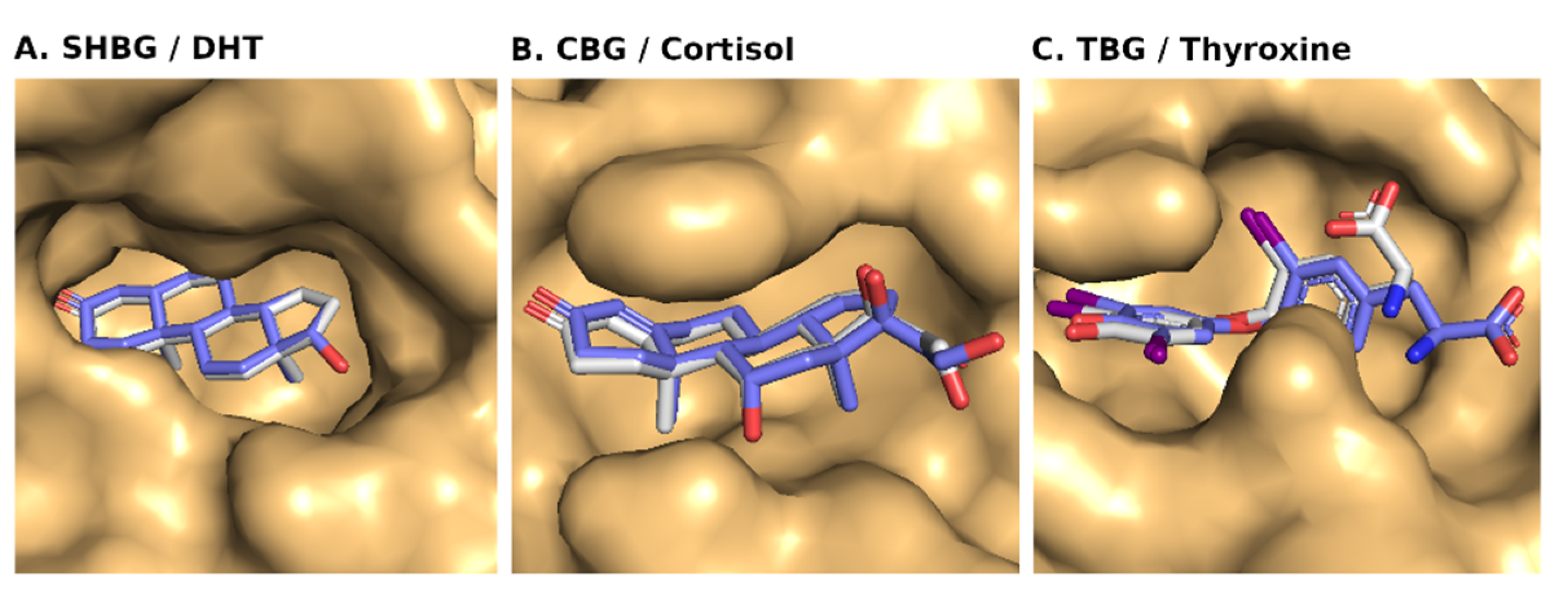
3.2. Molecular Docking of Nicotine, Cotinine, Trans-3′-Hydroxycotinine, and 5′-Hydroxycotinine with SHBG
All the tobacco ligands bound well within the binding site of SHBG (
Figure 3). The absolute values of the binding-strength scores, viz., dock score, binding energy, and dissociation constant were high and comparable among the ligands indicating a tight binding and similar binding strength to the carrier protein (
Table 2).
