A First-Tier Framework for Assessing Toxicological Risk from Vaporized Cannabis Concentrates
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Gathering the Data (Hazard Identification)
3.2. Dose Response Assessment
3.2.1. Respiratory Sensitizers
3.2.2. Genotoxic, Mutagenic, and Carcinogenic Substances
3.2.3. Other Substances to Avoid
3.3. Calculating a Safety Limit
3.3.1. Local Respiratory Toxicity
3.3.2. Developmental Toxicity
3.3.3. Calculating a Safety Limit with Available Data
3.3.4. Threshold of Toxicological Concern
3.4. Exposure Assessment
3.5. Risk Characterization
4. Discussion
4.1. Recommendations for Regulators
4.2. Recommendations for Manufacturers
4.3. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Selected Abbreviations and Definitions
| ACGIH | American Conference of Governmental Industrial Hygienists |
| Adverse effect | A harmful effect. Examples include a change in morphology or impairment of function. |
| CBD | Cannabidiol, a non-intoxicating phytocannabinoid in cannabis |
| Concentration | The level of a substance of interest in a medium (e.g., air, cell culture media, or solvent). Units may be in %, mg/L, or mg/m3 of air. |
| Dose | The exposure to a cannabis concentrate consumer (in mg per day or mg/kg body weight per day) or the test species in a toxicological study. |
| ECHA | European Chemicals Agency |
| EFSA | European Food Safety Authority |
| ENDS | Electronic nicotine delivery systems |
| EPA | U.S. Environmental Protection Agency |
| FEMA | Flavor and Extract Manufacturers Association of the U.S. FEMA is a trade organization supporting the U.S. FDA by establishing the GRAS status of food substances. |
| GCP | Good clinical practice. Guidelines are laid out in ICH E6 [101] to assure the quality and integrity of clinical studies. |
| GLP | Good laboratory practice—a set of principles to assure quality and integrity of non-clinical studies laid out in 21 CFR 58. |
| GRAS | Generally recognized as safe in food under certain conditions of use. GRAS status alone cannot demonstrate safety for inhalable cannabis concentrate additives; however, the underlying data to determine GRAS status may be useful in the risk assessment. |
| IFRA | International Fragrance Association |
| LOAEL | Lowest adverse effect level. The dose at which an adverse biological response is first observed. |
| NAMs | New approach methodologies. These are approaches used in risk assessment to fill information gaps that aim to reduce animal use. This generally includes in vitro, in chemico, and in silico. |
| NIOSH | U.S. National Institute for Occupational Safety & Health |
| NOAEC | No adverse effect concentration. The concentration at which no adverse effects are observed, typically in an inhalation study in units of mg/m3 or ppm. |
| NOAEL | No adverse effect level. The dose at which no adverse effects are observed. This is typically in units of mg/kg. |
| OEHHA | California Office of Environmental Health Hazard Assessment |
| OSHA | U.S. Occupational Safety and Health Administration |
| POD | Point of Departure. The dose chosen as a basis for making extrapolations needed for assessing risk, usually the NOAEL or LOAEL. |
| RIFM | Research Institute for Fragrance Materials |
| TCEQ | Texas Commission on Environmental Quality |
| THC | Delta-9 tetrahydrocannabinol, the substance in cannabis generally responsible for the intoxicating effect. |
| Toxicodynamics | The biological effects of a substance |
| Toxicokinetics | The disposition of substances in the body, including absorption, distribution, metabolism, and elimination. |
| TTC | Threshold of toxicological concern |
| WHO | World Health Organization |
References
- Bahlai, C.A.; Xue, Y.; McCreary, C.M.; Schaafsma, A.W.; Hallett, R.H. Choosing organic pesticides over synthetic pesticides may not effectively mitigate environmental risk in soybeans. PLoS ONE 2010, 5, e11250. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Profet, M.; Gold, L.S. Nature’s chemicals and synthetic chemicals: Comparative toxicology. Proc. Natl. Acad. Sci. USA 1990, 87, 7782–7786. [Google Scholar] [CrossRef] [PubMed]
- Botanical drug Development: Guidance for Industry; U.S. FDA: Silver Spring, MD, USA, 2016.
- Jameson, L.E.; Conrow, K.D.; Pinkhasova, D.V.; Boulanger, H.L.; Ha, H.; Jourabchian, N.; Johnson, S.A.; Simeone, M.P.; Afia, I.A.; Cahill, T.M.; et al. Comparison of State-Level Regulations for Cannabis Contaminants and Implications for Public Health. Environ. Health Perspect. 2022, 130, 97001. [Google Scholar] [CrossRef] [PubMed]
- CDC. Outbreak of Lung Injury Associate with the Use of E-Cigarette, or Vaping, Products. Available online: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed on 22 November 2022).
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2020, 382, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Meek, M.E.; Boobis, A.R.; Crofton, K.M.; Heinemeyer, G.; Raaij, M.V.; Vickers, C. Risk assessment of combined exposure to multiple chemicals: A WHO/IPCS framework. Regul. Toxicol. Pharmacol. 2011, 60, S1–S14. [Google Scholar] [CrossRef]
- U.S. EPA. Concepts, Methods and Data Sources for Cumulative Health Risk Assessment of Multiple Chemicals, Exposures and Effects: A Resource Document; EPA/600/R-06/013F; U.S. Environmental Protection Agency: Washington, DC, USA, 2007. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=190187 (accessed on 27 October 2022).
