Copper and Zinc Treatments Alter the Thyroid Endocrine System in Zebrafish Embryos/Larvae
Abstract
1. Introduction
2. Experimental Procedures
2.1. Embryo Culture and Exposure
2.2. RNA Extraction and Quantitative RT-PCR
2.3. Thyroid Hormone Assays
2.4. Statistical Analysis
3. Results
3.1. Developmental Toxicity Caused by Cu2+ and Zn2+
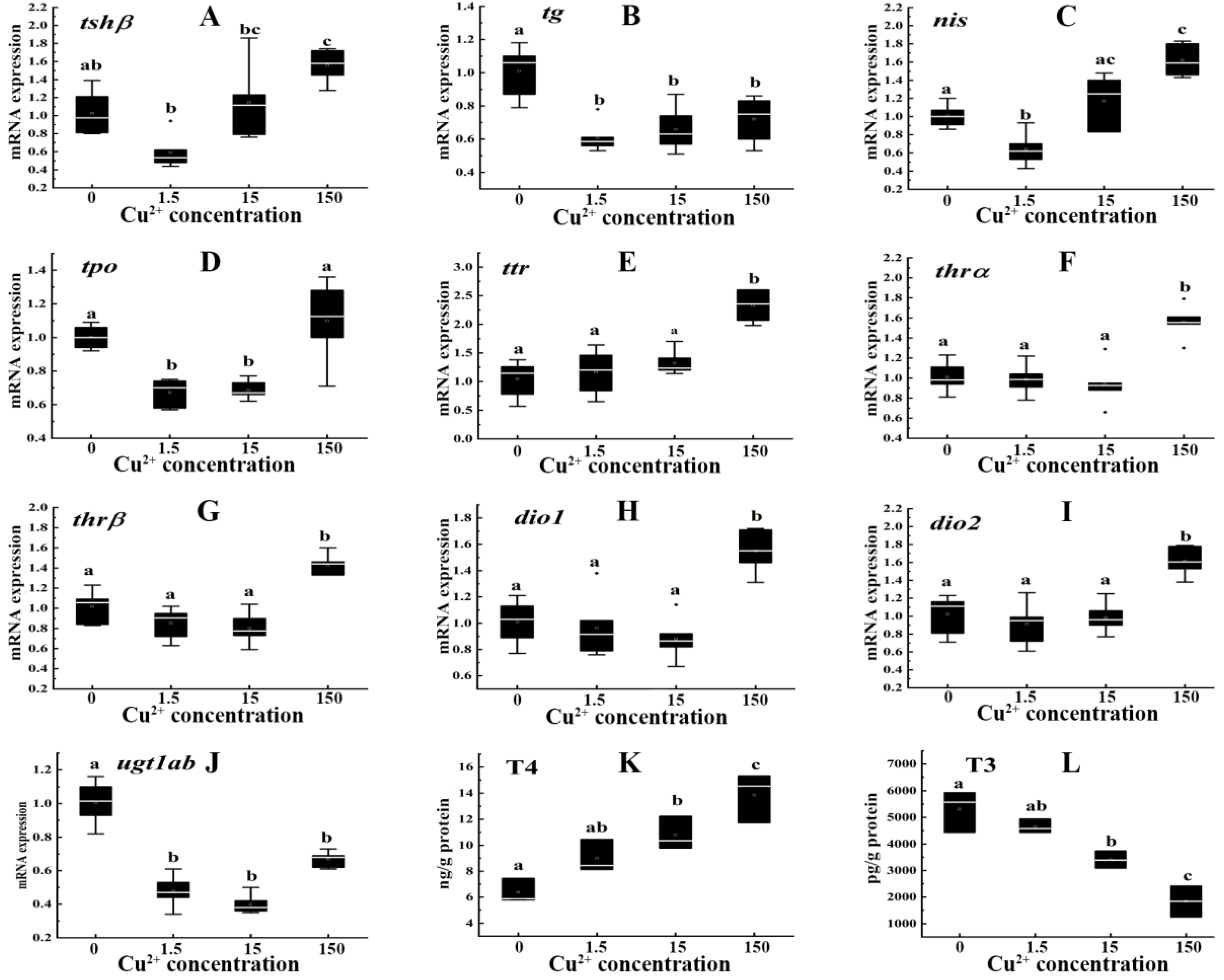
3.2. Influences of Cu2+ on Thyroid Endocrine System
3.3. Influences of Zn2+ on Thyroid Endocrine System
3.4. PCA and Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raychaudhuri, S.S.; Pramanick, P.; Talukder, P.; Basak, A. Polyamines, metallothioneins, and phytochelatins—Natural defense of plants to mitigate heavy metals. Stud. Nat. Prod. Chem. 2021, 69, 227–261. [Google Scholar]
- Ali, H.; Khan, E. What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’–proposal of a comprehensive definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Authman, M.M.; Zaki, M.S.; Khallaf, E.A.; Abbas, H.H. Use of fish as bio-indicator of the effects of heavy metals pollution. J. Aquac. Res. Dev. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 100, 133–164. [Google Scholar]
- Peng, Z.; Liu, X.; Zhang, W.; Zeng, Z.; Liu, Z.; Zhang, C.; Liu, Y.; Shao, B.; Liang, Q.; Tang, W. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2020, 134, 105298. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, P.; Esteban, M.Á.; Cuesta, A. Heavy metals produce toxicity, oxidative stress and apoptosis in the marine teleost fish SAF-1 cell line. Chemosphere 2016, 144, 225–233. [Google Scholar] [CrossRef]
- Thakare, M.; Sarma, H.; Datar, S.; Roy, A.; Pawar, P.; Gupta, K.; Pandit, S.; Prasad, R. Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr. Res. Biotechnol. 2021, 3, 84–98. [Google Scholar] [CrossRef]
- Clearwater, S.J.; Farag, A.M.; Meyer, J. Bioavailability and toxicity of dietborne copper and zinc to fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 132, 269–313. [Google Scholar] [CrossRef]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Ebrahimpour, M.; Alipour, H.; Rakhshah, S. Influence of water hardness on acute toxicity of copper and zinc on fish. Toxicol. Ind. Health 2010, 26, 361–365. [Google Scholar] [CrossRef]
- Kucharzewski, M.; Braziewicz, J.; Majewska, U.; Góźdź, S. Copper, zinc, and selenium in whole blood and thyroid tissue of people with various thyroid diseases. Biol. Trace Elem. Res. 2003, 93, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Ger, T.-R.; Uapipatanakul, B.; Huang, J.-C.; Chen, K.H.-C.; Hsiao, C.-D. Review of copper and copper nanoparticle toxicity in fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, B.; Ługowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2009, 35, 625–640. [Google Scholar] [CrossRef]
