Dietary Arthrospira platensis in Rainbow Trout (Oncorhynchus mykiss): A Means to Reduce Threats Caused by CdCl2 Exposure?
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of the Specimens
2.2. Experimental Design and Diet
- Group I, or the control group, was fed on a basal diet without any treatment;
- Group II was fed with a basal diet, and 0.2 mg CdCl2 per kg of commercial fish feed;
- Group III was fed simultaneously with 0.2 mg Kg−1 of CdCl2 and supplemented with 2.5 g A. platensis;
- Group IV was fed simultaneously with 0.2 mg Kg−1 CdCl2 and supplemented with 5 g A. platensis per kg commercial fish feed;
- Group V was fed simultaneously with 0.2 mg Kg−1 CdCl2 and supplemented with 10 g A. platensis per kg commercial fish feed.
2.3. Preparing Samples
2.4. Blood Biochemical Parameter Analyses Performed
2.5. Measurement of Cadmium Bioaccumulation
2.6. Data Analyses
3. Results
3.1. Comments on the Clinical Status
3.2. Serum Biochemical Parameters
3.3. Tissue Antioxidant and Oxidative Stress Markers
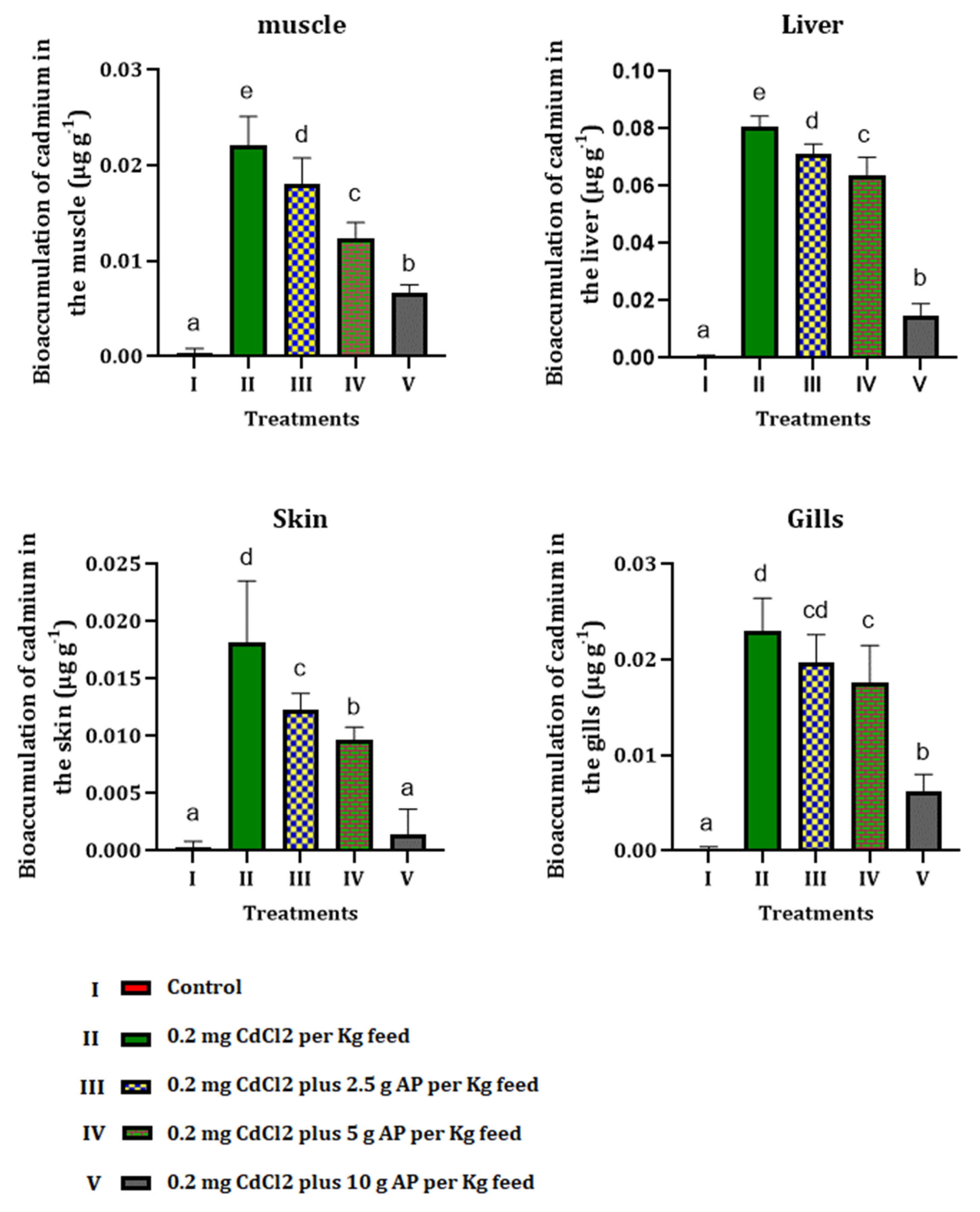
3.4. Bioaccumulation of Cadmium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agriculture Organization of the United Nations. The State of World Fisheries and Aquacuture; Fisheries Department, Agriculture Organization of the United Nations: Rome, Italy, 2000. [Google Scholar]
- Rashidian, G.; Gorji, S.B.; Farsani, M.N.; Prokić, M.D.; Faggio, C. The oak (Quercus brantii) acorn as a growth promotor for rainbow trout (Oncorhynchus mykiss): Growth performance, body composition, liver enzymes activity and blood biochemical parameters. Nat. Prod. Res. 2020, 34, 2413–2423. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, J.; Sehonova, P.; Hodkovicova, N.; Vecerkova, L.; Blahova, J.; Franc, A.; Marsalek, P.; Mares, J.; Tichy, F.; Svobodova, Z.; et al. The effect of foodborne sertraline on rainbow trout (Oncorhynchus mykiss). Sci. Total. Environ. 2020, 708, 135082. [Google Scholar] [CrossRef] [PubMed]
- Jeyavani, J.; Sibiya, A.; Sivakamavalli, J.; Divya, M.; Preetham, E.; Vaseeharan, B.; Faggio, C. Phytotherapy and combined nanoformulations as a promising disease management in aquaculture: A review. Aquac. Int. 2022, 30, 1071–1086. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Porcino, C.; Cerezuela, R.; Cuesta, A.; Faggio, C.; Esteban, M. Impact of date palm fruits extracts and probiotic enriched diet on antioxidant status, innate immune response and immune-related gene expression of European seabass (Dicentrarchus labrax). Fish Shellfish Immunol. 2016, 52, 298–308. [Google Scholar] [CrossRef]
- Tukmechi, A.; Andani, H.R.R.; Manaffar, R.; Sheikhzadeh, N. Dietary administration of beta-mercapto-ethanol treated Saccharomyces cerevisiae enhanced the growth, innate immune response and disease resistance of the rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol. 2011, 30, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, G.; Shahin, K.; Elshopakey, G.E.; Mahboub, H.H.; Fahim, A.; Elabd, H.; Prokić, M.D.; Faggio, C. The Dietary Effects of Nutmeg (Myristica fragrans) Extract on Growth, Hematological Parameters, Immunity, Antioxidant Status, and Disease Resistance of Common Carp (Cyprinus carpio) against Aeromonas hydrophila. J. Mar. Sci. Eng. 2022, 10, 325. [Google Scholar] [CrossRef]
