Separation of Minor Actinides from High-Level Liquid Waste Using Novel Silica-Based Butyl-BTP Adsorbents
Abstract
1. Introduction
2. Materials and Experiment Methods
2.1. Reagents
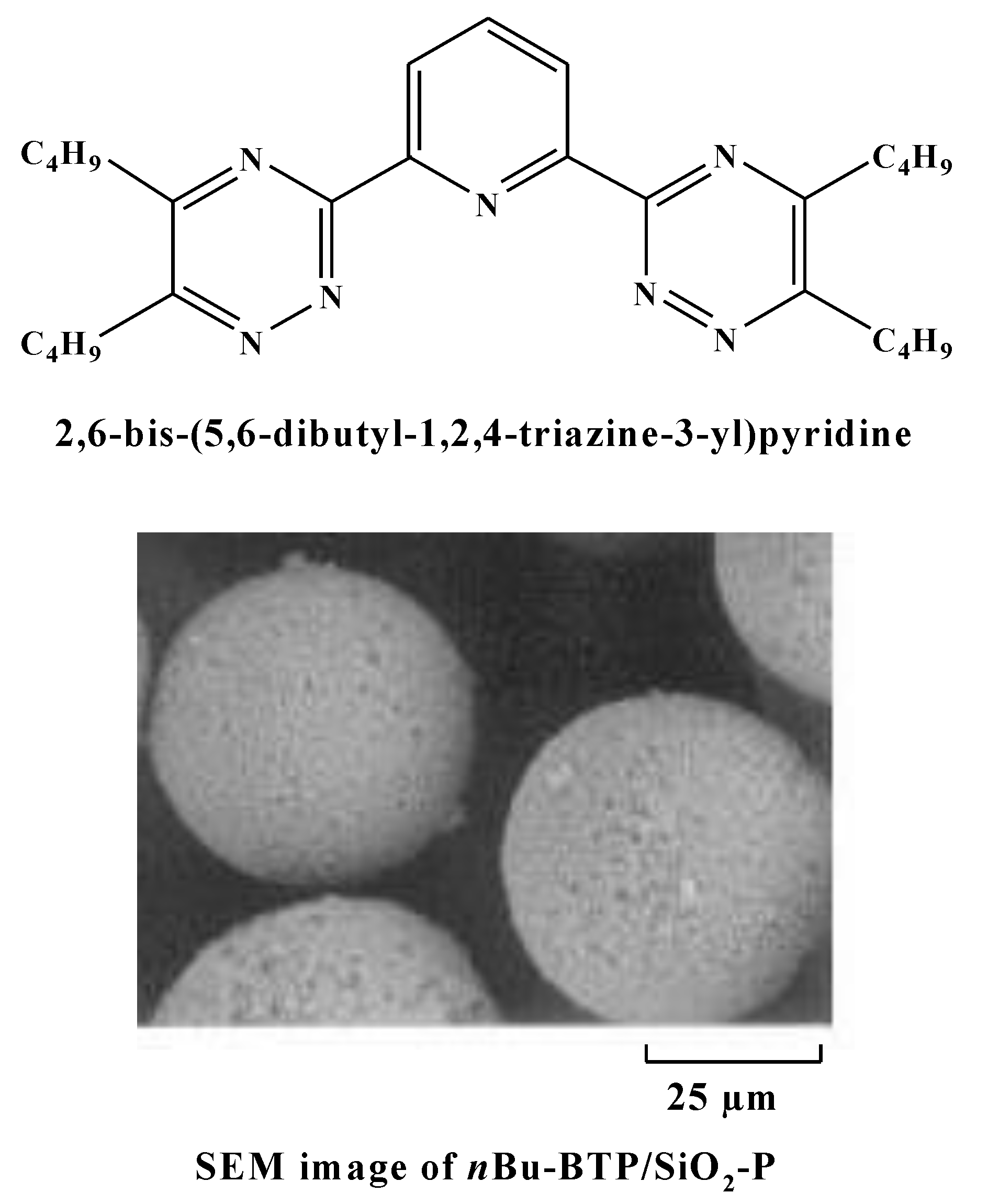
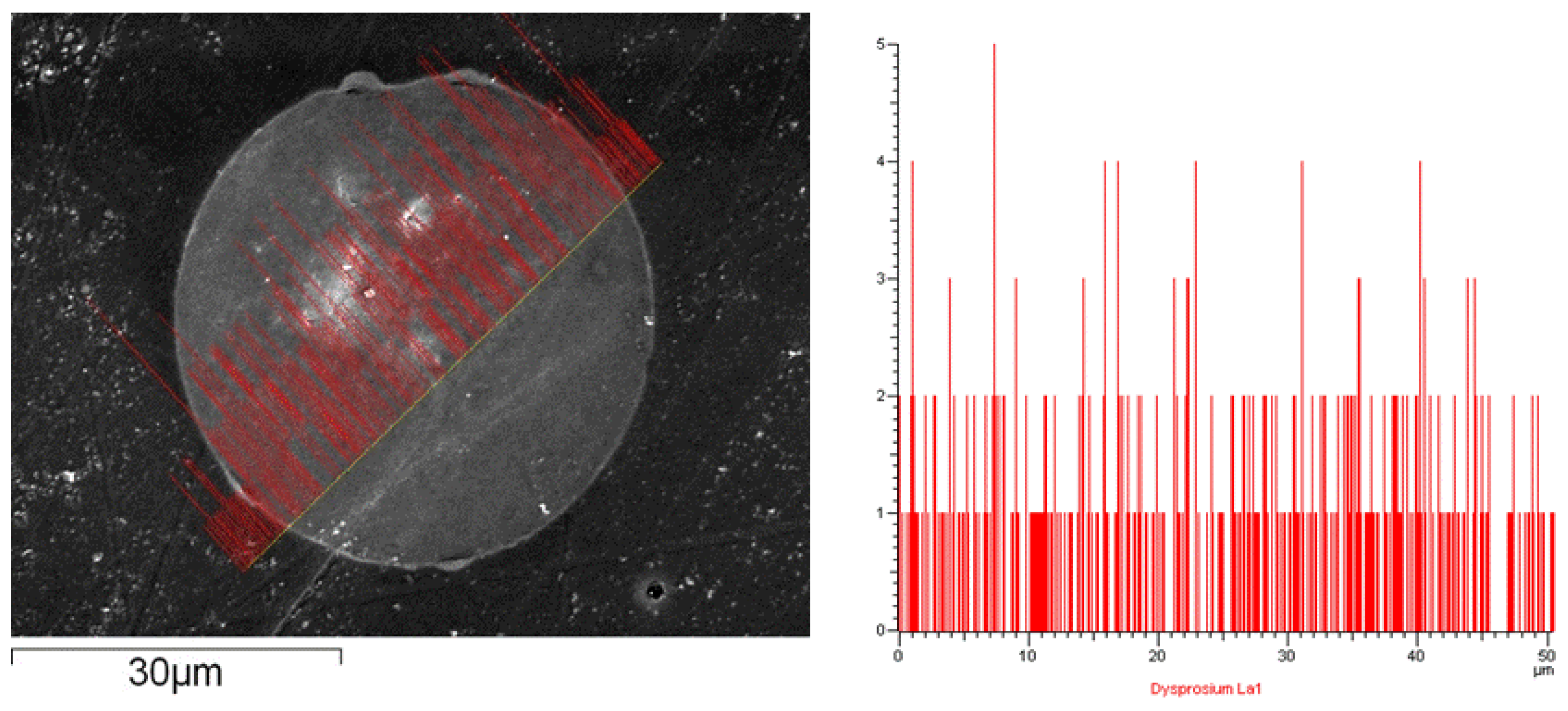
2.2. Synthesis and Characterization of Materials
2.3. Batch Adsorption Experiment
2.4. Hot Column Separation Experiment
3. Results and Discussion
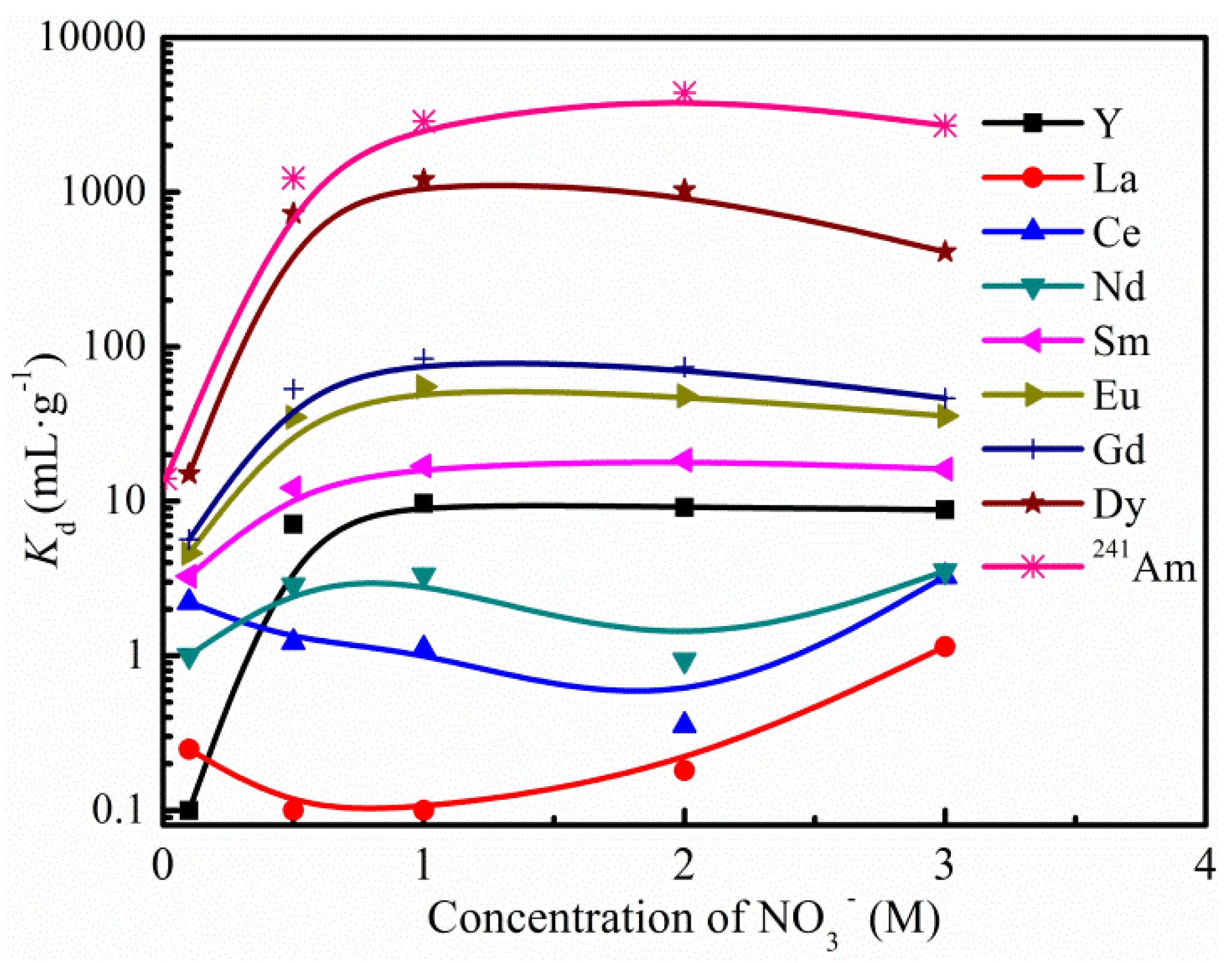
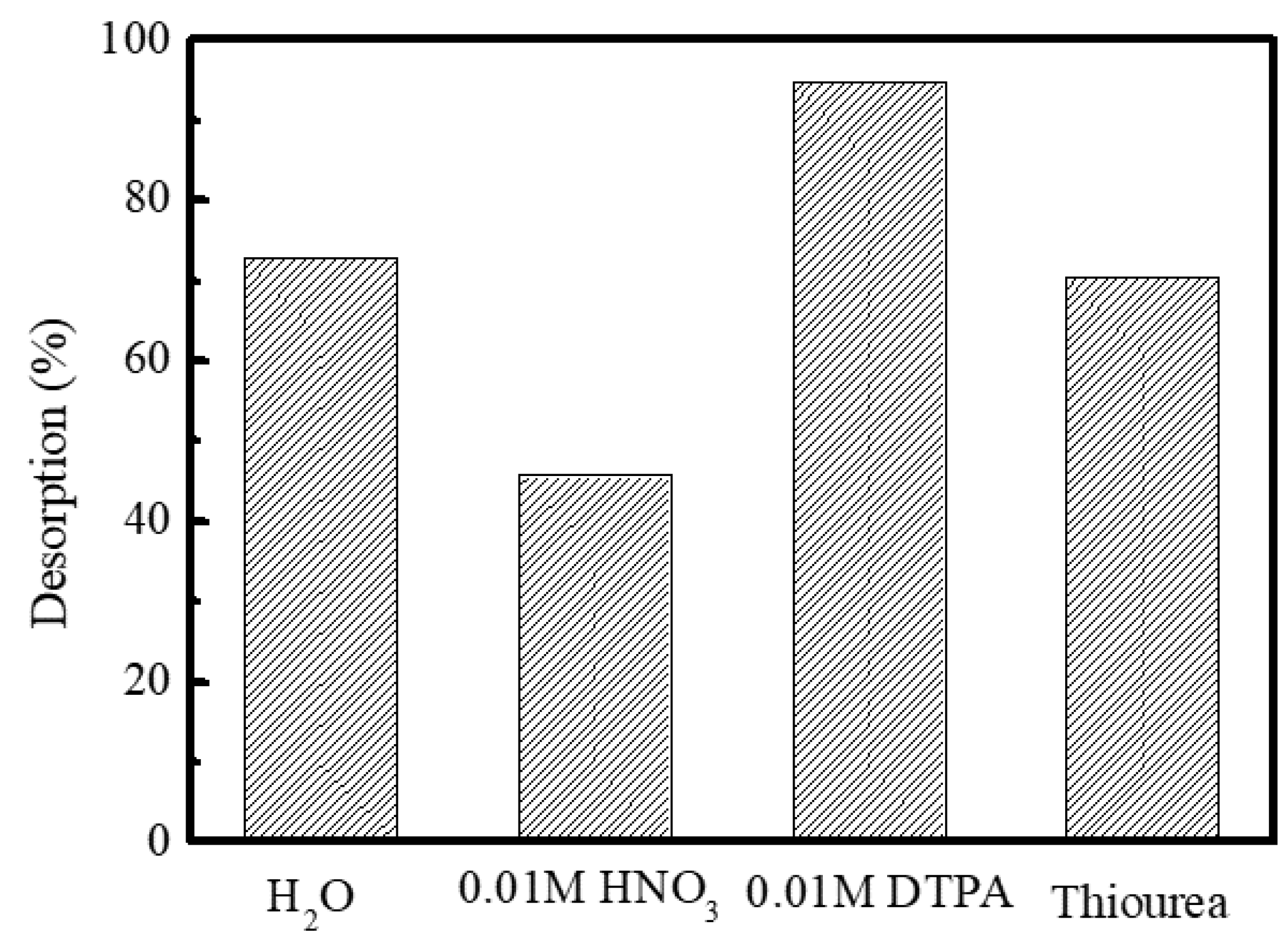
3.1. Adsorption and Desorption Behavior of Am(III) and Ln(III)
3.2. Chromatographic Separation of Am(III) from Ln(III)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; He, X.; Ye, G.; Yi, R.; Chen, J. Americium(III) capture using phosphonic acid-functionalized silicas with different mesoporous morphologies: Adsorption behavior study and mechanism investigation by EXAFS/XPS. Environ. Sci. Technol. 2014, 48, 6874–6881. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.Y.; Zhang, W.; Yu, S.Q.; Zhang, S.C.; Zhou, J.; Wang, X.P.; Wei, Y.Z. Selective separation of MA(III) from Ln(III) by highly stable silica-polymer based N-donor IsoBu-BTP/SiO2-P adsorbent. Solvent Extr. Ion Exch. 2019, 37, 126–139. [Google Scholar] [CrossRef]
- Madic, C. Overview of the hydrometallurgical and pyro-metallurgical processes studied world-wide for the partitioning of high active nuclear wastes. In Proceedings of the NEA/OECD 6th Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation, Madrid, Spain, 11–13 December 2000; pp. 53–64. [Google Scholar]
- Ning, S.Y.; Zhang, S.C.; Zhou, J.; Zhang, W.; Wei, Y.Z. Salt-free separation of 241Am(III) from lanthanides by highly stable macroporous silica-polymer based Me2-CA-BTP/SiO2-P adsorbent. J. Radioanal. Nucl. Chem. 2019, 322, 1023–1030. [Google Scholar] [CrossRef]
- Ning, S.Y.; Zou, Q.; Wang, X.P.; Liu, R.Q.; Wei, Y.-Z. Adsorption mechanism of silica/polymer-based 2,6-bis(5,6-diisohexyl-1,2,4-triazin-3-yl)pyridine adsorbent towards Ln(III) from nitric acid solution. J. Nucl. Sci. Technol. 2016, 53, 1417–1425. [Google Scholar] [CrossRef]
- Kolarik, Z.; Müllich, U.; Gassner, F. Selective extraction of Am(III) over Eu(III) by 2,6-ditriazolyl-and 2,6-ditriazolylpyridines. Solvent Extr. Ion Exch. 1999, 17, 23–32. [Google Scholar] [CrossRef]
- Kolarik, Z.; Müllich, U.; Gassner, F. Extraction of Am(III) and Eu(III) nitrates by 2,6-Di-(5,6-dipropyl-1,2,4-triazine-3-yl). Solvent Extr. Ion Exch. 1999, 17, 1155–1170. [Google Scholar] [CrossRef]
- Wei, Y.-Z.; Kumagai, M.; Takashima, Y.; Modolo, G.; Odoj, R. Studies on the separation of minor actinides from high-level wastes by extraction chromatography using novel silica-based extraction resins. Nucl. Technol. 2000, 132, 413–423. [Google Scholar] [CrossRef]