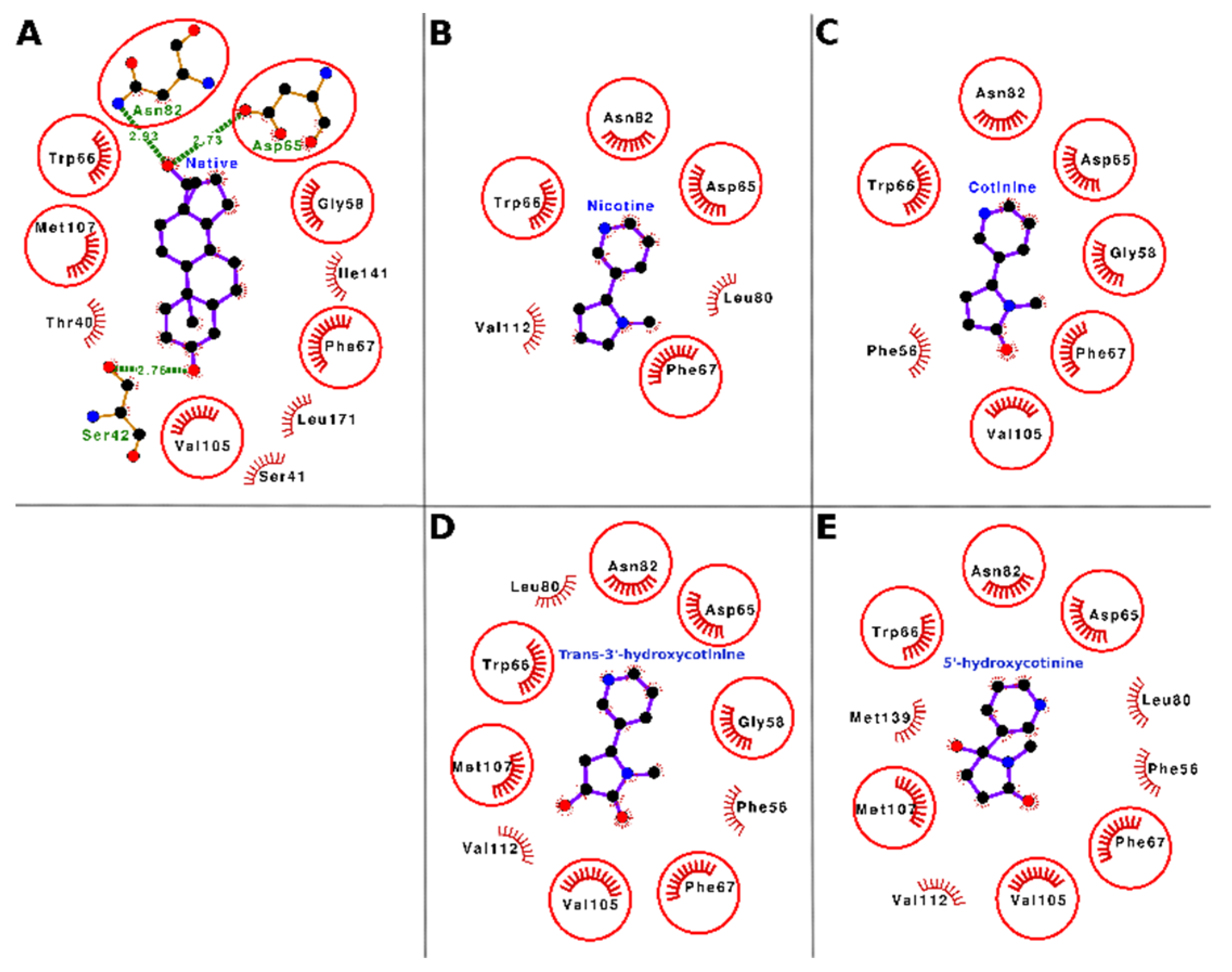
The docking interactions of tobacco ligands nicotine, cotinine, trans-3′-hydroxycotinine, and 5′-hydroxycotinine, and the native ligand (dihydrotestosterone) with SHBG are presented in
Figure 4 (Panels A–E) and
Table 3. Nicotine bound deep in the ligand-binding cavity and interacted with six residues of SHBG, i.e., Asp-65, Trp-66, Phe-67, Leu-80, Asn-82, and Val-112. These interacting residues formed 23 nonbonded contacts with nicotine and stabilized the nicotine–SHBG complex (
Figure 4, Panel B;
Table 3). Of these interacting residues, Phe-67 was proposed to be the most important residue as it was involved in the maximum number of nonbonded contacts (10) and showed the maximum loss in ASA (24.83 Å
2) due to binding. When the binding of nicotine was compared with that of the SHBG native ligand (dihydrotestosterone), four of six interacting residues were common between nicotine and the native ligand (
Figure 4, Panel A and B;
Supplementary Table S1). This indicated that the nicotine competed for the binding with the same residues to which the native ligand was bound. Thus, nicotine may potentially interfere with the binding of gonadal-steroid hormones (androgens and estrogens) to SHBG and cause a disturbance of their transport and homeostasis.
Cotinine sat well in the ligand binding cavity and interacted with seven residues of SHBG, i.e., Phe-56, Gly-58, Asp-65, Trp-66, Phe-67, Asn-82, and Val-105. These interacting residues formed 21 nonbonded contacts and thus stabilized the protein–ligand complex (
Figure 4, Panel C;
Table 3). Of these seven interacting residues, Phe-67 was proposed as the most important interacting residue as it stood out by forming the maximum number of nonbonded contacts (seven) and showing the maximum loss in ASA (24.83 Å
2) due to binding. The comparison of cotinine binding interactions with SHBG to those of the native ligand (dihydrotestosterone) interactions showed that six of seven interacting residues were common between cotinine and the native ligand (
Figure 4A,C,
Supplementary Table S1). On a preliminary basis, this indicated that cotinine also competed for the binding with the same set of residues that the native ligand bound to and thus, like nicotine, may interfere with the binding of the gonadal steroid hormones (androgens and estrogens) to SHBG resulting in disturbance of their transport and homeostasis.
