Evaluating Different Extraction Approaches for GC-MS Based Metabolomics Analysis of the Giant Pandas’ Fur
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. Hair Segmentation and Decontamination Procedures
2.4. Acid Hydrolysis
2.5. Base Hydrolysis
2.6. Liquid/Liquid Extraction
2.7. MCF Derivatization
2.8. GC/MS Analysis
2.9. Metabolite Identification, Data Extraction, and Statistical Analysis
3. Results
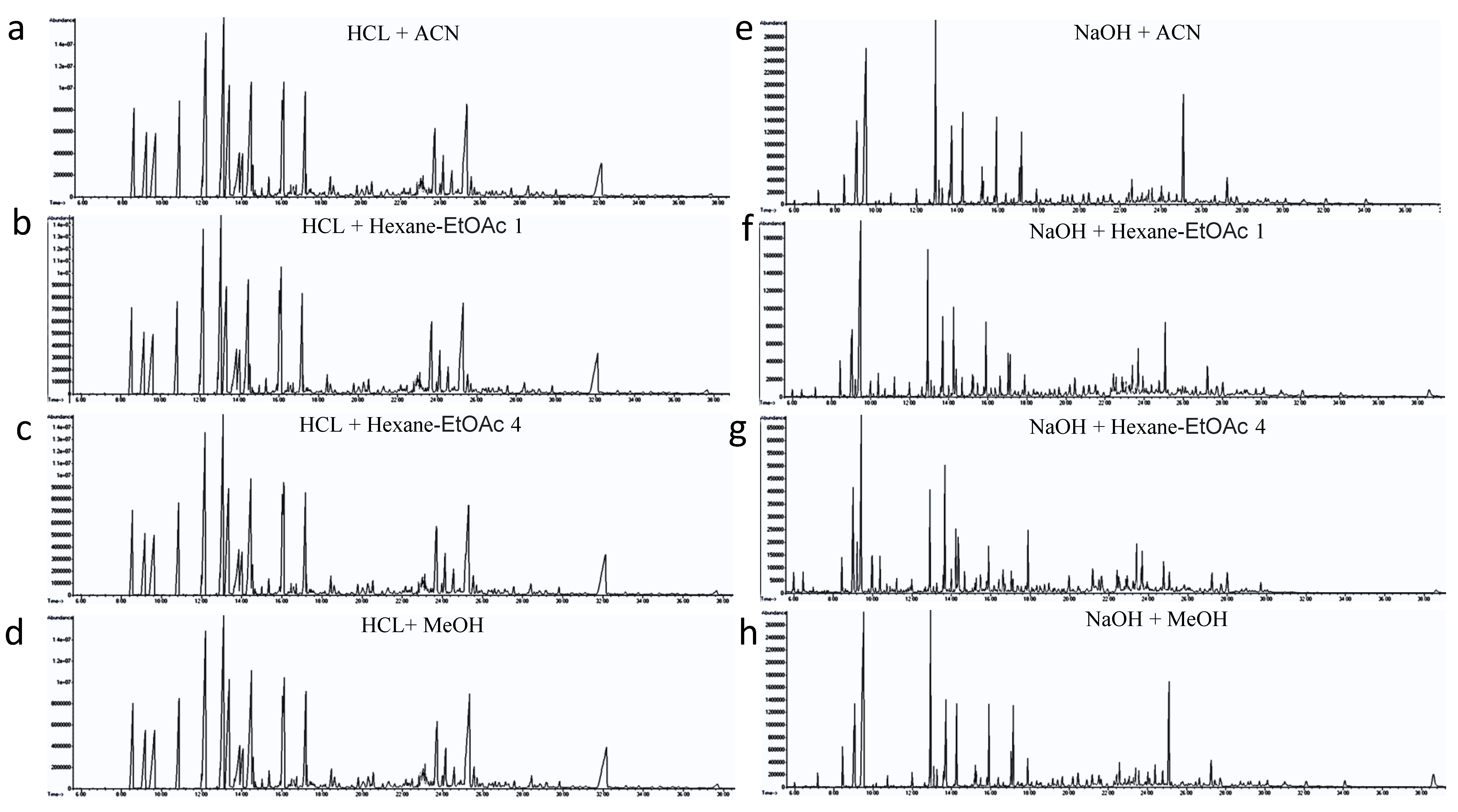
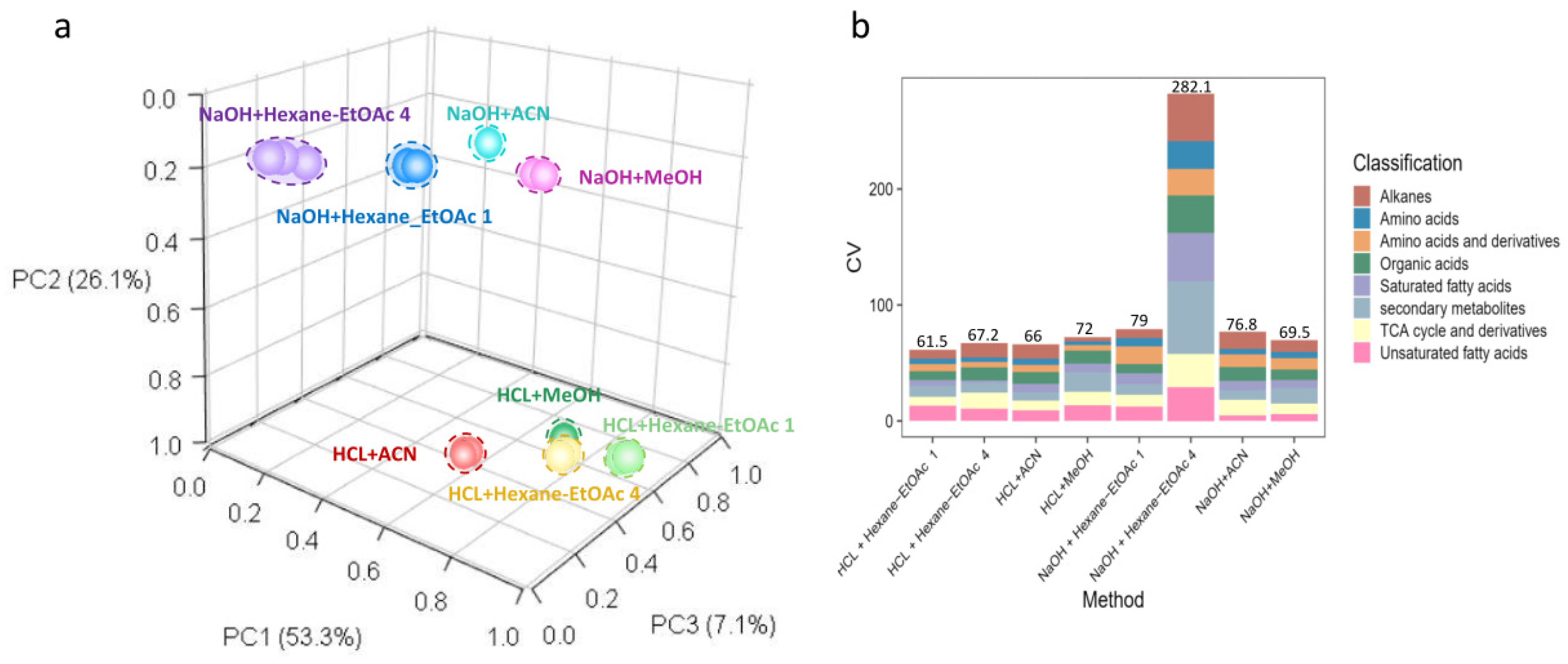
3.1. Analytical Qualities and Reproducibility of the Eight Different Extraction Methods
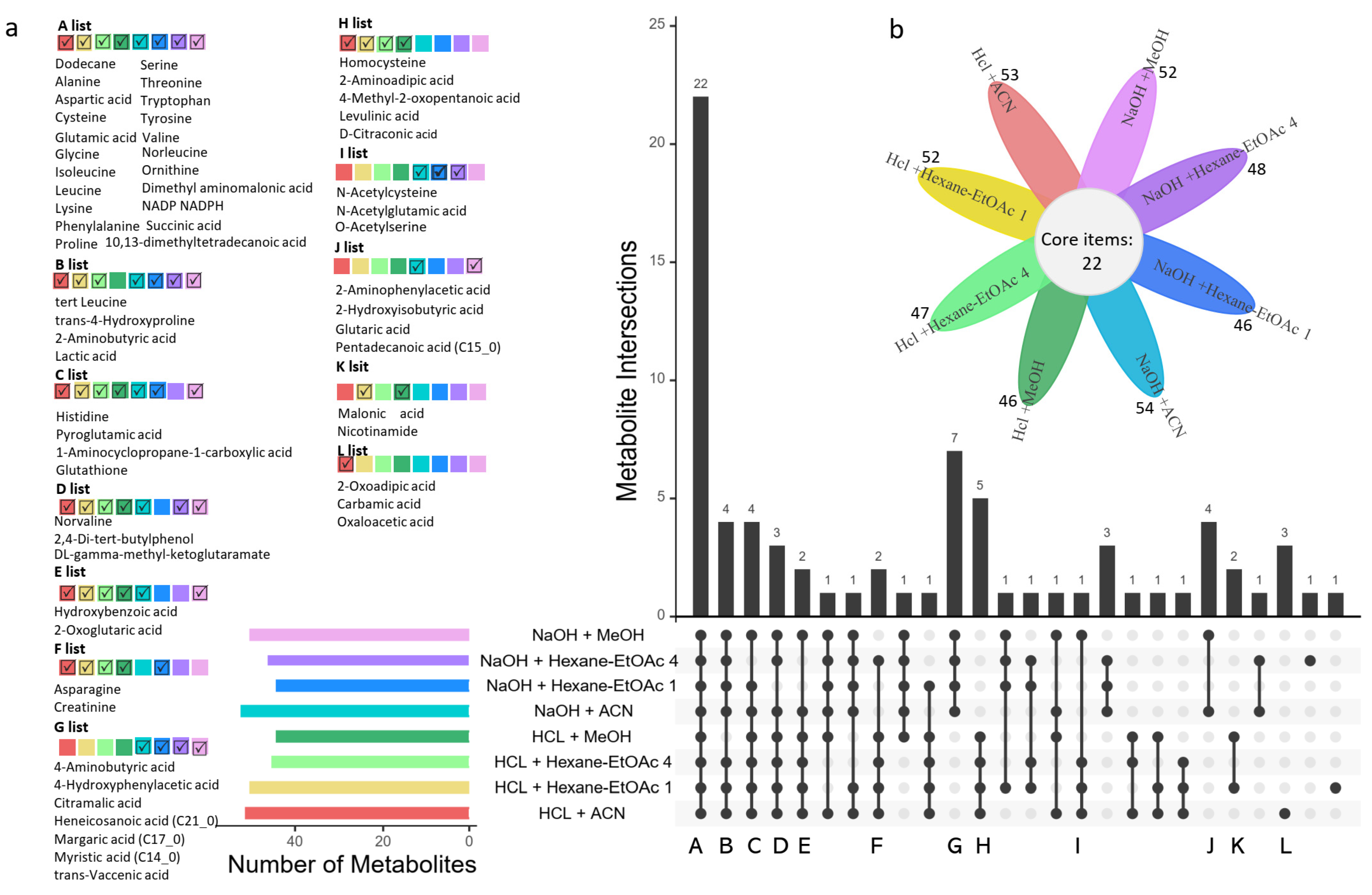
3.2. Metabolite Coverage of the Eight Different Extraction Methods for Panda Fur
3.3. Extraction Efficiency of Different Extraction Methods
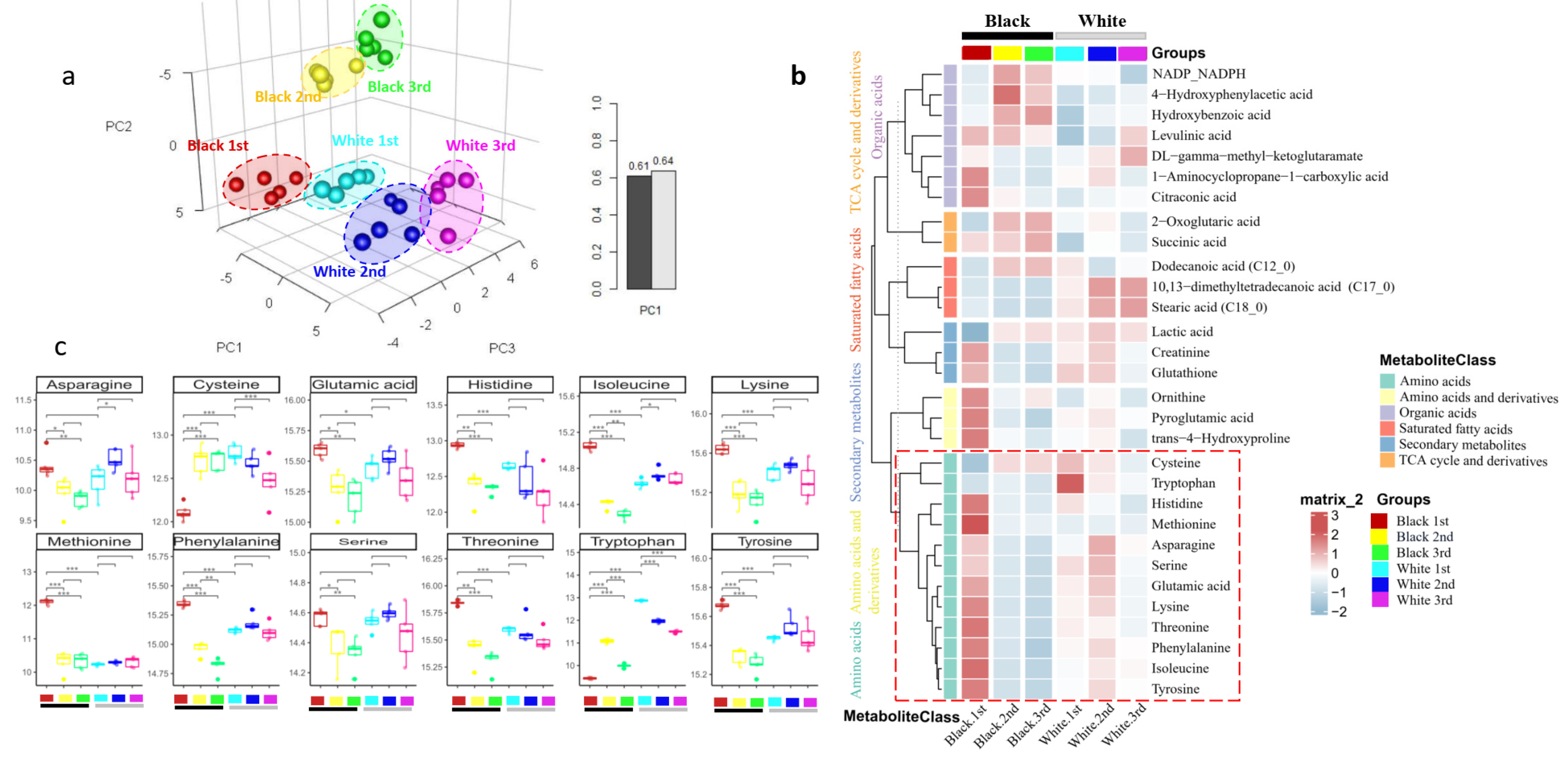
3.4. Validation Experiment: Metabolite Profile of Three Segments between White and Black Panda Fur
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.-P.; Liu, Q.; Ma, Q.-Y.; Maltby, L.; Ellison, A.M.; Zhao, Y. Environmental toxicants impair liver and kidney function and sperm quality of captive pandas. Ecotoxicol. Environ. Saf. 2018, 162, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, Z.; Liu, X.; Sun, W. The potential risks and exposure of Qinling giant pandas to polycyclic aromatic hydrocarbon (PAH) pollution. Environ. Pollut. 2021, 292, 118294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Y.