- Wei, Y.-Z.; Kumagai, M.; Takashima, Y.; Yokoi, H.; Hoshikawa, T.; Kawamura, F. Development of silica-based chelating exchangers and their separation behavior for lanthanides and americium by column chromatography. In Proceedings of the RECOD’98, Nice Acropolis, France, 25–28 October 1998; pp. 701–708. [Google Scholar]
- Zhang, H.; Li, C.M.; Chen, X.J.; Fu, H.; Chen, Y.L.; Ning, S.Y.; Fujita, T.; Wei, Y.Z.; Wang, X.P. Layered ammonium vanadate nanobelt as efficient adsorbents for removal of Sr2+ and Cs+ from contaminated water. J. Colloid Interf. Sci. 2022, 615, 110–123. [Google Scholar] [CrossRef]
- Wei, Y.; Ning, S.; Wang, Q.; Chen, Z.; Wu, Y.; Liu, R.; Mimura, H. Adsorption materials development for the separation of actinides and specific fission products from high level waste. Adv. Sci. Technol. 2014, 94, 103–110. [Google Scholar] [CrossRef]
- Wei, Y.-Z.; Hoshi, H.; Kumagai, M.; Goethals, P.; Bruggeman, A. A hot test on minor actinides separation from high-level-waste by CMPO/SiO2-P extraction resin. In Recent Advances in Actinide Science; Alvares, R., Bryan, N., May, I., Eds.; RSC: London, UK, 2006; pp. 647–649. [Google Scholar]
