Pt-N Co-Modified TiO2 Nanotube Electrode Photoelectrocatalytic Degradation of Oxytetracycline in Simulated Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Pt-N-TiO2 Nanotube Electrode
2.3. Photoelectrocatalytic Degradation of Oxytetracycline
2.4. Characterization Tests
3. Results and Discussion
3.1. Scanning Electron Microscopy
3.2. X-ray Photoelectron Spectroscopy
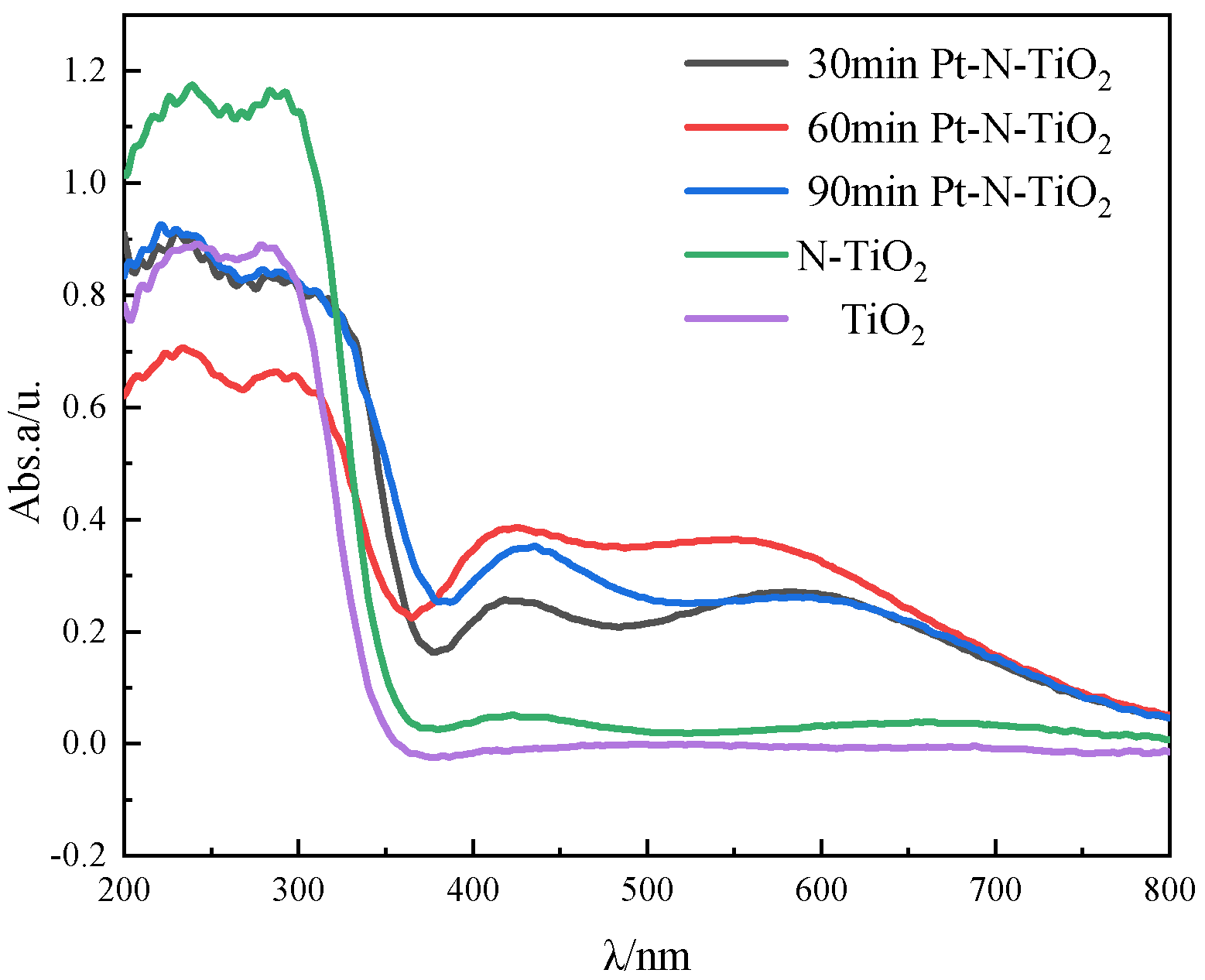
3.3. X-ray Diffraction and UV-VIS Diffuse Reflectance Specatrum
3.4. Photoluminescence Spectroscopy
3.5. Electrochemical Analyses
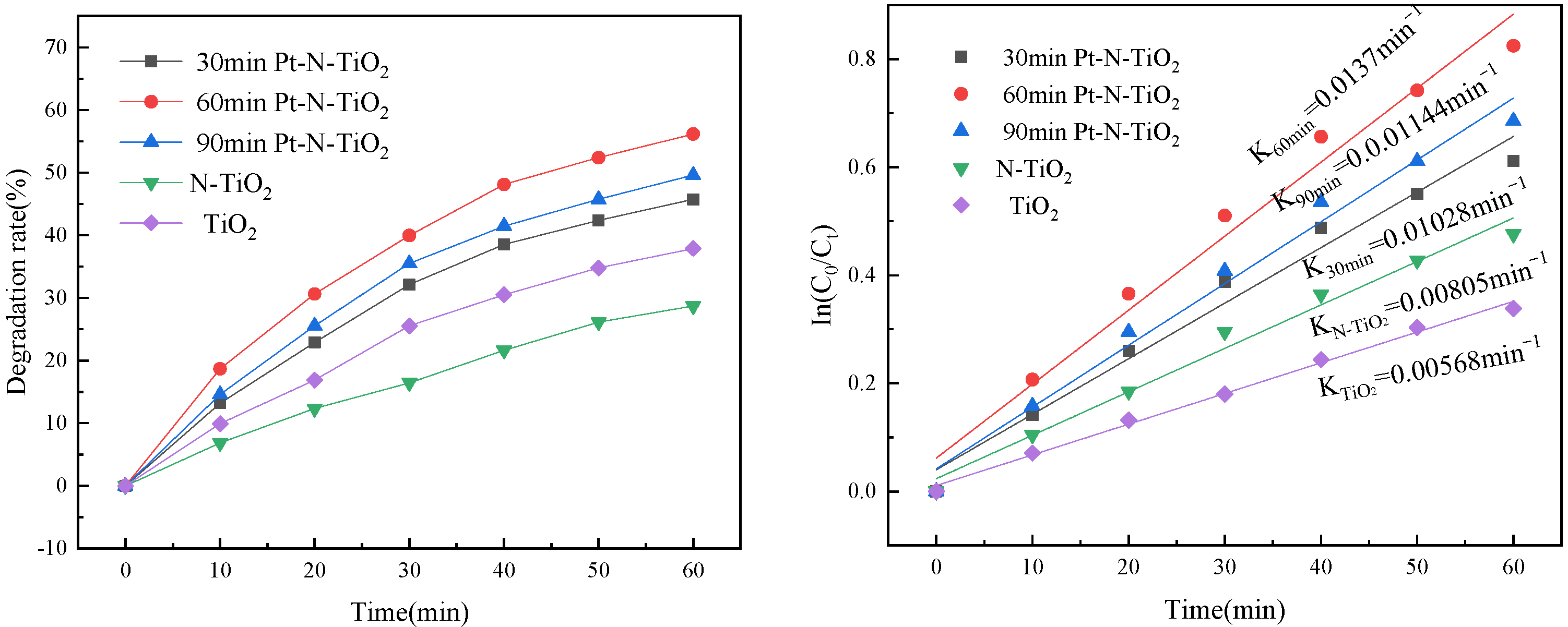
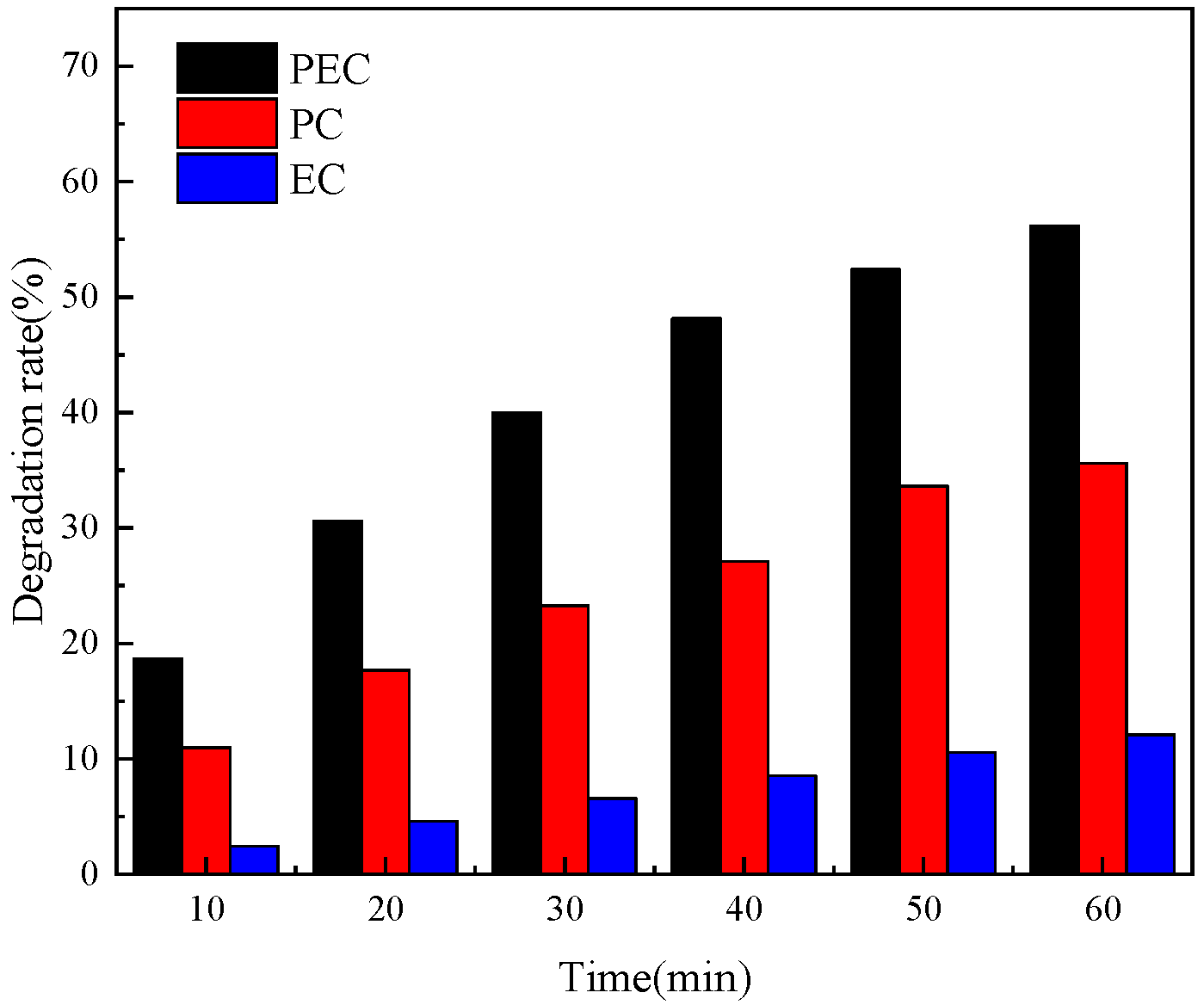
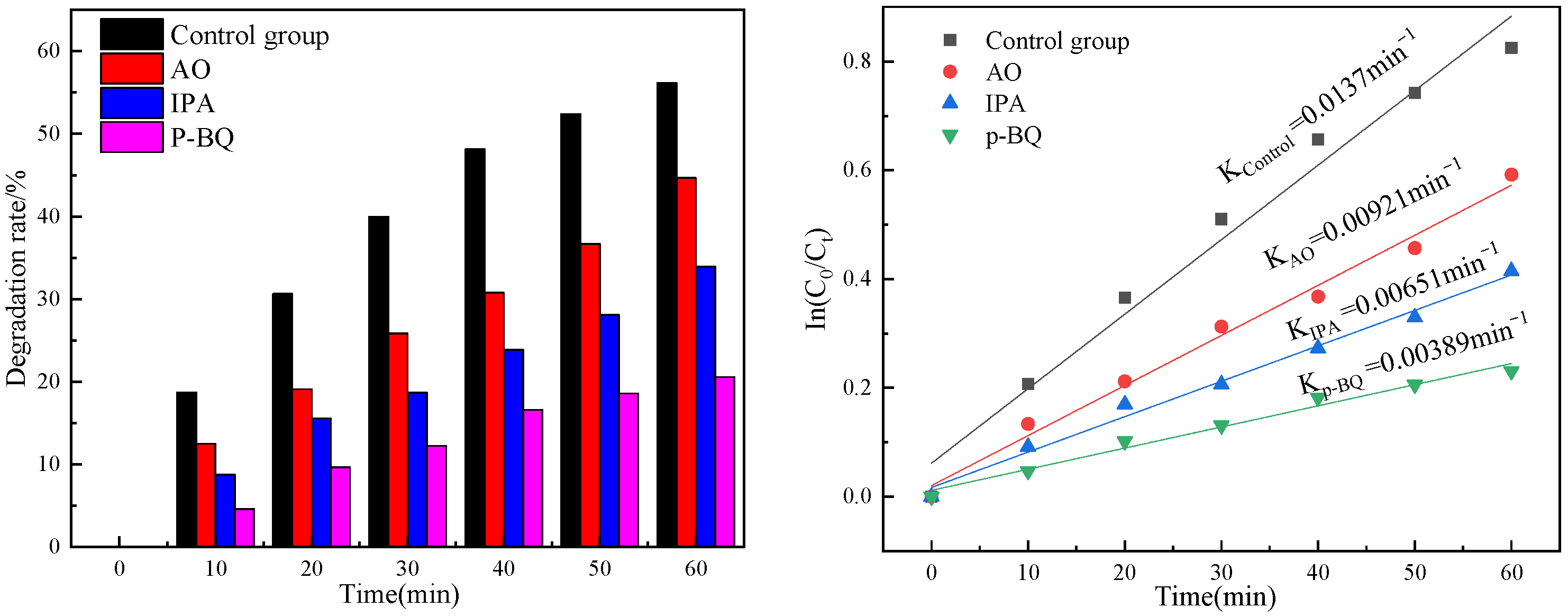
3.6. Photoelectrocatalytic Degradation of Oxytetracycline
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baran, W.; Adamek, E.; Jajko, M.; Sobczak, A. Removal of veterinary antibiotics from wastewater by electrocoagulation. Chemosphere 2018, 194, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.-L. Occurrence of antibiotics in the aquatic environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods. Bioengineered 2021, 12, 7376–7416. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, B.; Gao, Y.; Liu, W.; Zhao, X.; Huang, X.; Yu, J. Occurrence and fate of antibiotics in the aqueous environment and their removal by constructed wetlands in China: A review. Pedosphere 2017, 27, 42–51. [Google Scholar] [CrossRef]