-P.; Zheng, Y.; Ma, Q.; Jiang, Y. Quantifying the heavy metal risks from anthropogenic contributions in Sichuan panda (Ailuropoda melanoleuca melanoleuca) habitat. Sci. Total Environ. 2020, 745, 140941. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fang, R.; Wang, H.; Xu, D.-X.; Yang, J.; Huang, X.; Cozzolino, D.; Fang, M.; Huang, Y. A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: Where exposomics meets metabolomics. Environ. Int. 2021, 158, 106941. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.-L.; Miller, G.W. The exposome and health: Where chemistry meets biology. Science 2020, 367, 392–396. [Google Scholar] [CrossRef]
- Barbosa, J.; Faria, J.; Carvalho, F.; Pedro, M.; Queirós, O.; Moreira, R.; Dinis-Oliveira, R.J. Hair as an alternative matrix in bioanalysis. Bioanalysis 2013, 5, 895–914. [Google Scholar] [CrossRef]
- Jang, W.-J.; Choi, J.Y.; Park, B.; Seo, J.H.; Seo, Y.H.; Lee, S.; Jeong, C.-H.; Lee, S. Hair Metabolomics in Animal Studies and Clinical Settings. Molecules 2019, 24, 2195. [Google Scholar] [CrossRef]
- Kuwayama, K.; Miyaguchi, H.; Iwata, Y.T.; Kanamori, T.; Tsujikawa, K.; Yamamuro, T.; Segawa, H.; Inoue, H. Three-step drug extraction from a single sub-millimeter segment of hair and nail to determine the exact day of drug intake. Anal. Chim. Acta 2016, 948, 40–47. [Google Scholar] [CrossRef]