- Rashidian, G.; Boldaji, J.T.; Rainis, S.; Prokić, M.; Faggio, C. Oregano (Origanum vulgare) Extract Enhances Zebrafish (Danio rerio) Growth Performance, Serum and Mucus Innate Immune Responses and Resistance against Aeromonas hydrophila Challenge. Animals 2021, 11, 299. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Alagawany, M.; Faggio, C.; Nowosad, J.; Kucharczyk, D. Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish Shellfish Immunol. 2022, 122, 115–130. [Google Scholar] [CrossRef]
- Rashidian, G.; Kajbaf, K.; Prokić, M.D.; Faggio, C. Extract of common mallow (Malvae sylvestris) enhances growth, immunity, and resistance of rainbow trout (Oncorhynchus mykiss) fingerlings against Yersinia ruckeri infection. Fish Shellfish Immunol. 2020, 96, 254–261. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Shakouri, M.; Yousefi, S.; Van Doan, H.; Shafiei, S.; Yousefi, M.; Mazandarani, M.; Mozanzadeh, M.T.; Tulino, M.G.; Faggio, C. Humoral and skin mucosal immune parameters, intestinal immune related genes expression and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed olive (Olea europea L.) waste. Fish Shellfish Immunol. 2020, 100, 171–178. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Horn, P.; Hancz, C. Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev. Aquac. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Parajó, J. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Marhamati, A.; Rabiee, R.; Faggio, C. Immunomodulation, antioxidant enhancement and immune genes up-regulation in rainbow trout (Oncorhynchus mykiss) fed on seaweeds included diets. Fish Shellfish Immunol. 2020, 106, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Saranraj, P.; Sivasakthi, S. Spirulina platensis—Food For Future: A Review. Asian J. Pharm. Sci. Technol. 2014, 4, 26–33. [Google Scholar]
- Jung, F.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.-H. Spirulina platensis, a super food? J. Cell. Biotechnol. 2019, 5, 43–54. [Google Scholar] [CrossRef]
- Roohani, A.M.; Kenari, A.A.; Kapoorchali, M.F.; Borani, M.S.; Zoriezahra, S.J.; Smiley, A.H.; Esmaeili, M.; Rombenso, A.N. Effect of spirulina Spirulina platensis as a complementary ingredient to reduce dietary fish meal on the growth performance, whole-body composition, fatty acid and amino acid profiles, and pigmentation of Caspian brown trout (Salmo trutta caspius) juveniles. Aquac. Nutr. 2019, 25, 633–645. [Google Scholar] [CrossRef]
- Nandeesha, M.C.; Gangadhar, B.; Varghese, T.J.; Keshavanath, P. Effect of feeding Spirulina platensis on the growth, proximate composition and organoleptic quality of common carp, Cyprinus carpio L. Aquac. Res. 1998, 29, 305–312. [Google Scholar] [CrossRef]
- Kumar, A.; Ramamoorthy, D.; Verma, D.K.; Kumar, A.; Kumar, N.; Kanak, K.R.; Marwein, B.M.; Mohan, K. Antioxidant and phytonutrient activities of Spirulina platensis. Energy Nexus 2022, 6, 100070. [Google Scholar] [CrossRef]
- Mohammadiazarm, H.; Maniat, M.; Ghorbanijezeh, K.; Ghotbeddin, N. Effects of spirulina powder (Spirulina platensis) as a dietary additive on Oscar fish, Astronotus ocellatus: Assessing growth performance, body composition, digestive enzyme activity, immune-biochemical parameters, blood indices and total pigmentation. Aquac. Nutr. 2021, 27, 252–260. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Mousavi, S.; Oushani, A.K.; Firouzamandi, M.; Mardani, K. Spirulina platensis in rainbow trout (Oncorhynchus mykiss) feed: Effects on growth, fillet composition, and tissue antioxidant mechanisms. Aquac. Int. 2019, 27, 1613–1623. [Google Scholar] [CrossRef]