- EFSA. International Framework Dealing with Human Risk Assessment of Combined Exposure to Multiple Chemicals. Efsa J. 2013, 11, 3313. [Google Scholar]
- Neltner, T.G.; Alger, H.M.; Leonard, J.E.; Maffini, M.V. Data gaps in toxicity testing of chemicals allowed in food in the United States. Reprod. Toxicol. 2013, 42, 85–94. [Google Scholar] [CrossRef]
- Miller-Holt, J.; Behrsing, H.P.; Clippinger, A.J.; Hirn, C.; Stedeford, T.J.; Stucki, A.O. Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of tobacco and other nicotine-containing products. Front. Toxicol. 2022, 4, 943358. [Google Scholar] [CrossRef]
- U.S. EPA. EPA New Approach Methods Work Plan: Reducing Use of Vertebrate Animals in Chemical Testing. Available online: https://www.epa.gov/chemical-research/epa-new-approach-methods-work-plan-reducing-use-vertebrate-animals-chemical (accessed on 29 September 2022).
- Yakowicz, W. Weed vs. Greed: How America Botched Legalizing Pot. Forbes. 2022. August/September 2022. Available online: https://www.forbes.com/sites/willyakowicz/2022/08/02/how-america-botched-legalizing-cannabis-berner-cookies-kim-rivers-trulieve-steve-deangelo/?sh=7857a1204489 (accessed on 27 October 2022).
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.; Tierney, P.A.; Pankow, J.F.; Talbot, P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep. 2019, 9, 2468. [Google Scholar] [CrossRef]
- Vreeke, S.; Zhu, X.; Strongin, R.M. A simple predictive model for estimating relative e-cigarette toxic carbonyl levels. PLoS ONE 2020, 15, e0238172. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Liao, J.; Matsuo, T.; Ito, K.; Fowles, J.; Shusterman, D.; Mendell, M.; Kumagai, K. A Device-Independent Evaluation of Carbonyl Emissions from Heated Electronic Cigarette Solvents. PLoS ONE 2017, 12, e0169811. [Google Scholar] [CrossRef] [PubMed]
- Zelinkova, Z.; Wenzl, T. Influence of battery power setting on carbonyl emissions from electronic cigarettes. Tob. Induc. Dis. 2020, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, G.; Alibrando, F.; Vento, F.; Trovato, E.; Zoccali, M.; Guarnaccia, P.; Dugo, P.; Mondello, L. Development of a Novel Microwave Distillation Technique for the Isolation of Cannabis sativa L. Essential Oil and Gas Chromatography Analyses for the Comprehensive Characterization of Terpenes and Terpenoids, Including Their Enantio-Distribution. Molecules 2021, 26, 1588. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.; Nahtigal, I. The evolving landscape of cannabis edibles. Curr. Opin. Food Sci. 2019, 28, 25–31. [Google Scholar] [CrossRef]
- Bowen, J.K.; Chaparro, J.M.; McCorkle, A.M.; Palumbo, E.; Prenni, J.E. The impact of extraction protocol on the chemical profile of cannabis extracts from a single cultivar. Sci. Rep. 2021, 11, 21801. [Google Scholar] [CrossRef]
- Meehan-Atrash, J.; Rahman, I. Cannabis Vaping: Existing and Emerging Modalities, Chemistry, and Pulmonary Toxicology. Chem. Res. Tox. 2021, 34, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Afia, I.; Jourabchian. Vapes: What are you actually inhaling? Cannabis Sci. Technol. 2020, 3, 44–51. [Google Scholar]
- U.S. EPA. Procedures for Reviewing Relevant Effects Data Published in the Open Literature for Use in OPP’s Human Health Risk Assessments; U.S. EPA: Research Triangle Park, NC, USA, 2012.
- Oar, M.A.; Savage, C.H.; Rufer, E.S.; Rucker, R.P.; Guzman, J.A. Thermography of cannabis extract vaporization cartridge heating coils in temperature- and voltage-controlled systems during a simulated human puff. PLoS ONE 2022, 17, e0262265. [Google Scholar] [CrossRef]
- Dankovic, D.A.; Naumann, B.D.; Maier, A.; Dourson, M.L.; Levy, L.S. The scientific basis of uncertainty factors used in setting occupational exposure limits. J. Occup. Environ. Hyg. 2015, 12 (Suppl. 1), S55–S68. [Google Scholar] [CrossRef]
- NIOSH. p-cymene; 2017. Available online: https://www.cdc.gov/niosh-rtecs/gz5aca30.html (accessed on 5 August 2022).
- Norden. Existing Default Values and Recommendations for Exposure Assessment: A Nordice Exposure Group Project; Nordic Council of Ministers: Copenhagen, Denmark, 2011. [Google Scholar]
- Guidance on information requirements and chemical safety assessment. In Chapter R.8: Characterisation of Dose [Concentration]-Response for Human Health; ECHA: Helsinki, Finland, 2012.
- Guidance on Information Requirements and Chemical Safety Assessment. In Chapter R.15: Consumer Exposure Assessment; ECHA: Helsinki, Finland, 2016.
- Flavor and Extract Manufacturers Association of the U.S. (FEMA). Safety and Regulatory Authority to Use Flavors—Focus on Vaping Products. Available online: https://www.femaflavor.org/safety-regulatory-authority-use-flavors-focus-vaping-products (accessed on 4 August 2022).
- Blackburn, K.; Stuard, S.B. A framework to facilitate consistent characterization of read across uncertainty. Regul. Toxicol. Pharmacol. 2014, 68, 353–362. [Google Scholar] [CrossRef]
- Lester, C.; Reis, A.; Laufersweiler, M.; Wu, S.; Blackburn, K. Structure activity relationship (SAR) toxicological assessments: The role of expert judgment. Regul. Toxicol. Pharmacol. 2018, 92, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Blackburn, K.; Amburgey, J.; Jaworska, J.; Federle, T. A framework for using structural, reactivity, metabolic and physicochemical similarity to evaluate the suitability of analogs for SAR-based toxicological assessments. Regul. Toxicol. Pharmacol. 2010, 56, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Hand, A.; Blake, A.; Kerrigan, P.; Samuel, P.; Friedberg, J. History of medical cannabis. Cannabis: Med. Asp. 2016, 9, 387–394. [Google Scholar]
- CDC. Health Effects of Cigarette Smoking. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm (accessed on 13 September 2022).