- Takahashi, F.; Kobayashi, M.; Kobayashi, A.; Kobayashi, K.; Asamura, H. High-Frequency Heating Extraction Method for Sensitive Drug Analysis in Human Nails. Molecules 2018, 23, 3231. [Google Scholar] [CrossRef]
- Yang, J.; Wei, Y.; Qi, H.; Yin, N.; Yang, Y.; Li, Z.; Xu, L.; Wang, X.; Yuan, P.; Li, L.; et al. Neonatal hair profiling reveals a metabolic phenotype of monochorionic twins with selective intrauterine growth restriction and abnormal umbilical artery flow. Mol. Med. 2020, 26, 37. [Google Scholar] [CrossRef]
- Angeli, I.; Casati, S.; Ravelli, A.; Minoli, M.; Orioli, M. A novel single-step GC–MS/MS method for cannabinoids and 11-OH-THC metabolite analysis in hair. J. Pharm. Biomed. Anal. 2018, 155, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rashaid, A.H.B.; Harrington, P.D.B.; Jackson, G.P. Profiling Amino Acids of Jordanian Scalp Hair as a Tool for Diabetes Mellitus Diagnosis: A Pilot Study. Anal. Chem. 2015, 87, 7078–7084. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Schwartz, Z.; Jackson, G.P. δ13C analysis of amino acids in human hair using trimethylsilyl derivatives and gas chromatography/combustion/isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Gambelunghe, C.; Sommavilla, M.; Ferranti, C.; Rossi, R.; Aroni, K.; Manes, N.; Bacci, M. Analysis of anabolic steroids in hair by GC/MS/MS. Biomed. Chromatogr. 2007, 21, 369–375. [Google Scholar] [CrossRef]