- Lorenzo, P.; Adriana, A.; Jessica, S.; Caries, B.; Marinella, F.; Marta, L.; Jose Luis, B.; Pierre, S. Antibiotic resistance in urban and hospital wastewaters and their impact on a receiving freshwater ecosystem. Chemosphere 2018, 206, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Inyinbor, A.A.; Bello, O.S.; Fadiji, A.E.; Inyinbor, H.E. Threats from antibiotics: A serious environmental concern. J. Environ. Chem. Eng. 2018, 6, 784–793. [Google Scholar] [CrossRef]
- Chin, W.; Zhong, G.; Pu, Q.; Yang, C.; Lou, W.; De Sessions, P.F.; Periaswamy, B.; Lee, A.; Liang, Z.C.; Ding, X.; et al. A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset. Nat. Commun. 2018, 9, 917. [Google Scholar] [CrossRef]
- Chen, B.; Lin, L.; Fang, L.; Yang, Y.; Chen, E.; Yuan, K.; Zou, S.; Wang, X.; Luan, T. Complex pollution of antibiotic resistance genes due to beta-lactam and aminoglycoside use in aquaculture farming. Water Res. 2018, 134, 200–208. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions—A review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Luo, L.; Yang, Y.; Huang, H.; Peng, H.; Tang, L.; Mu, Y. Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: Mechanism study and research gaps. Chem. Eng. J. 2018, 347, 379–397. [Google Scholar] [CrossRef]
- Olea, M.A.U.; Bueno, J.D.J.P.; Pérez, A.X.M. Nanometric and surface properties of semiconductors correlated to photocatalysis and photoelectrocatalysis applied to organic pollutants—A review. J. Environ. Chem. Eng. 2021, 9, 106480. [Google Scholar] [CrossRef]