- Wei, Y.-Z.; Sabharwal, K.N.; Kumagai, M.; Asakura, T.; Uchiyama, G.; Fujine, S. Preparation of novel silica-based nitrogen donor extraction resins and their adsorption performance for trivalent americium and lanthanides. J. Nucl. Sci. Technol. 2000, 37, 1108–1110. [Google Scholar] [CrossRef]
- Wei, Y.-Z.; Hoshi, H.; Kumagai, M.; Asakura, T.; Uchiyama, G. Preparation of novel silica-based R-BTP extraction-resins and their application to trivalent actinides and lanthanides separation. J. Nucl. Sci. Technol. 2002, 39 (Suppl. S3), 761–764. [Google Scholar] [CrossRef]
- Wei, Y.-Z.; Hoshi, H.; Kumagai, M.; Asakura, T.; Morita, Y. Separation of Am(III) and Cm(III) from trivalent lanthanides by 2,6-bistriazinylpyridine extraction chromatography for radioactive waste management. J. Alloys Compd. 2004, 374, 447–450. [Google Scholar] [CrossRef]
- Wei, Y.-Z.; Arai, T.; Hoshi, H.; Kumagai, M.; Bruggeman, A.; Goethals, P. Development of a new aqueous process for nuclear fuel reprocessing: Hot tests on the recovery of U and Pu from a nitric acid solution of spent LWR fuel. Nucl. Technol. 2005, 149, 217–231. [Google Scholar] [CrossRef]
- Benedict, M.; Pigford, T.H.; Levi, H.W. Nuclear Chemical Engineering, 2nd ed.; McGraw-Hill: New York, NY, USA, 1981; Chapter 10. [Google Scholar]
- Geist, A.; Hill, C.; Modolo, G.; Foreman, M.R.S.J.; Weigl, M.; Gompper, K.; Hudson, M.J. 6,6′-Bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo [1,2,4]triazin-3-yl) [2.;2′]bipyridine, an effective extracting agent for the separation of americium(III) and curium(III) from the lanthanides. Solv. Extr. Ion Exch. 2006, 24, 463–482. [Google Scholar] [CrossRef]
- Trumm, S.; Geist, A.; Panak, P.J.; Fanghänel, T. An improved hydrolytically-stable bis-triazinyl-pyridine (BTP) for selective actinide extraction. Solv. Extr. Ion Exch. 2011, 29, 213–229. [Google Scholar] [CrossRef]