- Melamede, R. Cannabis and tobacco smoke are not equally carcinogenic. Harm Reduct. J. 2005, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Robinson, T.; Bullen, C.; Curran, V.; Jutras-Aswad, D.; Medina-Mora, M.E.; Pacula, R.L.; Rehm, J.; Room, R.; Brink, W.v.d.; et al. Lower-Risk Cannabis Use Guidelines (LRCUG) for reducing health harms from non-medical cannabis use: A comprehensive evidence and recommendations update. Int. J. Drug Policy 2022, 99, 103381. [Google Scholar] [CrossRef]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef]
- Wang, T.; Collet, J.P.; Shapiro, S.; Ware, M.A. Adverse effects of medical cannabinoids: A systematic review. CMAJ 2008, 178, 1669–1678. [Google Scholar] [CrossRef]
- LaFrance, E.M.; Stueber, A.; Glodosky, N.C.; Mauzay, D.; Cuttler, C. Overbaked: Assessing and predicting acute adverse reactions to Cannabis. J. Cannabis Res. 2020, 2, 3. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, S.; Aldington, D.; Wiffen, P.J. Adverse events associated with single dose oral analgesics for acute postoperative pain in adults—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2015, CD011407. [Google Scholar] [CrossRef]
- National Institute on Alcohol Abuse and Alcoholism. Alcohol Facts and Statistics. 2022; p. 26. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics (accessed on 26 September 2022).
- Gibson, L.P.; Karoly, H.C.; Ellingson, J.M.; Klawitter, J.; Sempio, C.; Squeri, J.E.; Bryan, A.D.; Bidwell, L.C.; Hutchison, K.E. Effects of cannabidiol in cannabis flower: Implications for harm reduction. Addict. Biol. 2022, 27, e13092. [Google Scholar] [CrossRef]
- Cuttler, C.; LaFrance, E.M.; Stueber, A. Acute effects of high-potency cannabis flower and cannabis concentrates on everyday life memory and decision making. Sci. Rep. 2021, 11, 13784. [Google Scholar] [CrossRef] [PubMed]
- Confident Cannabis. Wholesale—Marketplace. Available online: https://confidentcannabis.com/ (accessed on 17 August 2021).
- Gieringer, D.H. Cannabis “Vaporization”. J. Cannabis Ther. 2001, 1, 153–170. [Google Scholar] [CrossRef]
- Meehan-Atrash, J.; Luo, W.; McWhirter, K.J.; Strongin, R.M. Aerosol Gas-Phase Components from Cannabis E-Cigarettes and Dabbing: Mechanistic Insight and Quantitative Risk Analysis. ACS Omega 2019, 4, 16111–16120. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Spooner, C.; May, L.; Leslie, R.; Whiteley, V.L. Cannabinoid Hyperemesis Syndrome Survey and Genomic Investigation. Cannabis Cannabinoid Res. 2022, 7, 336–344. [Google Scholar] [CrossRef]
- Petrilli, K.; Ofori, S.; Hines, L.; Taylor, G.; Adams, S.; Freeman, T.P. Association of cannabis potency with mental ill health and addiction: A systematic review. Lancet Psychiatry 2022, 9, 736–750. [Google Scholar] [CrossRef]
- Chatkin, J.M.; Zani-Silva, L.; Ferreira, I.; Zamel, N. Cannabis-associated asthma and allergies. Clin. Rev. Allergy Immunol. 2019, 56, 196–206. [Google Scholar] [CrossRef]
- Konradsen, J.R.; Borres, M.P.; Nilsson, C. Unusual and Unexpected Allergic Reactions Can Be Unraveled by Molecular Allergy Diagnostics. Int. Arch. Allergy. Immunol. 2021, 182, 904–916. [Google Scholar] [CrossRef]
- Lu, S.J.; Li, L.; Duffy, B.C.; Dittmar, M.A.; Durocher, L.A.; Panawennage, D.; Delaney-Baldwin, E.R.; Spink, D.C. Investigation of Vaping Fluids Recovered From New York State E-Cigarette or Vaping Product Use-Associated Lung Injury Patients. Front Chem 2021, 9, 748935. [Google Scholar] [CrossRef]
- Attfield, K.R.; Chen, W.; Cummings, K.J.; Jacob, P., 3rd; O’Shea, D.F.; Wagner, J.; Wang, P.; Fowles, J. Potential of ethenone (ketene) to contribute to electronic cigarette, or vaping, product use-associated lung injury. Am. J. Respir. Crit. Care Med. 2020, 202, 1187–1189. [Google Scholar] [CrossRef]
- Matsumoto, S.; Fang, X.; Traber, M.G.; Jones, K.D.; Langelier, C.; Hayakawa Serpa, P.; Calfee, C.S.; Matthay, M.A.; Gotts, J.E. Dose-Dependent Pulmonary Toxicity of Aerosolized Vitamin E Acetate. Am. J. Respir. Cell Mol. Biol. 2020, 63, 748–757. [Google Scholar] [CrossRef]
- Lee, H. Vitamin E acetate as linactant in the pathophysiology of EVALI. Med. Hypotheses 2020, 144, 110182. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; O’Shea, D.F. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc. Natl. Acad. Sci. USA 2020, 117, 6349–6355. [Google Scholar] [CrossRef] [PubMed]
- Guidance on Assessment Factors to Derive a DNEL. ECETOC: ECETOC AISBL: Brussels, Belgium. 2010. Available online: https://www.ecetoc.org/wp-content/uploads/2014/08/ECETOC-TR-110-Guidance-on-assessment-factors-to-derive-a-DNEL.pdf (accessed on 27 October 2022).