- Li, H.; Wang, G.; Niu, J.; Wang, E.; Niu, G.; Xie, C. Preparation of TiO2 nanotube arrays with efficient photocatalytic performance and super-hydrophilic properties utilizing anodized voltage method. Results Phys. 2019, 14, 102499. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of titanium dioxide (TiO2) modification on its application to pollution treatment—A review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Park, K.-W.; Han, S.-B.; Lee, J.-M. Photo (UV)-enhanced performance of Pt–TiO2 nanostructure electrode for methanol oxidation. Electrochem. Commun. 2007, 9, 1578–1581. [Google Scholar] [CrossRef]
- Javed, F.; Javed, S.; Mujahid, M.; ul Inam, F.; Bhatti, A.S. Modified optical characteristics of TiO2/Au/TiO2 thin composite films. Ceram. Int. 2019, 45, 22336–22343. [Google Scholar] [CrossRef]
- Toe, E.D.; Kurniawan, W.; Mariquit, E.G.; Hinode, H. Synthesis of N-doped mesoporous TiO2 by facile one-step solvothermal process for visible light photocatalytic degradation of organic pollutant. J. Environ. Chem. Eng. 2018, 6, 5125–5134. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Li, C.; He, L. Electronic and photocatalytic properties of N/F co-doped anatase TiO2. RSC Adv. 2017, 7, 55282–55287. [Google Scholar] [CrossRef]
- Yang, G.; Yan, Z.; Xiao, T. Preparation and characterization of SnO2/ZnO/TiO2 composite semiconductor with enhanced photocatalytic activity. Appl. Surf. Sci. 2012, 258, 8704–8712. [Google Scholar] [CrossRef]