- Rayburn, J.; Aladdin, R. Developmental toxicity of copper, chromium, and aluminum using the shrimp embryo teratogenesis assay: Palaemonid with artificial seawater. Bull. Environ. Contam. Toxicol. 2003, 71, 0481–0488. [Google Scholar] [CrossRef]
- Kralik, A.; Kirchgessner, M.; Eder, K. Concentrations of thyroid hormones in serum and activity of hepatic 5' monodeiodinase in copper-deficient rats. Z. Ernahrungswiss. 1996, 35, 288–291. [Google Scholar] [CrossRef]
- Bastian, T.W.; Prohaska, J.R.; Georgieff, M.K.; Anderson, G.W. Perinatal iron and copper deficiencies alter neonatal rat circulating and brain thyroid hormone concentrations. Endocrinology. 2010, 151, 4055–4065. [Google Scholar] [CrossRef]
- Suvi, R.; Giovanna, M.; Katja, A. Experimental copper exposure, but not heat stress, leads to elevated intraovarian thyroid hormone levels in three-spined sticklebacks (Gasterosteus aculeatus). Ecotoxicology 2020, 29, 1431–1440. [Google Scholar] [CrossRef]
- Eyckmans, M.; Tudorache, C.; Darras, V.M.; Blust, R.; De Boeck, G. Hormonal and ion regulatory response in three freshwater fish species following waterborne copper exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 270–278. [Google Scholar]
- Eisler, R. Zinc Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; US Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1993. [Google Scholar]
- Song, Y.; Leonard, S.W.; Traber, M.G.; Ho, E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J. Nutr. 2009, 139, 1626–1631. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, L.; Li, D.-L.; Ling, F.; Wang, G.-X. Developmental toxicity in rare minnow (Gobiocypris rarus) embryos exposed to Cu, Zn and Cd. Ecotoxicol. Environ. Saf. 2014, 104, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Salvaggio, A.; Marino, F.; Albano, M.; Pecoraro, R.; Camiolo, G.; Tibullo, D.; Bramanti, V.; Lombardo, B.M.; Saccone, S.; Mazzei, V. Toxic effects of zinc chloride on the bone development in Danio rerio (Hamilton, 1822). Front. Physiol. 2016, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- McRae, N.K.; Gaw, S.; Glover, C.N. Mechanisms of zinc toxicity in the galaxiid fish, Galaxias maculatus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 179, 184–190. [Google Scholar] [CrossRef]
- Khanam, S. Impact of zinc on thyroid metabolism. J. Diabetes Metab. Disord. Control 2018, 5, 27–28. [Google Scholar] [CrossRef]
- Sinha, S.; Kar, K.; Dasgupta, A.; Basu, S.; Sen, S. Correlation of Serum zinc with TSH in hyperthyroidism. Asian J. Med. Sci. 2015, 7, 66–69. [Google Scholar] [CrossRef]