- U.S. FDA. Guidance on M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing. Auth. Pharm. 2010, 75, 3471–3472. [Google Scholar]
- Chemical-Specific Adjustment Factors for Interspecies Differences and Human Variability: Guidance Document for Use of Data in Dose/Concentration-Response Assessment; WHO: Geneva, Switzerland, 2005.
- Derelanko, M.J. The Toxicologist’s Pocket Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Arcus, A.; Blaisdell, R.; Brown, J.P.; Budroe, J.; DuTeaux, S.; Collins, J.F.; Dodge, D.E.; Lam, R.; Marty, M.A.; Miller, M.; et al. TSD for Noncancer Reference Exposure Levels. 2008. Available online: https://oehha.ca.gov/media/downloads/crnr/noncancertsdfinal.pdf (accessed on 27 October 2022).
- Schröder, K.; Escher, S.E.; Hoffmann-Dörr, S.; Kühne, R.; Simetska, N.; Mangelsdorf, I. Evaluation of route-to-route extrapolation factors based on assessment of repeated dose toxicity studies compiled in the database RepDose®. Toxicol. Lett. 2016, 261, 32–40. [Google Scholar] [CrossRef]
- Blackburn, K.; Daston, G.; Fisher, J.; Lester, C.; Naciff, J.M.; Rufer, E.S.; Stuard, S.B.; Woeller, K. A strategy for safety assessment of chemicals with data gaps for developmental and/or reproductive toxicity. Regul. Toxicol. Pharmacol. 2015, 72, 202–215. [Google Scholar] [CrossRef]
- Evidence on the Developmental Toxicity of Cannabis (Marijuana) Smoke and Delta-9THC; CAL EPA OEHHA: Sacramento, CA, USA, 2019.
- Conner, S.N.; Bedell, V.; Lipsey, K.; Macones, G.A.; Cahill, A.G.; Tuuli, M.G. Maternal Marijuana Use and Adverse Neonatal Outcomes: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2016, 128, 713–723. [Google Scholar] [CrossRef]
- Torres, C.A.; Medina-Kirchner, C.; O’Malley, K.Y.; Hart, C.L. Totality of the Evidence Suggests Prenatal Cannabis Exposure Does Not Lead to Cognitive Impairments: A Systematic and Critical Review. Front. Psychol. 2020, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.J.; Korach, N.J.; Kruger, J.S. Requirements for Cannabis Product Labeling by U.S. State. Cannabis Cannabinoid Res. 2022, 7, 156–160. [Google Scholar] [CrossRef]
- Fell, J.C.; Toomey, T.; Eichelberger, A.H.; Kubelka, J.; Schriemer, D.; Erickson, D. What is the likelihood that underage youth can obtain marijuana from licensed recreational marijuana outlets in California, a state where recreational marijuana is legal? J. Saf. Res. 2022, 82, 102–111. [Google Scholar] [CrossRef]
- A review of the Reference Dose (RfD) and Reference Concentration (RfC) processes. In Risk Assessment Forum; U.S. EPA: Washington, DC, USA, 2002. Available online: https://www.epa.gov/osa/review-reference-dose-and-reference-concentration-processes (accessed on 27 October 2022).
- Kroes, R.; Kleiner, J.; Renwick, A. The threshold of toxicological concern concept in risk assessment. Toxicol. Sci. 2005, 86, 226–230. [Google Scholar] [CrossRef]
- ISO. Biological Evaluation of Medical Devices—Application of the Threshold of Toxicological Concern (TTC) for Assessing Biocompatibility of Medical Device Constituents; ISO: Geneva, Switzerland, 2019; p. 21726. [Google Scholar]
- ICH Guideline M7(R1) on Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk; EMA: Amsterdam, The Netherlands, 2015.
- M7(R1) Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk Guidance for Industry; U.S. FDA: Silver Spring, MD, USA, 2018.
- Carthew, P.; Clapp, C.; Gutsell, S. Exposure based waiving: The application of the toxicological threshold of concern (TTC) to inhalation exposure for aerosol ingredients in consumer products. Food Chem. Toxicol. 2009, 47, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Nelms, M.D.; Patlewicz, G. Derivation of New Threshold of Toxicological Concern Values for Exposure via Inhalation for Environmentally-Relevant Chemicals. Front. Toxicol. 2020, 2, 580347. [Google Scholar] [CrossRef]
- Choosing a Percentile of Acute Dietary Exposure as a Threshold of Regulatory Concern; U.S. EPA: Research Triangle Park, NC, USA, 2000. Available online: https://www.epa.gov/sites/default/files/2015-07/documents/trac2b054_0.pdf (accessed on 27 October 2022).
- WHO. Dietary exposure assessment for chemicals in Food. In EHC 240: Principles for Risk Assessment of Chemicals in Food, 2nd ed.; World Health Organization & Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2020; Available online: https://inchem.org/documents/ehc/ehc/ehc240_index.htm (accessed on 27 October 2022).