Trans-3′-hydroxycotinine bound well in the ligand binding cavity of SHBG and interacted with ten amino acid residues, namely Phe-56, Gly-58, Asp-65, Trp-66, Phe-67, Leu-80, Asn-82, Val-105, Met-107, and Val-112. These residues formed 33 nonbonded contacts holding the ligand within the binding site (
Figure 4, Panel D;
Table 3). The Phe-67 residue stood out with the maximum number of nonbonded contacts (11) and the maximum ΔASA (24.83 Å
2) and thus was proposed as the most important residue required for trans-3′-hydroxycotinine binding to SHBG. When the binding of trans-3′-hydroxycotinine with SHBG was compared with that of the native ligand (dihydrotestosterone), seven of ten amino acid residues were common between the two ligands (
Figure 4, Panel A and D,
Supplementary Table S1). Thus, the data indicated that trans-3′-hydroxycotinine also bound to the same set of residues to which the native ligand bound. Hence, trans-3′-hydroxycotinine may potentially interfere with the binding of the gonadal steroid hormones (androgens and estrogens) to SHBG leading to a disruption of their transport and homeostasis.
The molecular docking results showed that 5′-hydroxycotinine sat well in the cavity and interacted with 10 residues, i.e., Phe-56, Asp-65, Trp-66, Phe-67, Leu-80, Asn-82, Val-105, Met-107, Val-112, and Met-139 forming 27 nonbonded contacts holding the ligand within the binding site (
Figure 4, Panel E;
Table 3). Among the interacting residues, Phe-67 stood out with the maximum number of nonbonded contacts (11) and the second maximum ΔASA (24.83 Å
2) due to binding. The maximum ΔASA (29.38 Å
2) due to binding was shown by Met-139. The comparison of 5′-hydroxycotinine binding with that of the native ligand (dihydrotestosterone) showed that six interacting residues (of a total of ten) were common between both ligands (
Figure 4, Panel A and E,
Supplementary Table S1). Again, these data suggested that 5′-hydroxycotinine also competed for binding to the same site involving the same set of interacting residues as those for the native ligand. Thus, on a preliminary basis, 5′-hydroxycotinine may potentially interfere with the binding of the gonadal steroid hormones (androgens and estrogens) to SHBG leading to a disruption of their transport and homeostasis.
3.3. Molecular Docking of Nicotine, Cotinine, Trans-3′-Hydroxycotinine, and 5′-Hydroxycotinine with CBG
The molecular docking results of nicotine and its important metabolites into the ligand-binding site of CBG showed that all the indicated ligands bound well within the binding site (
Figure 5). The absolute values of the binding-strength scores, viz., dock score, binding energy, and dissociation constant were reasonably high and comparable among ligands indicating a high and similar stability of the protein–ligand complexes (
Table 4).
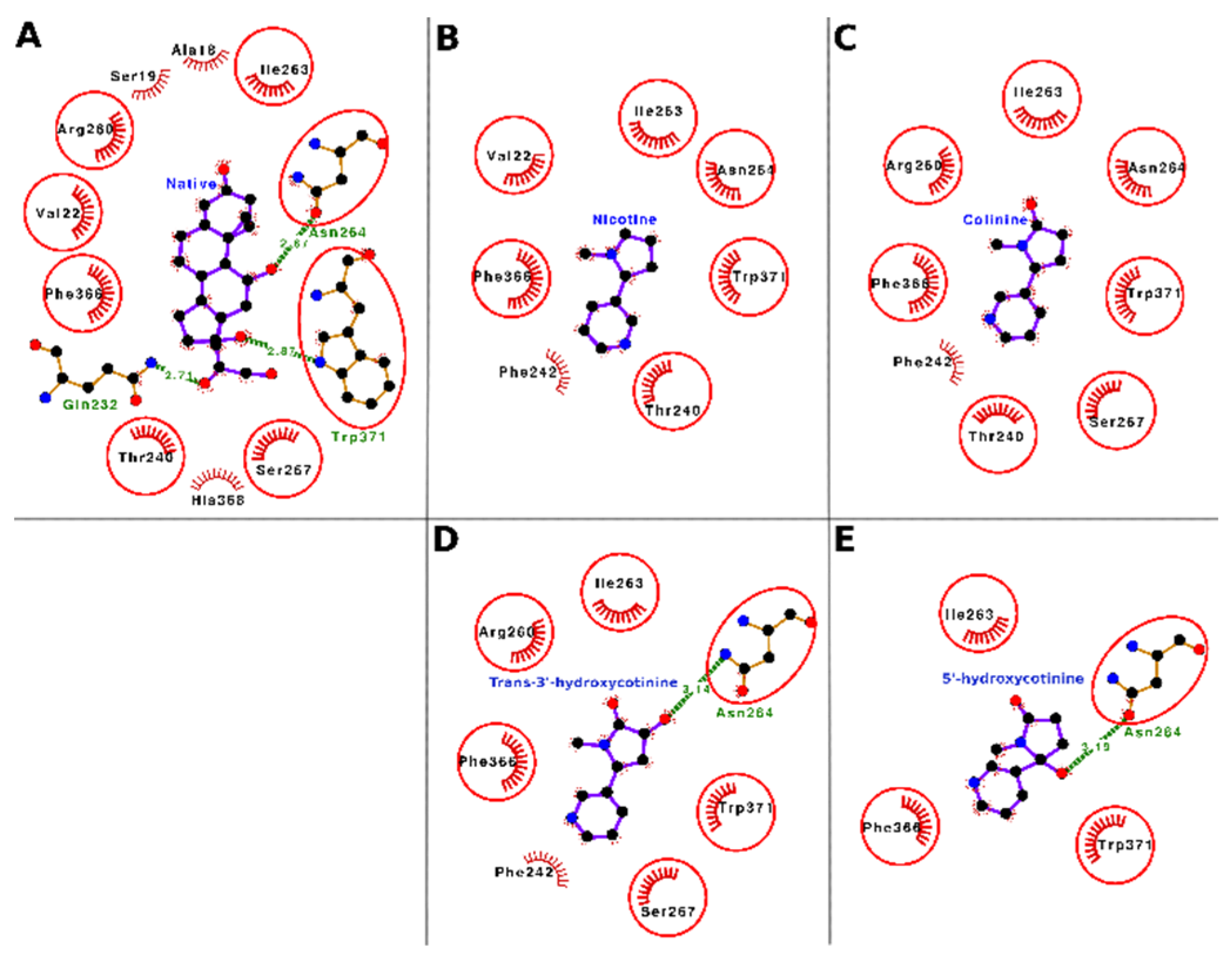
The docking interactions of tobacco ligands nicotine, cotinine, trans-3′-hydroxycotinine, and 5′-hydroxycotinine, and native ligand (cortisol) with CBG are presented in
Figure 6 (Panels A–E) and
Table 5. Nicotine bound deep in the ligand-binding cavity of CBG and interacted with seven residues, i.e., Val-22, Thr-240, Phe-242, Ile-263, Asn-264, Phe-366, and Trp-371. These seven interacting residues formed 14 nonbonded contacts and stabilized the protein–ligand complex (