- Morley, J.E.; Gordon, J.; Hershman, J.M. Zinc deficiency, chronic starvation, and hypothalamic-pituitary-thyroid function. Am. J. Clin. Nutr. 1980, 33, 1767–1770. [Google Scholar] [CrossRef]
- Severo, J.S.; Morais, J.B.S.; de Freitas, T.E.C.; Andrade, A.L.P.; Feitosa, M.M.; Fontenelle, L.C.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. The role of zinc in thyroid hormones metabolism. Int. J. Vitam. Nutr. Res. 2019, 89, 80–88. [Google Scholar] [CrossRef]
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef]
- Manchado, M.; Infante, C.; Asensio, E.; Planas, J.V.; Canavate, J.P. Thyroid hormones down-regulate thyrotropin beta subunit and thyroglobulin during metamorphosis in the flatfish Senegalese sole (Solea senegalensis Kaup). Gen. Comp. Endocrinol. 2008, 155, 447–455. [Google Scholar] [CrossRef]
- Blanco, J.; Mulero, M.; Heredia, L.; Pujol, A.; Domingo, J.L.; Sanchez, D.J. Perinatal exposure to BDE-99 causes learning disorders and decreases serum thyroid hormone levels and BDNF gene expression in hippocampus in rat offspring. Toxicology 2013, 308, 122–128. [Google Scholar] [CrossRef]
- Chen, A.; Kim, S.S.; Chung, E.; Dietrich, K.N. Thyroid hormones in relation to lead, mercury, and cadmium exposure in the National Health and Nutrition Examination Survey, 2007–2008. Environ. Health Perspect. 2013, 121, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Patiño, R. The hypothalamus–pituitary–thyroid axis in teleosts and amphibians: Endocrine disruption and its consequences to natural populations. Gen. Comp. Endocrinol. 2011, 170, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Li, H.B.; Xiang, P.; Zhang, X.; Ma, L.Q. Short-term exposure of arsenite disrupted thyroid endocrine system and altered gene transcription in the HPT axis in zebrafish. Environ. Pollut. 2015, 205, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wu, L.; Zhong, L.; Ru, H.; Yao, F.; Ni, Z.; Li, Y. Thyroid disruption and growth inhibition of zebrafish embryos/larvae by phenanthrene treatment at environmentally relevant concentrations. Aquat. Toxicol. 2022, 243, 106053. [Google Scholar] [CrossRef]
- Wu, L.; Ru, H.; Ni, Z.; Zhang, X.; Xie, H.; Yao, F.; Zhang, H.; Li, Y.; Zhong, L. Comparative thyroid disruption by o,p′-DDT and p,p′-DDE in zebrafish embryos/larvae. Aquat. Toxicol. 2019, 216, 105280. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sfakianakis, D.; Renieri, E.; Kentouri, M.; Tsatsakis, A. Effect of heavy metals on fish larvae deformities: A review. Environ. Res. 2015, 137, 246–255. [Google Scholar] [CrossRef]
- MacKenzie, D.S.; Jones, R.A.; Miller, T.C. Thyrotropin in teleost fish. Gen. Comp. Endocrinol. 2009, 161, 83–89. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Kul, A.; Bediz, C.S.; Ugur, A. Opposite effects of zinc and melatonin on thyroid hormones in rats. Toxicology 2004, 195, 69–75. [Google Scholar] [CrossRef]