- Kluxen, F.M.; Felkers, E.; Baumann, J.; Morgan, N.; Wiemann, C.; Stauber, F.; Strupp, C.; Adham, S.; Kuster, C.J. Compounded conservatism in European re-entry worker risk assessment of pesticides. Regul. Toxicol. Pharmacol. 2021, 121, 104864. [Google Scholar] [CrossRef] [PubMed]
- Bogen, K.T. A note on compounded conservatism. Risk Anal. 1994, 14, 379–381. [Google Scholar] [CrossRef]
- Polosa, R.; Caponnetto, P.; Maglia, M.; Morjaria, J.B.; Russo, C. Success rates with nicotine personal vaporizers: A prospective 6-month pilot study of smokers not intending to quit. BMC Public Health 2014, 14, 1159. [Google Scholar] [CrossRef]
- Smets, J.; Baeyens, F.; Chaumont, M.; Adriaens, K.; Van Gucht, D. When Less is More: Vaping Low-Nicotine vs. High-Nicotine E-Liquid is Compensated by Increased Wattage and Higher Liquid Consumption. Int. J. Environ. Res. Public Health 2019, 16, 723. [Google Scholar] [CrossRef]
- BlincGroup. Labstat. Cannabis Puffing Regimes. 2022. Available online: https://www.globenewswire.com/news-release/2022/01/25/2372346/0/en/The-Blinc-Group-Labstat-International-Inc-Study-Finds-Vape-Habits-Don-t-Cross-Borders.html (accessed on 17 May 2022).
- Morgan, J.; Gschwend, G.; Houston, M.; Jones, A.; Kelso, C. Vaping preferences of individuals who vaporise dry herb cannabis, cannabis liquids and cannabis concentrates. Drug Alcohol Depend. 2022, 240, 109632. [Google Scholar] [CrossRef]
- Daniulaityte, R.; Lamy, F.R.; Barratt, M.; Nahhas, R.W.; Martins, S.S.; Boyer, E.W.; Sheth, A.; Carlson, R.G. Characterizing marijuana concentrate users: A web-based survey. Drug Alcohol Depend. 2017, 178, 399–407. [Google Scholar] [CrossRef]
- Bolivar, L.E. Report on Cannabis Consumer Demographics and Consumption Habits; Cannabis Consumers Coalition: Lakewood, CO, USA, 2017. [Google Scholar]
- Bidwell, L.C.; YorkWilliams, S.L.; Mueller, R.L.; Bryan, A.D.; Hutchison, K.E. Exploring cannabis concentrates on the legal market: User profiles, product strength, and health-related outcomes. Addict. Behav. Rep. 2018, 8, 102–106. [Google Scholar] [CrossRef]
- Rotermann, M. Analysis of trends in the prevalence of cannabis use and related metrics in Canada. Health Rep. 2019, 30, 3–13. [Google Scholar] [CrossRef]
- Fehr, K.O.; Kalant, H. Analysis of Cannabis Smoke Obtained under Different Combustion Conditions. Can. J. Physiol. Pharmacol. 1972, 60, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Manno, J.E.; Kiplinger, G.F.; Haine, S.E.; Bennett, I.F.; Forney, R.B. Comparative effects of smoking marihuana or placebo on human motor and mental performance. Clin. Pharm. Ther. 1970, 11, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Perez-Reyes, M. Marijuana smoking: Factors that influence the bioavailability of tetrahydrocannabinol. NIDA Res. Monogr. 1990, 99, 42–62. [Google Scholar]
- Gieringer, D.; St. Laurent, J.; Goodrich, S. Cannabis Vaporizer Combines Efficient Delivery of THC with Effective Suppression of Pyrolytic Compounds. J. Cannabis Ther. 2004, 4, 7–27. [Google Scholar] [CrossRef]
- Exposure Factors Handbook 2011 Edition (Final Report); EPA/600/R-09/052F; U.S. EPA: Washington, DC, USA, 2011. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 (accessed on 27 October 2022).
- Colbert, M. Growing Health Concerns over Cannabis Vape Cartridge Additives. 2022. Available online: https://news.green-flower.com/vape-cartridge-additives/ (accessed on 1 October 2022).
- Troutt, W.D.; DiDonato, M.D. Carbonyl Compounds Produced by Vaporizing Cannabis Oil Thinning Agents. J. Altern. Complement. Med. 2017, 23, 879–884. [Google Scholar] [CrossRef]
- Meehan-Atrash, J.; Strongin, R.M. Pine rosin identified as a toxic cannabis extract adulterant. Forensic. Sci. Int. 2020, 312, 110301. [Google Scholar] [CrossRef]
- Medical Marijuana Access & Patient Safety, Inc., v. Pennsylvania Department of Health. 2022. Available online: https://assets.website-files.com/6205cfe07b880a38c2883917/621fa6f9dd0fe45cf908757d_Order%20Filed%20on%20Destruction%20of%20Product.pdf (accessed on 27 October 2022).
- Belushkin, M.; Tafin Djoko, D.; Esposito, M.; Korneliou, A.; Jeannet, C.; Lazzerini, M.; Jaccard, G. Selected harmful and potentially harmful constituents levels in commercial e-cigarettes. Chem. Res. Toxicol. 2020, 33, 657–668. [Google Scholar] [CrossRef]
- Earleywine, M.; Barnwell, S.S. Decreased respiratory symptoms in cannabis users who vaporize. Harm. Reduct. J. 2007, 4, 11. [Google Scholar] [CrossRef]
- Gilman, G.; Johnson, M.; Martin, A.; Myers, D.; Alston, B.; Mistra, M. Characterization of temperature regulation and HPHC profile of a nicotine-salt based ENDS Product. Available online: https://www.juullabsscience.com/wp-content/uploads/sites/8/2020/09/Characterization-of-Temperature-Regulation-and-HPHC-Profile-of-a-Nicotine-Salt-Based-ENDS-Product_SRNT_2018.pdf (accessed on 28 October 2022).