- Zhang, F.; Man, Y.B.; Mo, W.Y.; Wong, M.H. Application of Spirulina in aquaculture: A review on wastewater treatment and fish growth. Rev. Aquac. 2020, 12, 582–599. [Google Scholar] [CrossRef]
- Mahmoud, Y.I.; Shehata, A.M.M.; Fares, N.H.; Mahmoud, A.A. Spirulina inhibits hepatocellular carcinoma through activating p53 and apoptosis and suppressing oxidative stress and angiogenesis. Life Sci. 2021, 265, 118827. [Google Scholar] [CrossRef] [PubMed]
- McRae, N.K.; Gaw, S.; Glover, C.N. Effects of waterborne cadmium on metabolic rate, oxidative stress, and ion regulation in the freshwater fish, inanga (Galaxias maculatus). Aquat. Toxicol. 2018, 194, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Marquez, L.; Espinosa-Araujo, J.; Atencio-Garcia, V.; Olivero-Verbel, J. Effects of cadmium exposure on sperm and larvae of the neotropical fish Prochilodus magdalenae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 225, 108577. [Google Scholar] [CrossRef]
- Vicentini, M.; Fernandes, L.D.S.P.; Marques, A.E.M.L.; Osório, F.H.T.; Baika, L.M.; Risso, W.E.; Martinez, C.B.D.R.; Grassi, M.T.; Fávaro, L.F.; Mela, M.; et al. Effects of cadmium on the female reproductive axis of a Neotropical fish. Chemosphere 2022, 286, 131639. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-C.; Choi, Y.J.; Kim, J.-H. Toxic effects of waterborne cadmium exposure on hematological parameters, oxidative stress, neurotoxicity, and heat shock protein 70 in juvenile olive flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2022, 122, 476–483. [Google Scholar] [CrossRef]
- Pagano, M.; Savoca, S.; Impellitteri, F.; Albano, M.; Capillo, G.; Faggio, C. Toxicological Evaluation of Acetylsalicylic Acid in Non-Target Organisms: Chronic Exposure on Mytilus galloprovincialis (Lamarck, 1819). Front. Physiol. 2022, 13, 1165. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Pagano, M.; Porcino, C.; Briglia, M.; Fiorino, E.; Vazzana, M.; Silvestro, S.; Faggio, C. The Influence of Exposure of Cadmium Chloride and Zinc Chloride on Haemolymph and Digestive Gland Cells from Mytilus galloprovincialis. Int. J. Environ. Res. 2017, 11, 207–216. [Google Scholar] [CrossRef]
- Banni, M.; Chouchene, L.; Saïd, K.; Kerkeni, A.; Messaoudi, I. Mechanisms underlying the protective effect of zinc and selenium against cadmium-induced oxidative stress in zebrafish Danio rerio. BioMetals 2011, 24, 981–992. [Google Scholar] [CrossRef]
- Torre, A.; Trischitta, F.; Faggio, C. Effect of CdCl2 on Regulatory Volume Decrease (RVD) in Mytilus galloprovincialis digestive cells. Toxicol. Vitr. 2013, 27, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Curpan, A.-S.; Impellitteri, F.; Plavan, G.; Ciobica, A.; Faggio, C. Review: Mytilus galloprovincialis: An essential, low-cost model organism for the impact of xenobiotics on oxidative stress and public health. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109302. [Google Scholar] [CrossRef] [PubMed]
- Slobodskova, V.V.; Solodova, E.E.; Slinko, E.N.; Chelomin, V.P. Evaluation of the genotoxicity of cadmium in gill cells of the clam Corbicula japonica using the comet assay. Russ. J. Mar. Biol. 2010, 36, 311–315. [Google Scholar] [CrossRef]
- DeiviArunachalam, K.; Kuruva, J.K.; Pradhoshini, K.P.; Musthafa, M.S.; Faggio, C. Antioxidant and antigenotoxic potential of Morinda tinctoria Roxb. leaf extract succeeding cadmium exposure in Asian catfish, Pangasius sutchi. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 249, 109149. [Google Scholar] [CrossRef] [PubMed]