Figure 6, Panel B;
Table 5). Of the seven interacting residues, Trp-371 showed the maximum loss in ASA (43.56 Å
2) due to binding and was also involved in three nonbonded contacts. When the binding of nicotine was compared with that of the CBG native ligand (cortisol), six of seven interacting residues were common between both ligands (
Figure 6, Panel A and B;
Supplementary Table S2). These data indicated that nicotine was binding to the same location in the CBG binding site and interacting with similar residues as those for the native ligand. Thus, on a preliminary basis, nicotine may potentially interfere with the binding of cortisol and other hormones such as progesterone to CBG resulting in a disturbance of their transport and homeostasis.
Cotinine also bound well within the ligand-binding cavity of CBG and interacted with eight interacting residues, i.e., Thr-240, Phe-242, Arg-260, Ile-263, Asn-264, Ser-267, Phe-366, and Trp-371. These eight residues formed 25 nonbonded contacts and thus stabilized the protein–ligand complex (
Figure 6, Panel C;
Table 5). Among the interacting residues, the Trp-371 stood out with the maximum number of nonbonded contacts (nine) and the maximum ΔASA (45.77 Å
2) and was proposed as the most important interacting residue for cotinine binding. The comparison of cotinine binding interactions with CBG to those of the native ligand, cortisol, showed that seven of eight residues were common between the two ligands (
Figure 6, Panel A and C;
Supplementary Table S2). This finding suggested that cotinine also bound to the same set of residues in the ligand-binding cavity of CBG to which the native ligand bound. Thus, similar to nicotine, cotinine has the potential to interfere with the binding of cortisol and other hormones such as progesterone to CBG and cause a disturbance of their transport and homeostasis.
Trans-3′-hydroxycotinine sat well within the ligand-binding cavity of CBG and interacted with seven residues, i.e., Phe-242, Arg-260, Ile-263, Asn-264, Ser-267, Phe-366, and Trp-371 forming 22 nonbonded contacts and a hydrogen bond (
Figure 6, Panel D;
Table 5). These interactive forces stabilized the protein–ligand complex holding the ligand within the binding site. Among the interacting residues, Trp-371 stood out with the maximum ΔASA (48.85 Å
2) and the maximum number of nonbonded contacts (five) and was proposed as the important interacting residue playing a role in binding. Another residue, Asn-264, formed a hydrogen bond and two nonbonded contacts. When the binding of trans-3′-hydroxycotinine with CBG was compared with that of the native ligand (cortisol), six of seven interacting amino acid residues for trans-3′-hydroxycotinine were common between the two ligands (
Figure 6, Panel A and D,
Supplementary Table S2). This suggested that trans-3′-hydroxycotinine competed with the residues of CBG required for cortisol binding. Thus, trans-3′-hydroxycotinine may potentially interfere with the binding of the adrenal steroid hormones (cortisol, etc.) and progesterone to CBG leading to a disruption of their transport and homeostasis.