- Haddad, C.; Salman, R.; El-Hellani, A.; Talih, S.; Shihadeh, A.; Saliba, N.A. Reactive Oxygen Species Emissions from Supra- and Sub-Ohm Electronic Cigarettes. J. Anal. Toxicol. 2019, 43, 45–50. [Google Scholar] [CrossRef]
- E6(R2) Good Clinical Practice; ICH: Geneva, Switzerland, 2016; Available online: https://www.ich.org/page/efficacy-guidelines (accessed on 28 October 2022).
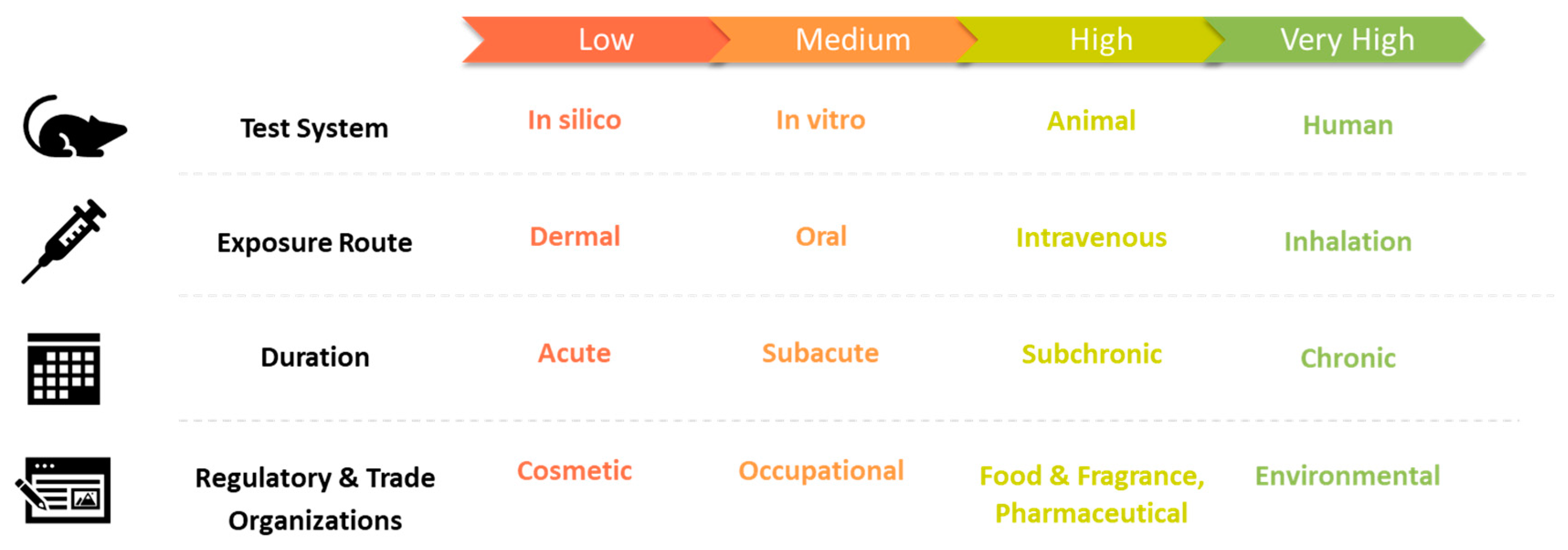
| Uncertainty Category | Value | Comment | References | ||
|---|---|---|---|---|---|
| 1 | Interspecies | Interspecies extrapolation | 10 | Extrapolation from animals to humans | [8,60,61] |
| 2 | Intraspecies | Intraspecies variation | 1, 3 or 10 | May be reduced to 3 or 1 if the study was conducted in a sensitive subpopulation (e.g., diseased individuals, children, elderly, etc.) | [8,60,61] |
| 3 | Route to route extrapolation | Oral to inhalation—toxicokinetics | 2 | If absorption data are available for inhalation or oral routes, they should be used instead. Combine with toxicodynamic differences if data are lacking. | [57,62] |
| Oral to inhalation—toxicodynamics | 3 | Combine with toxicokinetic differences if data are lacking. | [62] | ||
| 4 | LOAEL to NOAEL or LOAEC to NOAEC | Used if adverse effects were observed at the lowest dose tested | 10 | Benchmark dose modeling is preferred instead of a UF, but benchmark dose modeling is out of scope for a Tier 1 safety assessment. | [8,60,61] |
| 5 | Duration | Subacute to chronic | 10 | Subacute = 14–90 days. Chronic ≥ 1 year. | [57] |
| Subchronic to chronic | 3 | Subchronic = 90 days–1 year. Chronic ≥ 1 year. | [28,57,61] | ||
| 6 | Database | Database completeness | 1, 3 or 10 | Necessary if developmental or reproductive toxicity data are not available for a non-age-gated product (e.g., CBD in a convenience store). A UF of 3 can be used if the structural features of the substance are not suggestive of potential developmental toxicity as limits derived from repeat dose studies will suffice. | [63] |