- Lv, K.; Li, J.; Qing, X.; Li, W.; Chen, Q. Synthesis and photo-degradation application of WO3/TiO2 hollow spheres. J. Hazard. Mater. 2011, 189, 329–335. [Google Scholar] [CrossRef]
- Tio, F.; Mancuso, A.; Sacco, O.; Sannino, D.; Pragliola, S.; Vaiano, V. Enhanced visible-light-driven photodegradation of Acid Orange 7 azo dye in aqueous solution using Fe-N co-doped TiO2. Arab. J. Chem. 2020, 13, 8347–8360. [Google Scholar]
- Nzaba, S.K.M.; Ntsendwana, B.; Mamba, B.B.; Kuvarega, A.T. PAMAM templated N, Pt co-doped TiO2 for visible light photodegradation of brilliant black. Environ. Sci. Pollut. Res. 2018, 25, 15146–15158. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishian, S.M.; Ranjith, K.S.; Lee, H.; Park, B.; Norouzi, M.; Nikoo, S.Z.; Kim, W.-S.; Han, Y.-K.; Huh, Y.S. Tuning the phase composition of 1D TiO2 by Fe/Sn co-doping strategy for enhanced visible-light-driven photocatalytic and photoelectrochemical performances. J. Alloy. Compd. 2021, 851, 156826. [Google Scholar] [CrossRef]
- Soares, G.B.; Ribeiro, R.A.P.; De Lazaro, S.R.; Ribeiro, C. Photoelectrochemical and theoretical investigation of the photocatalytic activity of TiO2: N. RSC Adv. 2016, 6, 89687–89698. [Google Scholar] [CrossRef]
- Peighambardoust, N.S.; Asl, S.K.; Mohammadpour, R.; Asl, S.K. Band-gap narrowing and electrochemical properties in N-doped and reduced anodic TiO2 nanotube arrays. Electrochim. Acta 2018, 270, 245–255. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, H.; Zhang, P.; Xu, T. Pt deposited TiO2 films with exposed {0 0 1} facets for photocatalytic degradation of a pharmaceutical pollutant. Appl. Catal. A Gen. 2016, 521, 75–82. [Google Scholar] [CrossRef]