- Delplancke, T.D.J.; de Seymour, J.; Tong, C.; Sulek, K.; Xia, Y.; Zhang, H.; Han, T.-L.; Baker, P.N. Analysis of sequential hair segments reflects changes in the metabolome across the trimesters of pregnancy. Sci. Rep. 2018, 8, 36. [Google Scholar] [CrossRef]
- Lehmann, E.; Oltramare, C.; de Alencastro, L.F. Development of a modified QuEChERS method for multi-class pesticide analysis in human hair by GC-MS and UPLC-MS/MS. Anal. Chim. Acta 2018, 999, 87–98. [Google Scholar] [CrossRef]
- Jones, B.; Han, T.-L.; Delplancke, T.; McKenzie, E.J.; De Seymour, J.V.; Chua, M.C.; Tan, K.H.; Baker, P.N. Association between maternal exposure to phthalates and lower language ability in offspring derived from hair metabolome analysis. Sci. Rep. 2018, 8, 6745. [Google Scholar] [CrossRef]
- He, X.; de Seymour, J.; Sulek, K.; Qi, H.; Zhang, H.; Han, T.-L.; Villas-Boas, S.; Baker, P.N. Maternal hair metabolome analysis identifies a potential marker of lipid peroxidation in gestational diabetes mellitus. Geol. Rundsch. 2015, 53, 119–122. [Google Scholar] [CrossRef]
- Zeki, Ö.C.; Eylem, C.C.; Reçber, T.; Kır, S.; Nemutlu, E. Integration of GC-MS and LC-MS for untargeted metabolomics profiling. J. Pharm. Biomed. Anal. 2020, 190, 113509. [Google Scholar] [CrossRef]
- Heinl, S.; Lerch, O.; Erdmann, F. Automated GC-MS Determination of Delta9-Tetrahydrocannabinol, Cannabinol and Cannabidiol in Hair. J. Anal. Toxicol. 2016, 40, 498–503. [Google Scholar] [CrossRef]