The molecular docking results showed that 5′-hydroxycotinine bound well within the ligand-binding cavity of CBG and interacted with four residues, i.e., Ile-263, Asn-264, Phe-366, and Trp-371. These four interacting residues formed 26 nonbonded contacts and a hydrogen bond making the protein–ligand complex stable (
Figure 6, Panel E;
Table 5). The hydrogen bond was contributed to by the residue Asn-264, which was also involved in two nonbonded contacts. Among the interacting residues, Trp-371 played an important role in binding as it formed the maximum number of nonbonded contacts (18) and showed the maximum ΔASA (66.17 Å
2). The comparison of 5′-hydroxycotinine binding with CBG to that of the native ligand (cortisol) showed that all four interacting residues were common with the interacting residues for cortisol (
Figure 6, Panel A and E;
Supplementary Table S2). These results suggested that 5′-hydroxycotinine also competed for binding to the same site involving the same set of interacting residues as the native ligand. Thus, on a preliminary basis, 5′-hydroxycotinine may potentially interfere with the binding of the adrenal steroid hormones (cortisol, etc.) and progesterone to CBG leading to a disruption of their transport and homeostasis.
3.4. Molecular Docking of Nicotine, Cotinine, Trans-3′-Hydroxycotinine, and 5′-Hydroxycotinine with TBG
All the tobacco ligands bound well within the ligand-binding site of TBG (
Figure 7). Further, a high and similar stability of the protein–ligand complexes was evident from the high and comparable binding strength scores, viz., dock score, binding energy, and dissociation constant, among the ligands against the carrier protein, TBG (
Table 6).
The docking interactions of tobacco ligands nicotine, cotinine, trans-3′-hydroxycotinine, and 5′-hydroxycotinine, and the native ligand (thyroxine) with TBG are presented in
Figure 8 (Panels A–E) and
Table 7. The molecular docking results showed that nicotine bound well within the ligand-binding cavity and formed molecular interactions of 13 nonbonded contacts and a hydrogen bonding using four interacting residues including Leu-269, Lys-270, Asn-273, and Leu-376 (
Figure 8, Panel B;
Table 7). These residues through their molecular interactions stabilized the protein–ligand complex. Of the interacting residues, Asn-273 showed maximum ΔASA (66.17 Å
2) and two nonbonded contacts. While another residue Leu-269 showed the maximum number of nonbonded contacts (seven) and the second maximum ΔASA (29.65 Å
2). The hydrogen bond was contributed to by Lys-270, which also formed a nonbonded contact. The comparison of the binding modes of nicotine and the native ligand (thyroxine) showed that all four interacting residues were common between the two ligands (
Figure 8, Panel A and B;
Supplementary Table S3). This suggested that nicotine competed for binding with the same residues in the TBG ligand-binding site with which the native ligand, thyroxine, bound. Thus, nicotine may potentially interfere with the binding of thyroid hormones such as thyroxine and triiodothyronine to TBG resulting in a dysfunction of their transport and homeostasis.
Cotinine also bound well within the ligand-binding cavity of TBG and interacted with five residues Ser-23, Leu-269, Lys-270, Leu-376, and Arg-381. These five interacting residues formed 24 nonbonded contacts and a hydrogen bond stabilizing the protein–ligand complex (
Figure 8, Panel C;
Table 7). Of the interacting residue, Ser-23 contributed a hydrogen bond and two nonbonded contacts. Amino acid residue Arg-381 showed the maximum number of nonbonded contacts (14) and the maximum ΔASA (64.05 Å
2) and was proposed as the important interacting residue playing a role in binding. The comparison of the binding modes of cotinine and the native ligand (thyroxine) showed that four of five interacting residues were common between the two ligands (
Figure 8, Panel A and C,
Supplementary Table S3). On a preliminary basis, these results indicated that cotinine also competed for binding with the same set of residues that the native ligand bound to. Thus, like nicotine, cotinine may interfere with the binding of the thyroid hormones (thyroxine and triiodothyronine) to TBG and cause a disturbance of their transport and homeostasis.
The molecular docking results showed that trans-3′-hydroxycotinine also bound well within the ligand-binding cavity of TBG and interacted with seven residues, i.e., Leu-246, Leu-269, Asn-273, Leu-276, Leu-376, Glu-377, and Arg-381. These seven interacting residues contributed 15 nonbonded contacts providing stability to the protein–ligand complex (
Figure 8, Panel D;
Table 7). Of the interacting residues, Arg-381 showed the maximum number of nonbonded contacts (five) and the maximum ΔASA (44.89 Å
2), and Asn-273 also showed nonbonded contacts (four) and the second maximum ΔASA (40.32 Å
2). Both these residues were proposed as important interacting residues required for trans-3′-hydroxycotinine binding. The comparison of the binding modes of trans-3′-hydroxycotinine and the native ligand, thyroxine, showed that five of seven interacting residues were common between the two ligands (
Figure 8, Panel A and D;
Supplementary Table S3). This suggested that the binding of trans-3′-hydroxycotinine resulted in occupying the same subsite within the ligand-binding site of TBG which may have the potential to interfere with the binding of the native ligands (thyroxine and triiodothyronine) to the carrier protein. Thus, trans-3′-hydroxycotinine may lead to a disruption of thyroid hormone transport and homeostasis.
