 c | |||
| Jinan, China | Human serum | /13,800 a | 1670–42,700 a | /15,200 a | 1350–38,900 a | [60] |
| China | Human blood | /3500 a | 370–35,000 a | /740 a | 130–3200 a | [61] |
| China | Maternal serum, | /117,100 d | /38,900 d | [62] | ||
| Cord serum, | /70,000 d | /25,600 d | ||||
| Placenta | /30.3 c | /19.0 c | ||||
| Breast milk | /82,600 d | /26,100 d | ||||
| China | Human milk | /303 a | /35.7 a | [63] | ||
| China, Korea, Japan | Breast milk | nd–54 a nd–20 a nd–20 a | [64] | |||
| South China | Maternal | 3280–10,400 d | 1300–5500 d | [65] | ||
| cord blood | 890–4130 d | 890–1690 d | ||||
| placenta | 3.18–9.12 c | 1.91–4.89 c | ||||
| Taizhou, China | Paddy seeds | 17.6 b | 4.9–55.1 b | [46] | ||
| Jinan, China | Food samples | 69.3 ± 74.4 c | 8.27–268 c | [66] | ||
| China | Aquatic food samples | 1472 c | 215–4200 c | 80.5 c | 9.0–586 c | [67] |
| China | Cooking oil | <9–7500 c | [68] | |||
| China | Cooking oil | nd–16,055 c | nd–11,612 c | [69] | ||
| China | Meat | 129 ± 4.1 c | 15.7–469 c | 5.7 ± 0.59 c | 0.3–23.8 c | [70] |
| China | Infant formulas, | 7.95 c | 2.32–54.2 c | 1.67–20.9 c | [71] | |
| Cereals | 4.26 c | 2.73–8.81 c | 1.21–8.24 c | |||
| Purees | 4.66 c | 1.33–8.43 c | 0.53–5.41 c | |||
| China | Food samples | nd–120 c | nd–100 c | [72] | ||
| China | Green tea | 55.7 b | 4.99–717 b | 33.5 b | 2.55–543 b | [73] |
| China | Noodle | 1200 c | 59–3000 c | 140 c | 12–520 c | [74] |
| Seasoning | 1400 c | 160–3300 c | 160 c | 8–650 c | ||
| Noodle soup | 560 d | 160–1500 d | 540 d | 19–1500 d | ||
| China | Raw milk | 1470 a | 130–5770 a | 170 a | 6.8–800 a | [75] |
| German | Infant food | nd–190 a | Nd–32 a | [76] | ||
| Europe | Baby food | nd–3765 a | [77] | |||
| German | Vitamin E supplements | 3810 a | nd–61,100 a | 15,200 a | nd–151,000 a | [78] |
| UK | Human milk | /180 a | 49 to 820 a | /21 a | 6.2 to 320 a | [79] |
| Czech | Human serum | /370 a | 150–2600 a | /360 a | 200–2110 a | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Mai, B.; Luo, X. Bioaccumulation and Biotransformation of Chlorinated Paraffins. Toxics 2022, 10, 778. https://doi.org/10.3390/toxics10120778
Chen L, Mai B, Luo X. Bioaccumulation and Biotransformation of Chlorinated Paraffins. Toxics. 2022; 10(12):778. https://doi.org/10.3390/toxics10120778
Chicago/Turabian StyleChen, Liujun, Bixian Mai, and Xiaojun Luo. 2022. "Bioaccumulation and Biotransformation of Chlorinated Paraffins" Toxics 10, no. 12: 778. https://doi.org/10.3390/toxics10120778
APA StyleChen, L., Mai, B., & Luo, X. (2022). Bioaccumulation and Biotransformation of Chlorinated Paraffins. Toxics, 10(12), 778. https://doi.org/10.3390/toxics10120778