- Porazzi, P.; Calebiro, D.; Benato, F.; Tiso, N.; Persani, L. Thyroid gland development and function in the zebrafish model. Mol. Cell. Endocrinol. 2009, 312, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Targovnik, H.M.; Citterio, C.E.; Rivolta, C.M. Iodide handling disorders (NIS, TPO, TG, IYD). Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.T.; Dunn, A.D. Update on intrathyroidal iodine metabolism. Thyroid 2001, 11, 407–414. [Google Scholar] [CrossRef]
- Power, D.M.; Llewellyn, L.; Faustino, M.; Nowell, M.A.; Bjornsson, B.T.; Einarsdottir, I.E.; Canario, A.V.; Sweeney, G.E. Thyroid hormones in growth and development of fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, S.; You, H.; Liu, Z. Effects of ZnO nanoparticles on perfluorooctane sulfonate induced thyroid-disrupting on zebrafish larvae. J. Environ. Sci. (China) 2016, 47, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Yi, S.J.; Chen, P.Y.; Chen, M.; Zhong, W.J.; Yang, J.; Sun, B.B.; Zhu, L.Y. Thyroid endocrine disruption effects of perfluoroalkyl phosphinic acids on zebrafish at early development. Sci. Total Environ. 2019, 676, 290–297. [Google Scholar] [CrossRef]
- Yao, F.; Wu, J.; Ru, H.; Li, Y.; Wu, L.; Ni, Z.; Chen, D.; Zhong, L. Thyroid disruption and developmental toxicity caused by Cd2+ in Schizopygopsis younghusbandi larvae. Comp. Biochem. Physiol., C: Toxicol. Pharmacol. 2020, 235, 108783. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, Y.; Zhang, S.; Song, N.; Xu, H.; Dang, Y.; Liu, C.; Giesy, J.P.; Yu, H. Prenatal transfer of decabromodiphenyl ether (BDE-209) results in disruption of the thyroid system and developmental toxicity in zebrafish offspring. Aquat. Toxicol. 2017, 190, 46–52. [Google Scholar] [CrossRef]
- Li, Z.H.; Chen, L.; Wu, Y.H.; Li, P.; Li, Y.F.; Ni, Z.H. Alteration of thyroid hormone levels and related gene expression in Chinese rare minnow larvae exposed to mercury chloride. Environ. Toxicol. Pharmacol. 2014, 38, 325–331. [Google Scholar] [CrossRef]
- Olin, K.L.; Walter, R.M.; Keen, C.L. Copper deficiency affects selenoglutathione peroxidase and selenodeiodinase activities and antioxidant defense in weanling rats. Am. J. Clin. Nutr. 1994, 59, 654–658. [Google Scholar] [CrossRef]