| Questions | No | Yes | |
|---|---|---|---|
| 1 | Can the substance use the TTC approach? | If the substance fits into one of these categories, TTC cannot be used:
| Everything else. Proceed to Q2. |
| 2 | Is the substance an organophosphate or carbamate? | Move to Q3 | TTC of 18 µg/day |
| 3 | Is there a genotoxicity alert or other evidence of genotoxicity such as a positive Ames or micronucleus assay? | Move to Q4 | Exposure > 10 years to lifetime: 1.5 µg/day 1–10 years: 10 µg/day 1–12 months: 20 µg/day <1 month: 120 µg/day [71] |
| 4 | Is the substance a natural cannabis substance or GRAS? | Move to Q5 | Classify according to Cramer class and use the corresponding value: Cramer Class 1: 865 µg/day Cramer Class 2 or 3: 145 µg/day [75] |
| 5 | If the substance does not fit into the other categories above, TTC must be based on a wider range of substances (e.g., industrial chemicals, pesticides, etc.) | Acute aquatic toxicity MOA by OASIS in OECD QSAR Toolbox as defined by [75]: Basesurface narcotics: 22.39 µg/day Reactive: 4.286 µg/day | |
| Timeframe | Mean | Percentile | N | Notes | Reference | ||
|---|---|---|---|---|---|---|---|
| 50th (Median) | 90th | 95th | |||||
| Daily, mg | 64 (US) 56 (Canada) | - | - | - | 2000 users | [82] | |
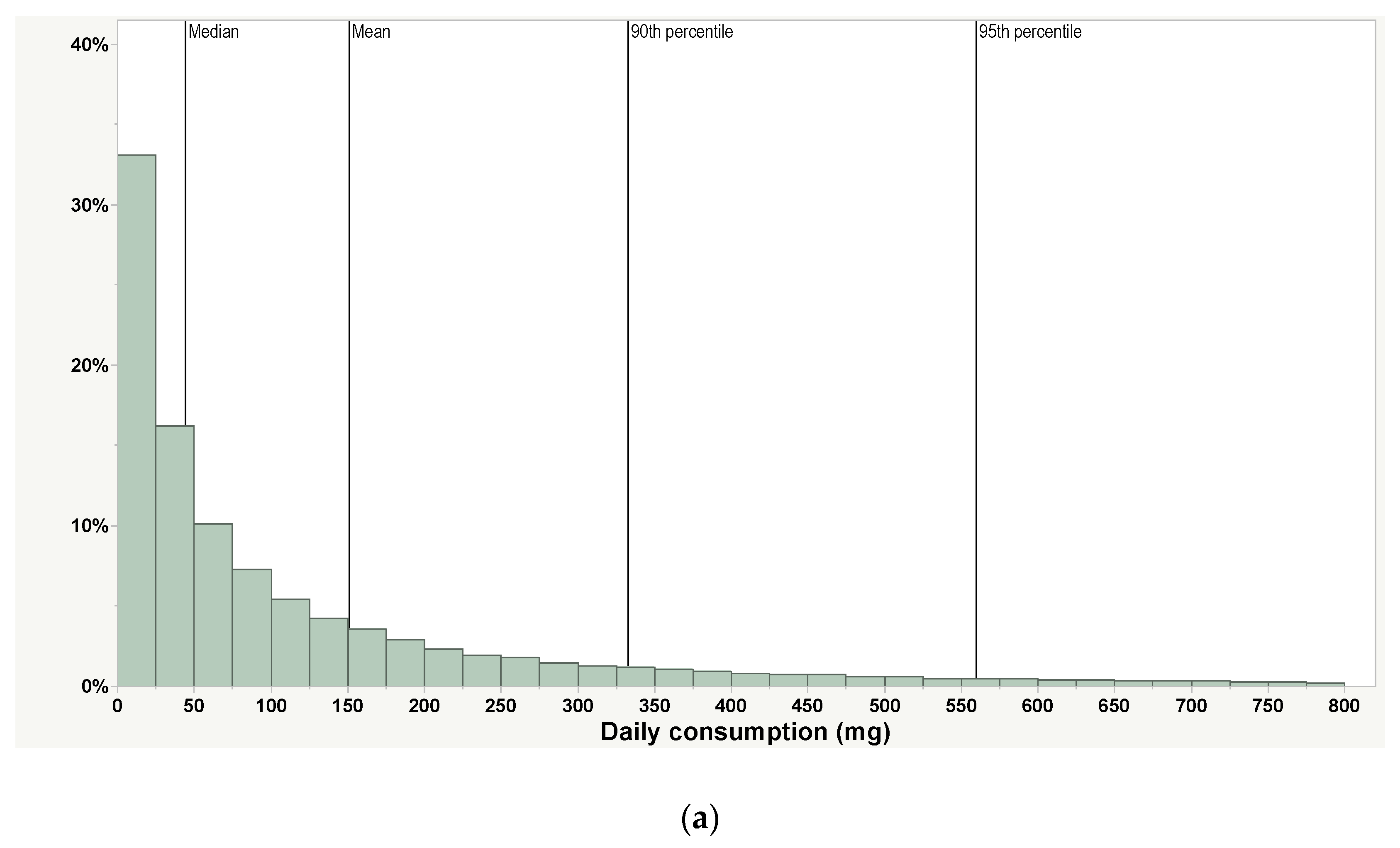
| Daily, mg1 | 151 | 44.0 | 333 | 560 | 54,500 devices | Only includes use days. Most people use their products less than daily | PAX Era App |
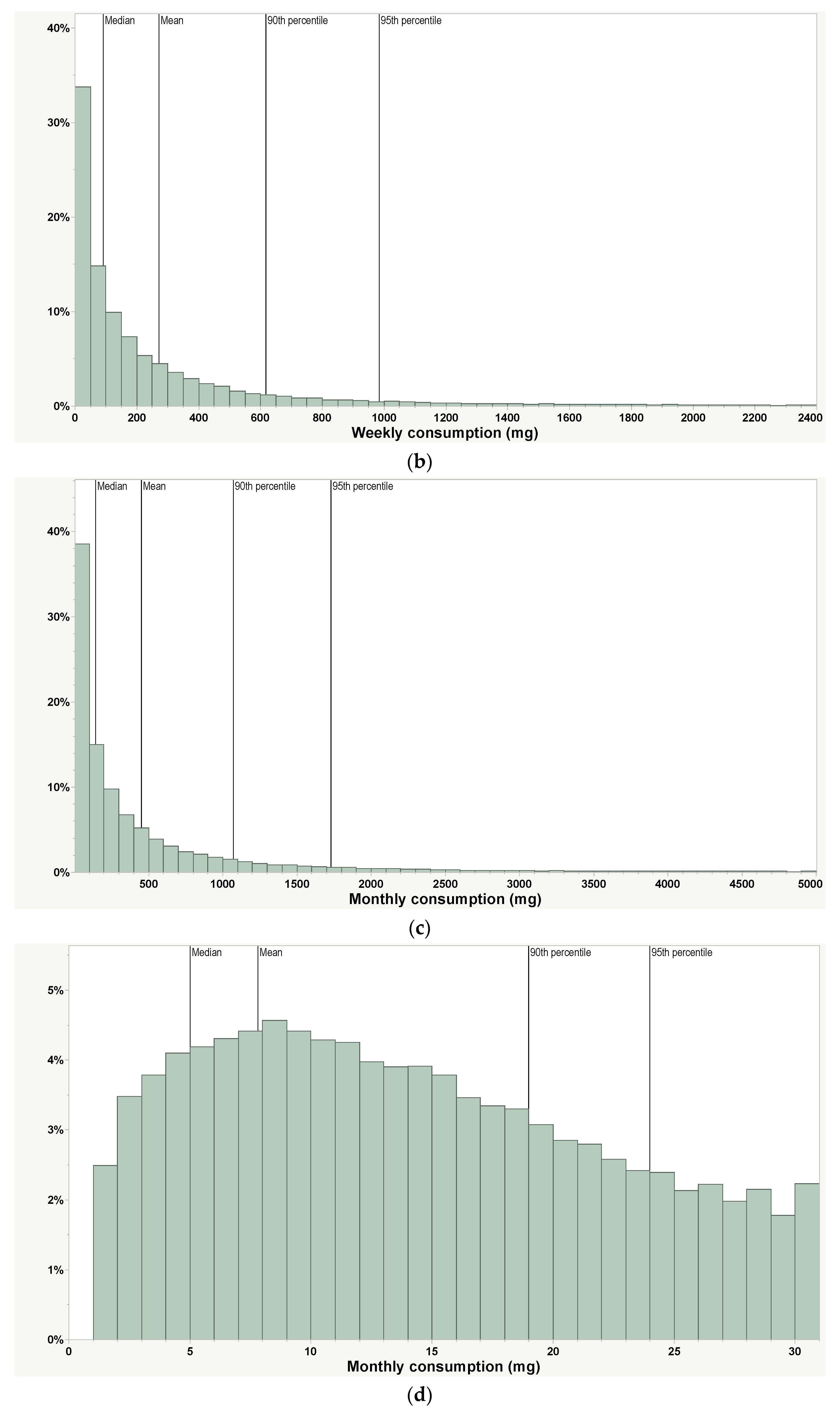
| Weekly (daily average), mg 1 | 271 (39) | 91.1 (13) | 617 (88) | 982 (140) | 54,500 devices | Includes non-use days | PAX Era App |
| Monthly (daily average), mg 1,2 | 452 (15) | 146 (5) | 1081 (35) | 1743 (57) | 54,500 devices | Includes non-use days | PAX Era App |
| Days of consumption per month, days | 7.8 | 5 | 19 | 24 | 54,500 devices | PAX Era App | |