- Mohiseni, M.; Sepidnameh, M.; Bagheri, D.; Banaee, M.; Haghi, B.N. Comparative effects of Shirazi thyme and vitamin E on some growth and plasma biochemical changes in common carp (Cyprinus carpio) during cadmium exposure. Aquac. Res. 2017, 48, 4811–4821. [Google Scholar] [CrossRef]
- Banaee, M.; Mohammadipour, S.; Madhani, S. Effects of sublethal concentrations of permethrin on bioaccumulation of cadmium in zebra cichlid (Cichlasoma nigrofasciatum). Toxicol. Environ. Chem. 2015, 97, 200–207. [Google Scholar] [CrossRef]
- Shiry, N.; Derakhshesh, N.; Gholamhosseini, A.; Pouladi, M.; Faggio, C. Heavy Metal Concentrations in Cynoglossus arel (Bloch & Schneider, 1801) and Sediment in the Chabahar Bay, Iran. Int. J. Environ. Res. 2021, 15, 773–784. [Google Scholar] [CrossRef]
- Gholamhosseini, A.; Hosseinzadeh, S.; Soltanian, S.; Banaee, M.; Sureda, A.; Rakhshaninejad, M.; Heidari, A.A.; Anbazpour, H. Effect of dietary supplements of Artemisia dracunculus extract on the haemato-immunological and biochemical response, and growth performance of the rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2021, 52, 2097–2109. [Google Scholar] [CrossRef]
- Soleimany, V.; Banaee, M.; Mohiseni, M.; Hagi, B.N.; Dehmourdi, L.M. Evaluation of pre-clinical safety and toxicology of Althaea officinalis extracts as naturopathic medicine for common carp (Cyprinus carpio). Iran. J. Fish. Sci. 2016, 15, 613–629. [Google Scholar] [CrossRef]
- Sacks, D.B. Carbohydrates. In Tietz Textbook of Clinical Chemistry, 3rd ed.; Burtis, C.A., Ashwood, E.R., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 1999; pp. 766–785. [Google Scholar]
- Rifai, N.; Bachorik, P.S.; Albers, J.J. Lipids, lipoproteins and apolipoproteins. In Tietz Textbook of Clinical Chemistry, 3rd ed.; Burtis, C.A., Ashwood, E.R., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 1999; pp. 809–861. [Google Scholar]
- Foster-Swanson, A.; Swartzentruber, M.; Roberts, P. Refrence interval studies of the rate-blancked creatinine, Jaffe method on BM/Hitachi Systems in Six U.S. Laboratories. Clin. Chem. 1994, 40, 361. [Google Scholar]
- Johnson, A.M.; Rohlfs, E.M.; Silverman, L.M. Proteins. In Tietz Textbook of Clinical Chemistry, 3rd ed.; Burtis, C.A., Ashwood, E.R., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 1999; pp. 477–540. [Google Scholar]
- Moss, D.V.; Henderson, A.R. Clinical enzymology. In Tietz Textbook of Clinical Chemistry, 3rd ed.; Burtis, C.A., Ashwood, E.R., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 1999; pp. 617–677. [Google Scholar]
- Chuiko, G.M. Comparative study of acetylcholinesterase and butyrylcholinesterase in brain and serum of several freshwater fish: Specific activities and in vitro inhibition by DDVP, an organophosphorus pesticide. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2000, 127, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 2019, 236, 124335. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1996, 16, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Góth, L. A Simple Method for Determination of Serum Catalase Activity and Revision of Reference Range. Clin. Chim. Acta 1991, 196, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Derikvandy, A.; Pourkhabbaz, H.R.; Banaee, M.; Sureda, A.; Haghi, N. Genotoxicity and oxidative damage in zebrafish (Danio rerio) after exposure to effluent from ethyl alcohol industry. Chemosphere 2020, 251, 126609. [Google Scholar] [CrossRef]