The molecular docking results showed that 5′-hydroxycotinine also bound deep in the ligand-binding cavity of TBG and interacted with four residues, i.e., Asn-273, Leu-276, Leu-376, and Arg-381. These four interacting residues formed 14 nonbonded contacts and a hydrogen bond which stabilized the protein–ligand complex (
Figure 8, Panel E;
Table 7). The hydrogen bond was contributed to by Asn-273 which also formed five nonbonded contacts and showed the second maximum ΔASA (41.78 Å
2), while another important residue Arg-381 contributed the maximum number of nonbonded contacts (six) and showed the maximum ΔASA (46.26 Å
2). Both residues Asn-273 and Arg-381 were proposed as important interacting residues. A comparison of the binding modes of 5′-hydroxycotinine and the native ligand (thyroxine) showed that three of four interacting residues were common among the interacting residues between both ligands (
Figure 8, Panel A and E;
Supplementary Table S3). These results suggested that 5′-hydroxycotinine competed for binding to the same site of TBG and involving the same set of interacting residues as those for the native ligand, thyroxine. Thus, 5′-hydroxycotinine has the potential to exert interference in the binding of the thyroid hormones, thyroxine and triiodothyronine, to TBG leading to a disruption of their transport and homeostasis.
3.5. Hormone Relationships, Relevance, and Conclusions
Previous studies involving nicotine and/or its metabolites on structural binding interactions against hormone carrier proteins such as SHBG, CBG, and TBG are not available to the best of our knowledge. However, recent structural binding and previous in vitro competitive binding studies have shown inhibitory interactions of nicotine and/or cotinine against steroid nuclear receptors [
40,
50,
51,
52,
53]. Nicotine is a powerful and addictive toxicant and has been reported to disturb the reproductive hormone levels including the SHBG levels in the circulation [
54,
55]. In vitro binding studies on nicotine and/or its metabolites to SHBG have also not been reported. Our results from this study on the structural binding of nicotine and its important metabolites against SHBG showed that the indicated tobacco ligands had the potential to interfere in the binding of endogenous native ligands such as testosterone and estradiol with SHBG. Hence, this may result in a dysfunction of their transport, homeostasis, and availability at the target tissues. The functional relevance of these nicotine effects has been shown in several epidemiological studies on fertility problems in both men and women due to smoking/nicotine [
56]. Briefly, in women, the adverse effects are shorter cycles, delayed conception and reduced fecundity [
57,
58], an accelerated loss of reproductive function and early menopause [
59,
60], an increased risk of ectopic pregnancy, miscarriage, abortion, a reduced fecundity due to mutagenesis of gametes [
61], and decreased success of ART and IVF [
62]. Nicotine use has been associated with lower estrogen concentrations in women and higher concentrations of androgens in men and women [
63,
64]. Smoking was also associated with higher SHBG levels in postmenopausal women [
65]. Gender differences for the effects of nicotine on SHBG are not available; however, important gender differences for nicotine have been observed with women being more prone to addiction to nicotine than men; women even take less time to develop a dependence after initial use and maintain the addiction at lower nicotine intake levels [
66]. Progesterone has been shown to have protective effects during the initiation and maintenance of nicotine addiction. Nicotine induces a suppression of estrogens which is either due to an increased metabolism of estrogens in the liver and/or an inhibition of the aromatase enzyme, which reduces the aromatization of androgens to estrogens in tissues; the inhibition of aromatase due to nicotine has been shown in baboons [
53]. In men, nicotine and cotinine have been detected in the seminal fluid [
67] and tobacco/nicotine use was associated with testosterone imbalance, a poor quality of semen, and erectile dysfunction [
68,
69]. These studies have also been supported by experimental studies in laboratory animals [
70]. Further, gestational nicotine exposure was associated with testicular and ovarian dysplasia and decreased testosterone and estradiol levels in male and female rat fetuses [
71,
72]. In addition, in male offspring, the exposure reprogrammed the testicular steroidogenic mechanisms leading to a lower expression of steroidogenic enzymes such as steroidogenic acute regulatory protein (StAR) and 3β-hydroxysteroid dehydrogenase (3β-HSD), a higher expression of fetal testicular nicotinic acetylcholine receptors (nAChRs) and histone deacetylase 4, along with a lower histone 3 lysine 9 acetylation (H3K9ac) of the StAR/3β-HSD promoter in adult rats; this steroidogenic reprogramming was observed even in subsequent generations indicating epigenetic heritability [
72]. In female offspring, the fetal nicotine exposure was associated with ovarian developmental problems, a lower estradiol and lower cytochrome P450 aromatase (P450arom) expression in fetal and adult life, an increased fetal ovarian nAChRs expression, and lower H3K9ac and H3K27ac levels in the P450arom promoter region during fetal or adult life [
71].