| Daily, mg | 80 | - | - | - | - | Estimated based on THC consumed per joint year | [47] |
| Monthly (daily average), mg 2 | 4600 +/− 7000 (150.8) | 1000 (32.8) | - | - | 83 users | Self-reported use from a U.S., EU, Australia, and New Zealand | [83] |
| Exposure Assumption | Value | Notes and References |
|---|---|---|
| Daily cannabis concentrate product consumption 1 | 100 mg/day | Average of 95th percentile between weekly and monthly from PAX Era device (Table 3). This is expected to be a conservative estimate of average daily use of a cannabis concentrate product for a high-level consumer. This value can be used to estimate additive exposure as indicated in Equation (2) |
| Bioavailability | 100% | Conservative assumption |
| Body weight | 60 kg | Conservative body weight assumption for American adult female. This would be more conservative than assuming an adult male of higher body weight |
| Inhalation volume: 1 full day (24 h) | 20 m3 | Average daily inhalation rate [27,28,29] |
| Inhalation volume: 8 h or 1 workday | 6.7 m3 | Average inhalation rate over a workday [27,28,29] |
| Inhalation volume: 15 min | 0.3 m3 | Average inhalation over 1 day or 1 h adjusted for time [28,92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vreeke, S.; Faulkner, D.M.; Strongin, R.M.; Rufer, E. A First-Tier Framework for Assessing Toxicological Risk from Vaporized Cannabis Concentrates. Toxics 2022, 10, 771. https://doi.org/10.3390/toxics10120771
Vreeke S, Faulkner DM, Strongin RM, Rufer E. A First-Tier Framework for Assessing Toxicological Risk from Vaporized Cannabis Concentrates. Toxics. 2022; 10(12):771. https://doi.org/10.3390/toxics10120771
Chicago/Turabian StyleVreeke, Shawna, David M. Faulkner, Robert M. Strongin, and Echoleah Rufer. 2022. "A First-Tier Framework for Assessing Toxicological Risk from Vaporized Cannabis Concentrates" Toxics 10, no. 12: 771. https://doi.org/10.3390/toxics10120771