- Capoluongo, E.; Giardina, B.; Minucci, A. Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Stanley Maloy, K.H., Ed.; Elsevier/Academic Press: London, UK, 2013; pp. 340–342. [Google Scholar] [CrossRef]
- Banaee, M.; Sureda, A.; Taheri, S.; Hedayatzadeh, F. Sub-lethal effects of dimethoate alone and in combination with cadmium on biochemical parameters in freshwater snail, Galba truncatula. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 220, 62–70. [Google Scholar] [CrossRef]
- Cicik, B.; Engin, K. The Effects of Cadmium on Levels of Glucose in Serum and Glycogen Reserves in the Liver and Muscle Tissues of Cyprinus carpio (L., 1758). Turk. J. Vet. Anim Sci. 2005, 29, 113–117. [Google Scholar]
- Hussein, S.Y.; Mekkawy, I.A.A. The effect of lead-exposure and lead-clay interaction on the growth performance, biochemical and physiological characteristics and histopathology of Tilapia zillii. Bull. Fac. Sci. Assiut Univ. 2001, 30, 65–97. [Google Scholar]
- Shaikh, Z.A.; Vu, T.T.; Zaman, K. Oxidative Stress as a Mechanism of Chronic Cadmium-Induced Hepatotoxicity and Renal Toxicity and Protection by Antioxidants. Toxicol. Appl. Pharmacol. 1999, 154, 256–263. [Google Scholar] [CrossRef]
- Mekkawy, I.A.A.; Lashein, F.E. The effect of lead and cadmium on LDH and G-6-PDH isozyme patterns exhibited during the early embryonic development of the teleost fish, Ctenopharyngodon idellus with emphasis on the corresponding morphological variations. In Proceedings of the 26th Annual Larval Fish Conference (LFC2002), Bergen, Norway, 22–26 July 2002; pp. 275–292. [Google Scholar]
- Karadeniz, A.; Cemek, M.; Simsek, N. The effects of Panax ginseng and Spirulina platensis on hepatotoxicity induced by cadmium in rats. Ecotoxicol. Environ. Saf. 2009, 72, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Mekkawy, I.A.A.; Mahmoud, U.M.; Wassif, E.T.; Naguib, M. Effects of cadmium on some haematological and biochemical characteristics of Oreochromis niloticus (Linnaeus, 1758) dietary supplemented with tomato paste and vitamin E. Fish Physiol. Biochem. 2010, 37, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Philippe, C.; Grégoir, A.F.; Thoré, E.S.J.; Brendonck, L.; De Boeck, G.; Pinceel, T. Acute sensitivity of the killifish Nothobranchius furzeri to a combination of temperature and reference toxicants (cadmium, chlorpyrifos and 3,4-dichloroaniline). Environ. Sci. Pollut. Res. 2018, 25, 10029–10038. [Google Scholar] [CrossRef]
- Philippe, C.; Hautekiet, P.; Grégoir, A.F.; Thoré, E.S.J.; Pinceel, T.; Stoks, R.; Brendonck, L.; De Boeck, G. Combined effects of cadmium exposure and temperature on the annual killifish (Nothobranchius furzeri). Environ. Toxicol. Chem. 2018, 37, 2361–2371. [Google Scholar] [CrossRef]
- Jyoti, D.; Sinha, R.; Faggio, C. Advances in biological methods for the sequestration of heavy metals from water bodies: A review. Environ. Toxicol. Pharmacol. 2022, 94, 103927. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Emran, A.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology—A review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Ikeda, H.; Haramaki, N.; Yoshida, N.; Imaizumi, T. Oxidative stress is related to exercise intolerance in patients with heart failure. Am. Hear. J. 1998, 135, 115–120. [Google Scholar] [CrossRef]