- Orozco, A.; Valverde, R.C. Thyroid hormone deiodination in fish. Thyroid 2005, 15, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Y.; Ru, H.; Xie, H.; Yao, F.; Ni, Z.; Zhong, L. Parental exposure to 2,2′,4,4′5-pentain polybrominated diphenyl ethers (BDE-99) causes thyroid disruption and developmental toxicity in zebrafish. Toxicol. Appl. Pharmacol. 2019, 372, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Van der Geyten, S.; Byamungu, N.; Reyns, G.E.; Kuhn, E.R.; Darras, V.M. Iodothyronine deiodinases and the control of plasma and tissue thyroid hormone levels in hyperthyroid tilapia (Oreochromis niloticus). J. Endocrinol. 2005, 184, 467–479. [Google Scholar] [CrossRef]
- Dhawan, D.; Singh Baweja, M.; Dani, V. Zinc sulphate following the administration of iodine-131 on the regulation of thyroid function, in rats. Hell J. Nucl. Med. 2007, 10, 167–171. [Google Scholar]
- Chen, Q.; Yu, L.; Yang, L.; Zhou, B. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat. Toxicol. 2012, 110–111, 141–148. [Google Scholar] [CrossRef]
- Wang, X.; Ling, S.; Guan, K.; Luo, X.; Chen, L.; Han, J.; Zhang, W.; Mai, B.; Zhou, B. Bioconcentration, biotransformation, and thyroid endocrine disruption of decabromodiphenyl ethane (Dbdpe), a novel brominated flame retardant, in zebrafish larvae. Environ. Sci. Technol. 2019, 53, 8437–8446. [Google Scholar] [CrossRef] [PubMed]



| Cu2+ (μg/L) | 0 | 1.5 | 15 | 150 |
|---|---|---|---|---|
| Hatching (%) | 89.83 ± 1.29 | 88.25 ± 1.32 | 87.50 ± 2.14 | 85.58 ± 1.87 |
| Malformation (%) | 0.83 ± 0.14 | 1.92 ± 0.38 | 2.25 ± 0.66 | 5.12 ± 0.80 * |
| Survival (%) | 89.25 ± 1.40 | 87.50 ± 1.40 | 87.50 ± 2.4 | 84.83 ± 2.13 |
| Zn2+ (μg/L) | 0 | 20 | 200 | 2000 |
| Hatching (%) | 88.83 ± 1.53 | 87.08 ± 1.23 | 87.00 ± 2.29 | 83.83 ± 1.61 |
| Malformation (%) | 1.08 ± 0.38 | 1.75 ± 0.66 | 3.5 ± 0.75 | 4.67 ± 1.13 * |
| Survival (%) | 88.33 ± 1.53 | 86.25 ± 0.86 | 85.92 ± 2.47 | 83.33 ± 1.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, L.; Zhang, H.; Wu, L.; Ru, H.; Wei, N.; Yao, F.; Ni, Z.; Duan, X.; Li, Y. Copper and Zinc Treatments Alter the Thyroid Endocrine System in Zebrafish Embryos/Larvae. Toxics 2022, 10, 756. https://doi.org/10.3390/toxics10120756
Zhong L, Zhang H, Wu L, Ru H, Wei N, Yao F, Ni Z, Duan X, Li Y. Copper and Zinc Treatments Alter the Thyroid Endocrine System in Zebrafish Embryos/Larvae. Toxics. 2022; 10(12):756. https://doi.org/10.3390/toxics10120756
Chicago/Turabian StyleZhong, Liqiao, He Zhang, Luyin Wu, Huijun Ru, Nian Wei, Fan Yao, Zhaohui Ni, Xinbin Duan, and Yunfeng Li. 2022. "Copper and Zinc Treatments Alter the Thyroid Endocrine System in Zebrafish Embryos/Larvae" Toxics 10, no. 12: 756. https://doi.org/10.3390/toxics10120756
APA StyleZhong, L., Zhang, H., Wu, L., Ru, H., Wei, N., Yao, F., Ni, Z., Duan, X., & Li, Y. (2022). Copper and Zinc Treatments Alter the Thyroid Endocrine System in Zebrafish Embryos/Larvae. Toxics, 10(12), 756. https://doi.org/10.3390/toxics10120756