- Usuda, S.; Wei, Y.-Z.; Xu, Y.; Li, Z.; Liu, R.; Kim, S.-Y.; Wakui, Y.; Hayashi, H.; Yamazaki, H. Development of a simplified separation process of trivalent minor actinides from fission products using novel R-BTP/SiO2-P adsorbents. J. Nucl. Sci. Technol. 2012, 49, 334–342. [Google Scholar] [CrossRef]


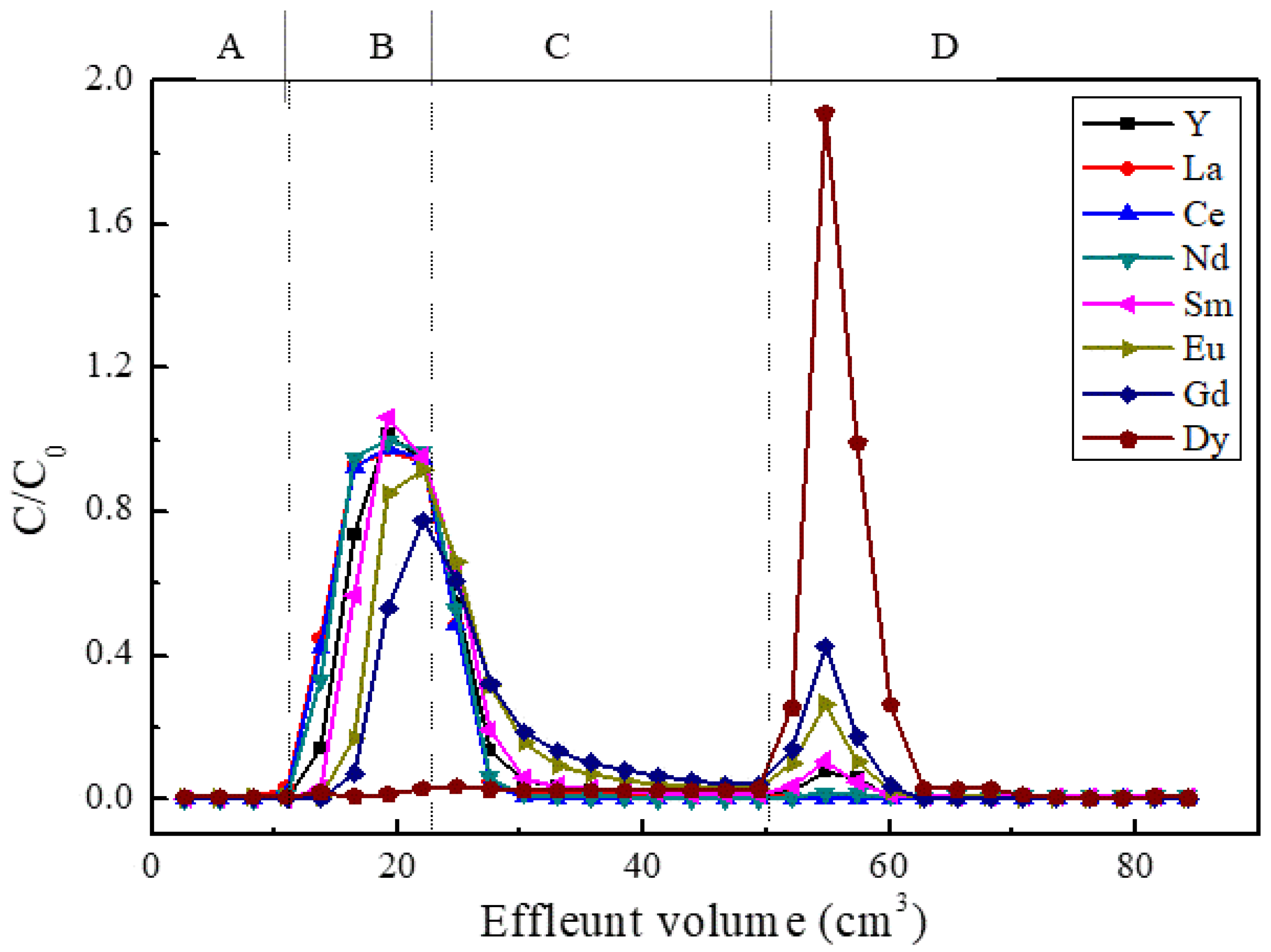
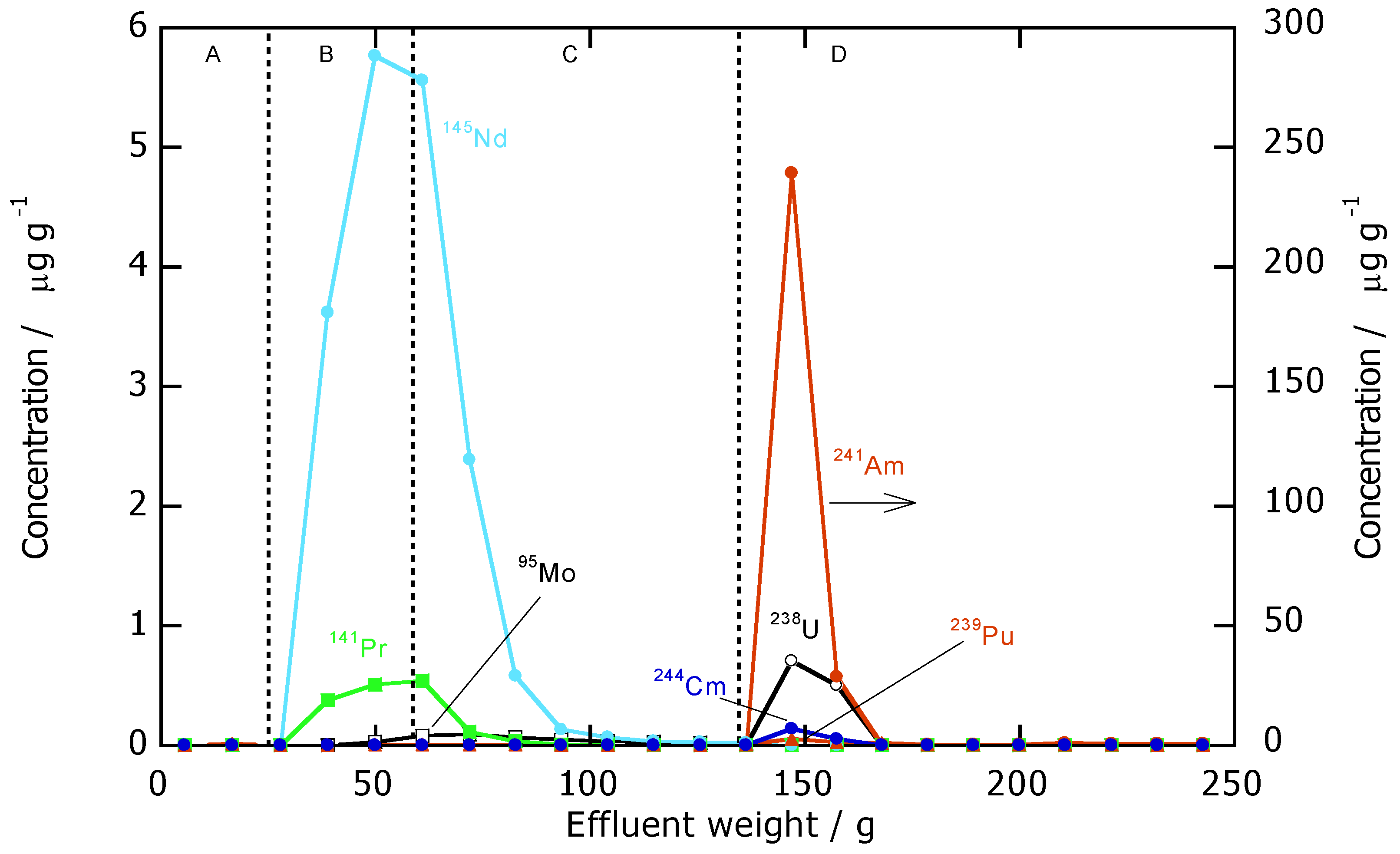
| Nuclide | Concentration | Analysis |
|---|---|---|
| (µg/g-soln) | Method | |
| 95Mo | 0.2 | ICP-MS |
| 141Pr | 0.7 | ICP-MS |
| 145Nd | 10.9 | ICP-MS |
| Nd-total | 62.9 | ICP-MS |
| 238U | 6.7 | ICP-MS |
| 239Pu | 0.2 | ICP-MS |
| 241Am | 84.4 | γ spectrometry |
| 244Cm | 0.1 | α spectrometry |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Ning, S.; Yu, T.; Wang, J.; Wei, Y.; Wu, Y.; He, H.; Chen, F.; Wang, Q. Separation of Minor Actinides from High-Level Liquid Waste Using Novel Silica-Based Butyl-BTP Adsorbents. Toxics 2022, 10, 741. https://doi.org/10.3390/toxics10120741
Jiang T, Ning S, Yu T, Wang J, Wei Y, Wu Y, He H, Chen F, Wang Q. Separation of Minor Actinides from High-Level Liquid Waste Using Novel Silica-Based Butyl-BTP Adsorbents. Toxics. 2022; 10(12):741. https://doi.org/10.3390/toxics10120741
Chicago/Turabian StyleJiang, Tianjiao, Shunyan Ning, Tao Yu, Ji Wang, Yuezhou Wei, Yan Wu, Hui He, Fangqiang Chen, and Qingsong Wang. 2022. "Separation of Minor Actinides from High-Level Liquid Waste Using Novel Silica-Based Butyl-BTP Adsorbents" Toxics 10, no. 12: 741. https://doi.org/10.3390/toxics10120741
APA StyleJiang, T., Ning, S., Yu, T., Wang, J., Wei, Y., Wu, Y., He, H., Chen, F., & Wang, Q. (2022). Separation of Minor Actinides from High-Level Liquid Waste Using Novel Silica-Based Butyl-BTP Adsorbents. Toxics, 10(12), 741. https://doi.org/10.3390/toxics10120741