CBG binds about 90% of the cortisol in the circulation with a high affinity and only a small amount (5%) is free [
49]. CBG has been shown to regulate the availability of cortisol in circulation both by acting as a cortisol reservoir and modulating its release [
73]. CBG helps prevent large fluctuations of free cortisol in the circulation to maintain its steady state [
34]. Any changes in the levels of CBG or interference in the binding of cortisol with CBG will cause a disruption of cortisol’s transport, homeostasis, and availability at the target tissues. The direct effects of nicotine on CBG levels or its conformation are not known. The results from this study on the structural binding of nicotine and its important metabolites against CBG have shown that all four nicotine ligands have the potential to interfere in the binding of endogenous native ligands such as cortisol with CBG. In vitro binding studies on nicotine and/or its metabolites to CBG have not been reported. In addition, epidemiological studies showing direct associations of nicotine-related adverse effects on CBG have not been reported. However, the functional relevance of our results and interrelationships of nicotine ligand interactions with CBG support previously reported nicotine-related HPA axis dysfunctions and an adrenal corticosteroid hormone imbalance. Cigarette smoking was associated with dose-related increase in nicotine, cortisol, and ACTH in blood circulation [
33,
74,
75]. An increase in nicotine in the circulation acutely stimulated the HPA axis through the hypothalamus causing the secretion of ACTH from the anterior pituitary, which resulted in an increase in cortisol levels [
21,
75,
76]. In another study, nicotine was shown to modulate the function of the HPA axis through an increase in the excitation of neurons expressing corticotropin-releasing hormone (CRH) in the hypothalamus [
77]. Chronically high cortisol levels lead to problems of stress, energy metabolism, blood sugar regulation, and inflammation [
35]. The constant activation of the HPA axis in habitual smokers is a cause of chronic inflammation of the airways, which is consequently associated with low-grade systemic inflammation in smokers [
78]. Other studies have suggested that smoking-induced hypertension may have its causal origins in the effects of nicotine on the HPA axis [
34]. In experimental studies on rats, nicotine caused swelling, cytoplasmic vacuolation, pyknotic nuclei, and an increased caspase 3 expression along with lipid deposition in the cortisol-producing adrenal zona fasciculata cells [
79]. In addition, gestational nicotine exposure was associated with the intrauterine growth retardation of rat fetuses and aberrant HPA development and metabolic disorder affecting many systems such as adrenal gland, testis, hippocampus, etc. [
80,
81,
82]. Nicotine affected the intrauterine neuroendocrine metabolic programming in fetuses by overexposure to maternal glucocorticoids which further affected the regulatory physiology of HPA, glucocorticoid-insulin-like growth factor (IGF)-1 axis, renin–angiotensin system, and other endocrine systems. The fetal HPA axis is especially sensitive to long-term modulation and programing of glucocorticoids, the effects of which can persist through life [
83,
84]. The dysregulation of the HPA axis due to this reprogramming has been shown to lead to neurodevelopmental and behavioral problems besides predisposing to many chronic diseases such as metabolic and cardiovascular problems. In this regard, nicotine exposure in fetuses increased blood corticosteroids, decreased placental 11β-hydroxysteroid dehydrogenase-2 expression, increased the expression of glucocorticoid receptor, decreased fetal hypothalamic CRH, decreased the expression of adrenal StAR and cholesterol side-chain cleavage enzyme, and dysregulated fetal liver IGF-1, its receptor and insulin receptor [
85]. Prenatally exposed rats showed a propensity for metabolic disorder during adult age which was suggested to be due to the in utero dysregulation of hippocampal developmental physiology [
80,
82,
86]. These fetal-originated neuro-metabolic modulations leading to an adulthood onset of disorders also exhibited epigenetic heritability in subsequent generations probably through the effects of glucocorticoids on the epigenome (DNA methylation, histone acetylation, and microRNA) [
83,
84]. Gender differences of nicotine exposure on abnormal adrenal development along with the upregulation of the adrenal IGF1 signaling pathway and steroidogenic function in male rats compared to a decreased adrenal IGF1 signaling pathway and steroidogenic function in female rats during fetal and postnatal life was reported [
81]. The gender differences in the IGF1 signaling pathway and steroidogenic function after gestational nicotine exposure were suggested to be due to the differences in nAChRβ1 expression. The adrenal dysfunction was downregulated even in subsequent generations of the prenatally exposed females. In this regard, it was previously reported that lactational nicotine exposure of rats was associated with hyperleptinemia, adrenal dysfunction, and a higher adiposity along with a higher adrenal catecholamine content, higher serum corticosterone, CRH, and ACTH in male offspring, which persisted into adult life; female did not show such adrenal phenotype [
87].