- Eisenbeiss, L.; Steuer, A.E.; Binz, T.M.; Baumgartner, M.R.; Kraemer, T. (Un)targeted hair metabolomics: First considerations and systematic evaluation on the impact of sample preparation. Anal. Bioanal. Chem. 2019, 411, 3963–3977. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.F.; Aggio, R.B.M.; Van Houtte, J.R.; Villas-Bôas, S.G. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 1709–1729. [Google Scholar] [CrossRef]
- Han, T.-L.; Cannon, R.D.; Gallo, S.M.; Villas-Bôas, S.G. A metabolomic study of the effect of Candida albicans glutamate dehydrogenase deletion on growth and morphogenesis. NPJ Biofilms Microbiomes 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; McKenzie, E.J.; Jones, M.B.; Zarate, E.; Seymour, D.J.; Baker, P.N.; Villas-Bôas, S.G.; Han, T.L. MassOmics: An R package of a cross-platform data processing pipeline for large-scale GC-MS untargeted metabolomics datasets. Zenodo 2021. [Google Scholar] [CrossRef]
- Gekko, K.; Ohmae, E.; Kameyama, K.; Takagi, T. Acetonitrile-protein interactions: Amino acid solubility and preferential solvation. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzym. 1998, 1387, 195–205. [Google Scholar] [CrossRef]
- Kurbanova, M.; Maslennikova, S. Acid Hydrolysis of Casein. Foods Raw Mater. 2014, 2, 27–30. [Google Scholar] [CrossRef]
- Lambert, M.A.; Moss, C.W. Comparison of the effects of acid and base hydrolyses on hydroxy and cyclopropane fatty acids in bacteria. J. Clin. Microbiol. 1983, 18, 1370–1377. [Google Scholar] [CrossRef]