- Lyu, J.; Zhou, Z.; Wang, Y.; Li, J.; Li, Q.; Zhang, Y.; Ma, X.; Guan, J.; Wei, X. Platinum-enhanced amorphous TiO2-filled mesoporous TiO2 crystals for the photocatalytic mineralization of tetracycline hydrochloride. J. Hazard. Mater. 2019, 373, 278–284. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Wan, J. Enhanced photocatalytic activity of nitrogen doped TiO2 anatase nano-particle under simulated sunlight irradiation. Energy Procedia 2012, 16, 598–605. [Google Scholar] [CrossRef]
- Özcan, L.; Mutlu, T.; Yurdakal, S. Photoelectrocatalytic degradation of paraquat by Pt loaded TiO2 nanotubes on Ti anodes. Materials 2018, 11, 1715. [Google Scholar] [CrossRef]
- Borowska, E.; Gomes, J.F.; Martins, R.C.; Quinta-Ferreira, R.M.; Horn, H.; Gmurek, M. Solar photocatalytic degradation of sulfamethoxazole by TiO2 modified with noble metals. Catalysts 2019, 9, 500. [Google Scholar] [CrossRef]
- Chen, H.W.; Ku, Y.; Kuo, Y.L. Effect of Pt/TiO2 characteristics on temporal behavior of o-cresol decomposition by visible light-induced photocatalysis. Water Res. 2007, 41, 2069–2078. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Liu, L.; Hu, J.; Cui, W. Highly ordered TiO2 nanotube arrays wrapped with g-C3N4 nanoparticles for efficient charge separation and increased photoelectrocatalytic degradation of phenol. J. Hazard. Mater. 