TBG is a circulatory glycoprotein that binds thyroxine with a high affinity [
29]. The majority of the amount of thyroxine (70%) in the blood is bound to TBG and the remaining is bound to transthyretin or albumin. A very tiny portion, about 0.1%, is unbound. Any changes in the levels of TBG or interference in the binding of thyroxine with TBG will lead to an acute disruption of thyroxin’s transport, homeostasis, and availability in the body tissues. In vitro binding studies on nicotine and/or its metabolites against TBG have not been reported. The direct effects of nicotine on TBG levels or on thyroxine binding to TBG are also not known. Our results from this study on the structural binding of nicotine and its important metabolites against TBG showed that all four nicotine ligands, nicotine, cotinine, trans 3′-hydroxycotinine, and 5′-hydroxycotinine, had the potential to interfere in the binding of endogenous native ligands, thyroxine and triiodothyronine, with TBG. The functional relevance of our results and the interrelationships of nicotine ligand interactions with TBG support the reported nicotine-related HPT axis imbalance and disorders. Cigarette smoking/nicotine has been associated with thyroid hormone dysfunction [
88]. Several studies have reported that smoking/nicotine is associated with decreased levels of TSH and higher serum free thyroxine and free triiodothyronine levels [
31,
89,
90]. These associations were again recently confirmed in a large cohort of subjects and these changes were also reflected by sequential negative and positive correlations with urinary cotinine [
32]. One of the possibilities proposed was that the nicotine-induced decrease in serum estrogens may decrease TBG levels and, hence, more thyroxine is available in circulation [
29]. The other mechanisms suggested were through nicotine causing sympathetic activation leading to increased thyroid hormone levels [
32,
88,
90] and a direct stimulatory effect of nicotine on the thyroid gland and/or a stimulatory effect of nicotine on hepatic oxidative metabolism, which could result in the increased conversion of thyroxine to triiodothyronine [
90]. Apparently, gender differences for the effects of nicotine on TBG and thyroid hormones have not been reported. Smokers in general have a lower prevalence of hypothyroidism [
91]. A meta-analysis of many studies on the effects of smoking on thyroid diseases revealed that nicotine was associated with postpartum thyroid disease, nontoxic goiter, Hashimoto’s thyroiditis, Graves’ disease, and Graves’ ophthalmopathy [
92]. Nicotine has been associated with further enhancing thyroid-associated ophthalmopathy in Grave’s patients [
93].
In conclusion, molecular docking simulations of nicotine and its three important metabolites, cotinine, trans 3′-hydroxycotinine, and 5′-hydroxycotinine against three circulatory endocrine transport proteins, SHBG, CBG, and TBG were performed. The self-docking analyses of the native ligands to the respective transport proteins demonstrated a -quality docking and reliability of the results. In addition, it also showed that the chosen 3-D structures were working well with our docking software and were suitable for exploring binding poses of other ligands using molecular docking. The docking results showed that all four nicotine ligands interacted with each of the carrier proteins and bound deep into their ligand-binding pockets. Nicotine and its metabolites formed nonbonded contacts and/or hydrogen bonds with amino acid residues of the carrier proteins. For SHBG, Phe-67 and Met-139 were the most important amino acid residues for nicotine ligand binding showing the maximum number of interactions and maximum ΔASA, and for CBG, Trp-371 and Asn-264 were the most important amino acid residues. For TBG, Ser-23, Leu-269, Lys-270, Asn-273, and Arg-381 were the most important amino acid residues for nicotine ligand binding. In general, the majority of the amino acid residues of carrier proteins interacting with nicotine ligands showed a commonality with the interacting residues for the native ligands of the carrier protein. Taken together, the binding energies, amino acid interactions, Dock scores, and dissociation constants suggested that nicotine and its three metabolites competed with native (endogenous) ligands (hormones) for binding to their carrier proteins. The binding pose analyses of nicotine ligands and their comparison with native ligands showed that in spite of having a large search space for binding (10 Å around the native ligand), the nicotine ligands bound to the same location where the native ligand was binding and were also interacting with a similar set of residues. In addition to the dock score from the docking software tool, the binding energy and dissociation constant values were calculated using another independent piece of software and these scores also corroborated the dock score. Further, the importance of interacting residues was checked by two different software tools using different criteria, and their results were also found to be consistent. Taken together, all of these corroborating results provided a high credence to our study. Thus, nicotine and its three metabolites may potentially interfere with the binding of endogenous native ligands of SHBG (testosterone, estradiol), CBG (cortisol, progesterone), and TBG (thyroxine, triiodothyronine) and result in the disbalance of their transport and homeostasis in the blood circulation.