- Borges, C.R.; Wilkins, D.G.; Rollins, D.E. Amphetamine and N-acetylamphetamine incorporation into hair: An investigation of the potential role of drug basicity in hair color bias. J. Anal. Toxicol. 2001, 25, 221–227. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yin, Y.; Tang, X.; Xia, Y.; Zhang, J.; Yan, C.; Zhang, W.; Zhang, H.; Han, T.-L. Evaluating Different Extraction Approaches for GC-MS Based Metabolomics Analysis of the Giant Pandas’ Fur. Toxics 2022, 10, 688. https://doi.org/10.3390/toxics10110688
Yang Y, Yin Y, Tang X, Xia Y, Zhang J, Yan C, Zhang W, Zhang H, Han T-L. Evaluating Different Extraction Approaches for GC-MS Based Metabolomics Analysis of the Giant Pandas’ Fur. Toxics. 2022; 10(11):688. https://doi.org/10.3390/toxics10110688
Chicago/Turabian StyleYang, Yang, Yanqiang Yin, Xianglan Tang, Yinyin Xia, Jinya Zhang, Chun Yan, Weixuan Zhang, Hua Zhang, and Ting-Li Han. 2022. "Evaluating Different Extraction Approaches for GC-MS Based Metabolomics Analysis of the Giant Pandas’ Fur" Toxics 10, no. 11: 688. https://doi.org/10.3390/toxics10110688
APA StyleYang, Y., Yin, Y., Tang, X., Xia, Y., Zhang, J., Yan, C., Zhang, W., Zhang, H., & Han, T.-L. (2022). Evaluating Different Extraction Approaches for GC-MS Based Metabolomics Analysis of the Giant Pandas’ Fur. Toxics, 10(11), 688. https://doi.org/10.3390/toxics10110688