2018, 344, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarasimhan, N.; Bokare, A.D.; Choi, W. Effect of agglomerated state in mesoporous TiO2 on the morphology of photodeposited Pt and photocatalytic activity. J. Phys. Chem. C 2012, 116, 17531–17539. [Google Scholar] [CrossRef]
- Lu, Q.; Dong, C.; Wei, F.; Li, J.; Wang, Z.; Mu, W.; Han, X. Rational fabrication of Bi2WO6 decorated TiO2 nanotube arrays for photocatalytic degradation of organic pollutants. Mater. Res. Bull. 2022, 145, 111563. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, M.; Pei, L.; Liu, T.; Zhang, T.; Ao, D. Pt-N Co-Modified TiO2 Nanotube Electrode Photoelectrocatalytic Degradation of Oxytetracycline in Simulated Wastewater. Toxics 2022, 10, 635. https://doi.org/10.3390/toxics10110635
Wang L, Li M, Pei L, Liu T, Zhang T, Ao D. Pt-N Co-Modified TiO2 Nanotube Electrode Photoelectrocatalytic Degradation of Oxytetracycline in Simulated Wastewater. Toxics. 2022; 10(11):635. https://doi.org/10.3390/toxics10110635
Chicago/Turabian StyleWang, Liming, Mengyao Li, Liang Pei, Tingting Liu, Tian Zhang, and Dong Ao. 2022. "Pt-N Co-Modified TiO2 Nanotube Electrode Photoelectrocatalytic Degradation of Oxytetracycline in Simulated Wastewater" Toxics 10, no. 11: 635. https://doi.org/10.3390/toxics10110635
APA StyleWang, L., Li, M., Pei, L., Liu, T., Zhang, T., & Ao, D. (2022). Pt-N Co-Modified TiO2 Nanotube Electrode Photoelectrocatalytic Degradation of Oxytetracycline in Simulated Wastewater. Toxics, 10(11), 635. https://doi.org/10.3390/toxics10110635