- Al-Asgah, N.A.; Abdel-Warith, A.-W.A.; Younis, E.-S.M.; Allam, H.Y. Haematological and biochemical parameters and tissue accumulations of cadmium in Oreochromis niloticus exposed to various concentrations of cadmium chloride. Saudi J. Biol. Sci. 2015, 22, 543–550. [Google Scholar] [CrossRef]
- Üner, N.; Oruç, E.; Sevgiler, Y.; Şahin, N.; Durmaz, H.; Usta, D. Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2006, 21, 241–245. [Google Scholar] [CrossRef]
- Heydarnejad, M.S.; Khosravian-Hemamai, M.; Nematollahi, A. Effects of cadmium at sub-lethal concentration on growth and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Ir. Vet. J. 2013, 66, 11. [Google Scholar] [CrossRef]
- Abdelkhalek, N.K.M.; Eissa, I.A.M.; Ahmed, E.; Kilany, O.E.; El-Adl, M.; Dawood, M.A.; Hassan, A.M.; Abdel-Daim, M.M. Protective role of dietary Spirulina platensis against diazinon-induced Oxidative damage in Nile tilapia; Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2017, 54, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Levesque, H.M.; Moon, T.W.; Campbell, P.G.C.; Hontela, A. Seasonal variation in carbohydrate and lipid metabolism of yellow perch (Perca flavescens) chronically exposed to metals in the field. Aquat. Toxicol. 2002, 60, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Wooster, G.A.; Bowser, P.R. Comparative blood chemistry and histopathology of tilapia infected with Vibrio vulnificus or Streptococcus iniae or exposed to carbon tetrachloride, gentamicin, or copper sulfate. Aquaculture 2004, 239, 421–443. [Google Scholar] [CrossRef]
- Shalaby, A.M.E. Effect of EDTA on Toxicity Reduction of Cadmium in Relation to Growth, Some Haematological and Biochemical Profiles of Nile Tilapia (Oreochromis niloticus). J. Fish. Aquat. Sci. 2007, 2, 100–109. [Google Scholar] [CrossRef]
- Banaee, M.; Akhlaghi, M.; Soltanian, S.; Sureda, A.; GholamHosseini, A.; Rakhshaninejad, M. Combined effects of exposure to sub-lethal concentration of the insecticide chlorpyrifos and the herbicide glyphosate on the biochemical changes in the freshwater crayfish Pontastacus leptodactylus. Ecotoxicology 2020, 29, 1500–1515. [Google Scholar] [CrossRef]
- Banaei, M.; Forouzanfar, M.; Jafarinia, M. Toxic effects of polyethylene microplastics on transcriptional changes, biochemical response, and oxidative stress in common carp (Cyprinus carpio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 261, 109423. [Google Scholar] [CrossRef]
- Banaee, M.; Tahery, S.; Haghi, B.N.; Shahafve, S.; Vaziriyan, M. Blood biochemical changes in common carp (Cyprinus carpio) upon co-exposure to titanium dioxide nanoparticles and paraquat. Iran. J. Fish. Sci. 2019, 18, 242–255. [Google Scholar] [CrossRef]
- Hatami, M.; Banaee, M.; Haghi, B.N. Sub-lethal toxicity of chlorpyrifos alone and in combination with polyethylene glycol to common carp (Cyprinus carpio). Chemosphere 2019, 219, 981–988. [Google Scholar] [CrossRef]
- Han, S.-H.; Park, J.-C.; Byun, M.S.; Yi, D.; Lee, J.H.; Lee, D.Y.; Mook-Jung, I. Blood acetylcholinesterase level is a potential biomarker for the early detection of cerebral amyloid deposition in cognitively normal individuals. Neurobiol. Aging 2019, 73, 21–29. [Google Scholar] [CrossRef]
- Perrault, J.R.; Bauman, K.D.; Greenan, T.M.; Blum, P.C.; Henry, M.S.; Walsh, C.J. Maternal transfer and sublethal immune system effects of brevetoxin exposure in nesting loggerhead sea turtles (Caretta caretta) from western Florida. Aquat. Toxicol. 2016, 180, 131–140. [Google Scholar] [CrossRef]
- Atli, G.; Canli, M. Enzymatic responses to metal exposures in a freshwater fish Oreochromis niloticus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.; Qayyum, A.; Sulehria, K.; Tariq, M.; Khadjia, I.; Fiaz, A.; Saeed, M. Effect of sublethal dose of CdCl2 on biochemical profile and catalase activity in fresh water fish Oreochromis niloticus. Environ. Sci. 2012, 58, 73–79. [Google Scholar]
- Cao, L.; Huang, W.; Liu, J.; Yin, X.; Dou, S. Accumulation and oxidative stress biomarkers in Japanese flounder larvae and juveniles under chronic cadmium exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, S.; Priyadarshinee, S.; Bhattacharjee, M.; Kadirvelu, K.; Ramesh, M. Exposure to polystyrene microplastics induced gene modulated biological responses in zebrafish (Danio rerio). Chemosphere 2021, 281, 128592. [Google Scholar] [CrossRef] [PubMed]
- Song, J.A.; Choi, C.Y.; Park, H.-S. Exposure of bay scallop Argopecten irradians to micro-polystyrene: Bioaccumulation and toxicity. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 236, 108801. [Google Scholar] [CrossRef]
- Messaoudi, I.; Barhoumi, S.; Saïd, K.; Kerken, A. Study on the sensitivity to cadmium of marine fish Salaria basilisca (Pisces: Blennidae). J. Environ. Sci. 2009, 21, 1620–1624. [Google Scholar] [CrossRef]
- Ibrahim, A.T.A.; Banaee, M.; Sureda, A. Genotoxicity, oxidative stress, and biochemical biomarkers of exposure to green synthesized cadmium nanoparticles in Oreochromis niloticus (L.). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 242, 108942. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Álvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-term exposure to microplastics induces oxidative stress and a pro-inflammatory response in the gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 2020, 266, 115295. [Google Scholar] [CrossRef]
- Li, Z.; Chang, X.; Hu, M.; Fang, J.K.-H.; Sokolova, I.M.; Huang, W.; Xu, E.G.; Wang, Y. Is microplastic an oxidative stressor? Evidence from a meta-analysis on bivalves. J. Hazard. Mater. 2021, 423, 127211. [Google Scholar] [CrossRef]
- Hamidi, S.; Banaee, M.; Pourkhabbaz, H.R.; Sureda, A.; Khodadoust, S. Effect of petroleum wastewater treated with gravity separation and magnetite nanoparticles adsorption methods on the blood biochemical response of mrigal fish (Cirrhinus cirrhosus). Environ. Sci. Pollut. Res. 2021, 29, 3718–3732. [Google Scholar] [CrossRef]
- Banihashemi, E.A.; Soltanian, S.; Gholamhosseini, A.; Banaee, M. Effect of microplastics on Yersinia ruckeri infection in rainbow trout (Oncorhynchus mykiss). Environ. Sci. Pollut. Res. 2022, 29, 11939–11950. [Google Scholar] [CrossRef] [PubMed]
- Fırat, Ö.; Çogun, H.Y.; Aslanyavrusu, S.; Kargın, F. Antioxidant responses and metal accumulation in tissues of Nile tilapia Oreochromis niloticus under Zn, Cd and Zn + Cd exposures. J. Appl. Toxicol. 2009, 29, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Scully, C. Metabolic disorders. In Scully’s Medical Problems in Dentistry, 7th ed.; Elsevier/Churchill Livingstone: London, UK, 2014; pp. 594–606. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. The Role of Spirulina (Arthrospira) in the Mitigation of Heavy-Metal Toxicity: An Appraisal. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 149–157. [Google Scholar] [CrossRef]

| Crude Protein | Control/ Group I | Group II: 0.2 mg Kg−1 CdCl2 | Group III: 0.2 mg Kg−1 CdCl2 2.5 g Kg−1 AP | Group IV: 0.2 mg Kg−1 CdCl2 5.0 g Kg−1 AP | Group V: 0.2 mg Kg−1 CdCl2 10.0 g Kg−1 AP |
|---|---|---|---|---|---|
| Dry matter % | 90 | 90 | 90.52 | 90.75 | 91.2 |
| Metabolizable energy (Kcal/g) | 2.68 | 2.68 | 2.79 | 2.83 | 2.91 |
| Crude protein % | 40.33 | 40.33 | 41.09 | 41.83 | 42.63 |
| Crude lipid % | 11 | 11 | 11.09 | 11.18 | 11.27 |
| Crude fiber % | 2 | 2 | 2.26 | 2.51 | 2.76 |
| Serum Biochemical Parameters | Control/ Group I | Group II: 0.2 mg Kg−1 CdCl2 | Group III: 0.2 mg Kg−1 CdCl2 + 2.5 g Kg−1 AP | Group IV: 0.2 mg Kg−1 CdCl2 + 5.0 g Kg−1 AP | Group V: 0.2 mg Kg−1 CdCl2 + 10.0 g Kg−1 AP |
|---|---|---|---|---|---|
| Total protein (g dL−1) | 4.4 ± 0.4 b,c | 3.7 ± 0.3 a | 4.0 ± 0.3 a,b | 4.5 ± 0.5 c | 4.6 ± 0.4 c |
| Albumin (g dL−1) | 2.9 ± 0.2 a | 2.9 ± 0.3 a | 2.9 ± 0.4 a | 3.0 ± 0.3 a | 3.0 ± 0.4 a |
| Globulins (g dL−1) | 1.5 ± 0.4 b | 0.8 ± 0.3 a | 1.1 ± 0.6 a | 1.5 ± 0.4 b | 1.6 ± 0.6 b |
| Total immunoglobulins (g dL−1) | 1.4 ± 0.2 b | 0.9 ± 0.3 a | 1.3 ± 0.2 b | 1.2 ± 0.2 b | 1.4 ± 0.2 b |
| Glucose (mg dL−1) | 61.8 ± 9.7 a | 110.1 ± 10.5 c | 99.7 ± 5.7 b | 66.9 ± 11.2 a | 69.1 ± 9.8 a |
| Cholesterol (mg dL−1) | 204.2 ± 14.5 a | 281.6 ± 19.1 b | 237.1 ± 7.9 b | 270.5 ± 9.9 b | 204.9 ± 42.3 a |
| Triglycerides (mg dL−1) | 172.1 ± 29.3 a | 223.9 ± 23.8 b | 226.6 ± 12.2 b | 172.7 ± 27.3 a | 170.8 ± 27.7 a |
| Creatinine (mg dL−1) | 0.5 ± 0.3 a | 1.7 ± 0.2 c | 1.0 ± 0.2 b | 0.4 ± 0.0 a | 0.7 ± 0.1 a |
| Serum Biochemical Parameters | Control/ Group I | Group II: 0.2 mg Kg−1 CdCl2 | Group III: 0.2 mg Kg−1 CdCl2 + 2.5 g Kg−1 AP | Group IV: 0.2 mg Kg−1 CdCl2 + 5.0 g Kg−1 AP | Group V: 0.2 mg Kg−1 CdCl2 + 10.0 g Kg−1 AP |
|---|---|---|---|---|---|
| AST (U·L−1) | 117.8 ± 9.7 a,b | 177.3 ± 60.7 c | 141.1 ± 18.2 b | 141.1 ± 20.5 b | 108.3 ± 5.0 a |
| ALT (U·L−1) | 13.1 ± 1.1 a | 27.3 ± 0.9 c | 19.1 ± 1.0 b | 12.5 ± 0.6 a | 12.9 ± 1.1 a |
| ALP (U·L−1) | 132.2 ± 12.6 a | 302.1 ± 55.8 c | 192.8 ± 42.5 b | 150.5 ± 22.0 a,b | 132.4 ± 17.8 a |
| LDH (U·L−1) | 386.5 ± 21.2 a | 743.8 ± 16.7 d | 580.8 ± 13.3 c | 428.5 ± 17.8 b | 363.9 ± 16.4 a |
| GGT (U·L−1) | 33.6 ± 3.2 a,b | 47.1 ± 7.9 c | 39.1 ± 5.5 b | 26.4 ± 5.1 a | 29.4 ± 3.7 a |
| BchE (U·L−1) | 1158.9 ± 45.2 d | 586.5 ± 91.3 a | 788.5 ± 52.6 b | 971.3 ± 45.5 c | 1023.6 ± 72.5 c |
| CPK (U·L−1) | 567.0 ± 89.3 a | 774.2 ± 152.8 b | 657.3 ± 119.7 a | 561.6 ± 64.2 a | 572.2 ± 64 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaee, M.; Impellitteri, F.; Evaz-Zadeh Samani, H.; Piccione, G.; Faggio, C. Dietary Arthrospira platensis in Rainbow Trout (Oncorhynchus mykiss): A Means to Reduce Threats Caused by CdCl2 Exposure? Toxics 2022, 10, 731. https://doi.org/10.3390/toxics10120731
Banaee M, Impellitteri F, Evaz-Zadeh Samani H, Piccione G, Faggio C. Dietary Arthrospira platensis in Rainbow Trout (Oncorhynchus mykiss): A Means to Reduce Threats Caused by CdCl2 Exposure? Toxics. 2022; 10(12):731. https://doi.org/10.3390/toxics10120731
Chicago/Turabian StyleBanaee, Mahdi, Federica Impellitteri, Hamid Evaz-Zadeh Samani, Giuseppe Piccione, and Caterina Faggio. 2022. "Dietary Arthrospira platensis in Rainbow Trout (Oncorhynchus mykiss): A Means to Reduce Threats Caused by CdCl2 Exposure?" Toxics 10, no. 12: 731. https://doi.org/10.3390/toxics10120731
APA StyleBanaee, M., Impellitteri, F., Evaz-Zadeh Samani, H., Piccione, G., & Faggio, C. (2022). Dietary Arthrospira platensis in Rainbow Trout (Oncorhynchus mykiss): A Means to Reduce Threats Caused by CdCl2 Exposure? Toxics, 10(12), 731. https://doi.org/10.3390/toxics10120731