Highly Selective Adsorption of 99TcO4−/ReO4− by a Novel Polyamide-Functionalized Polyacrylamide Polymer Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
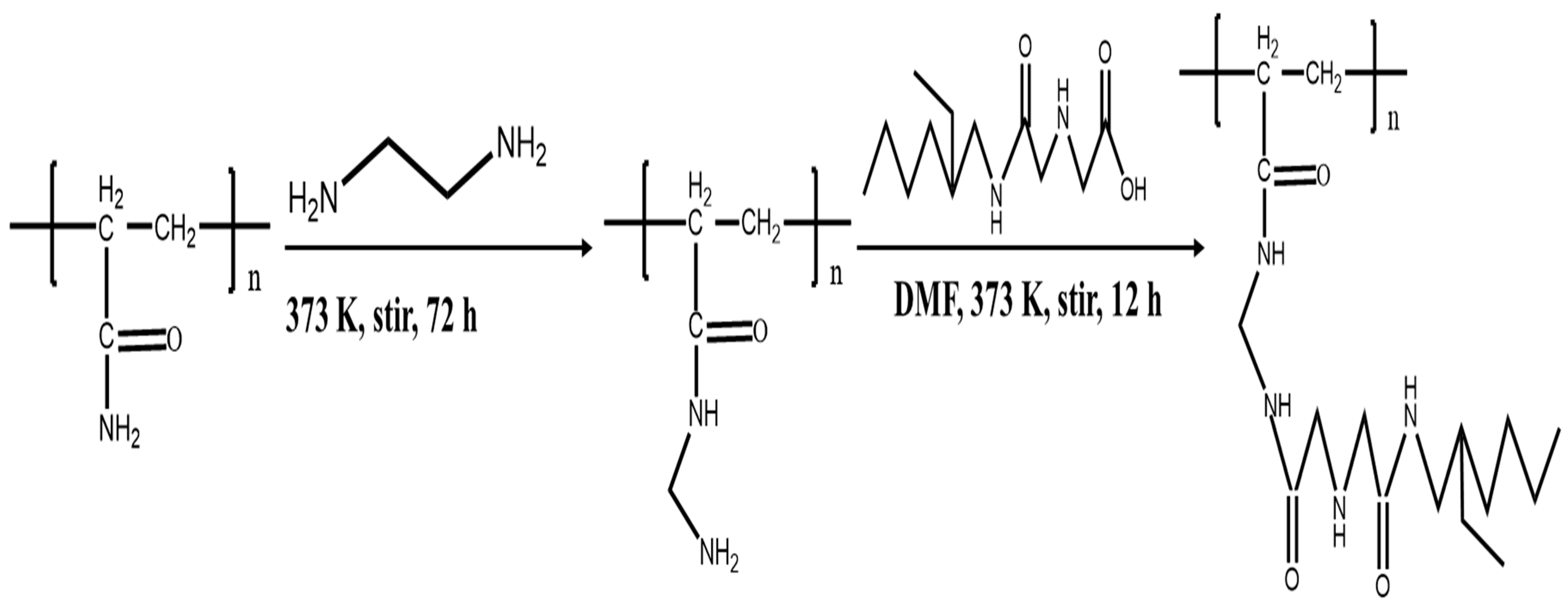
2.2. Synthesis of p-(Amide)-PAM
2.3. Characterization of PAM, N-PAM, and p-(Amide)-PAM
2.4. Batch Adsorption Experiments
3. Results and Discussion
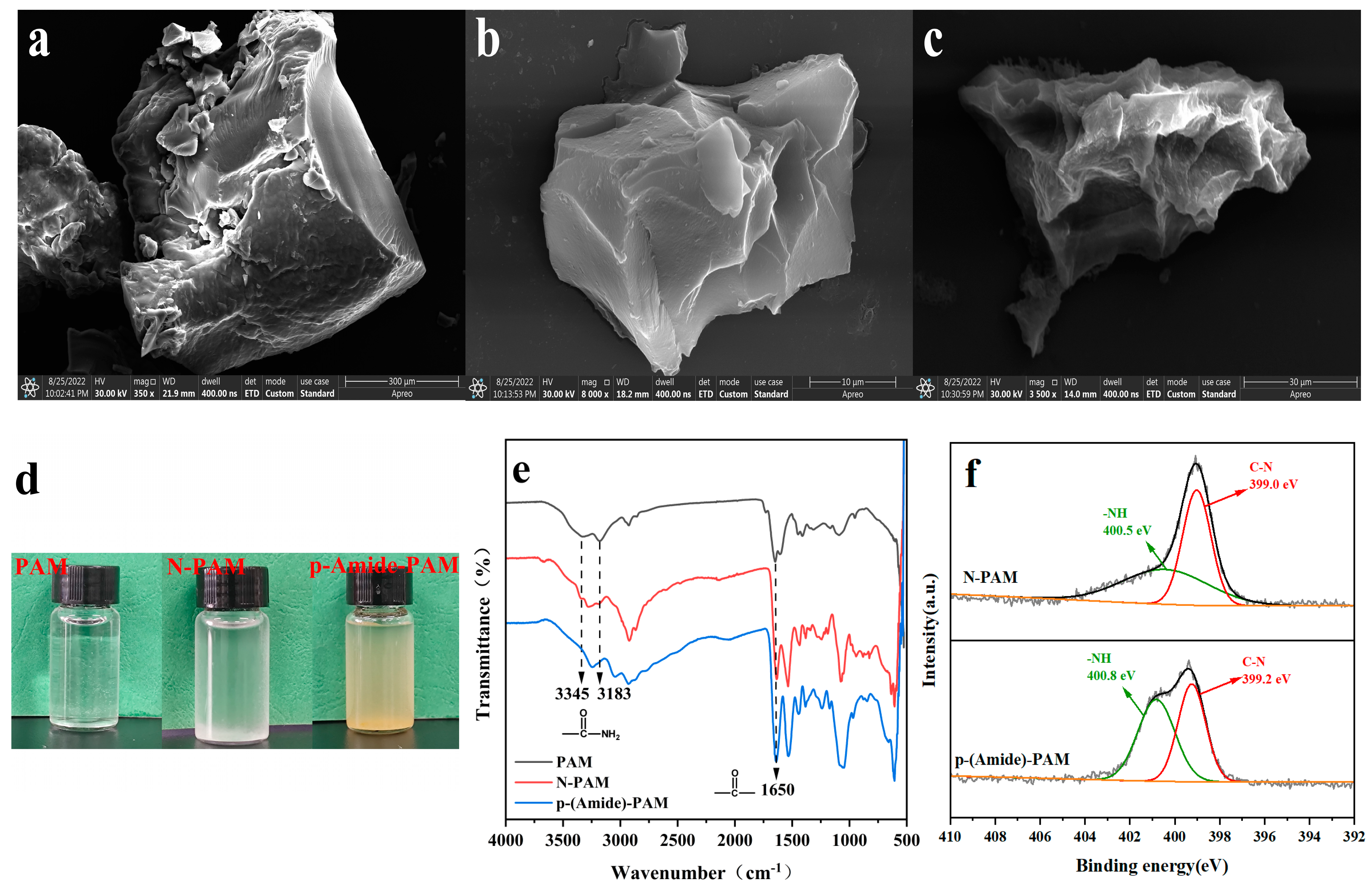
3.1. Characterizations of PAM, N-PAM, and p-(Amide)-PAM
3.2. Adsorption Experiment of ReO4− by p-(Amide)-PAM
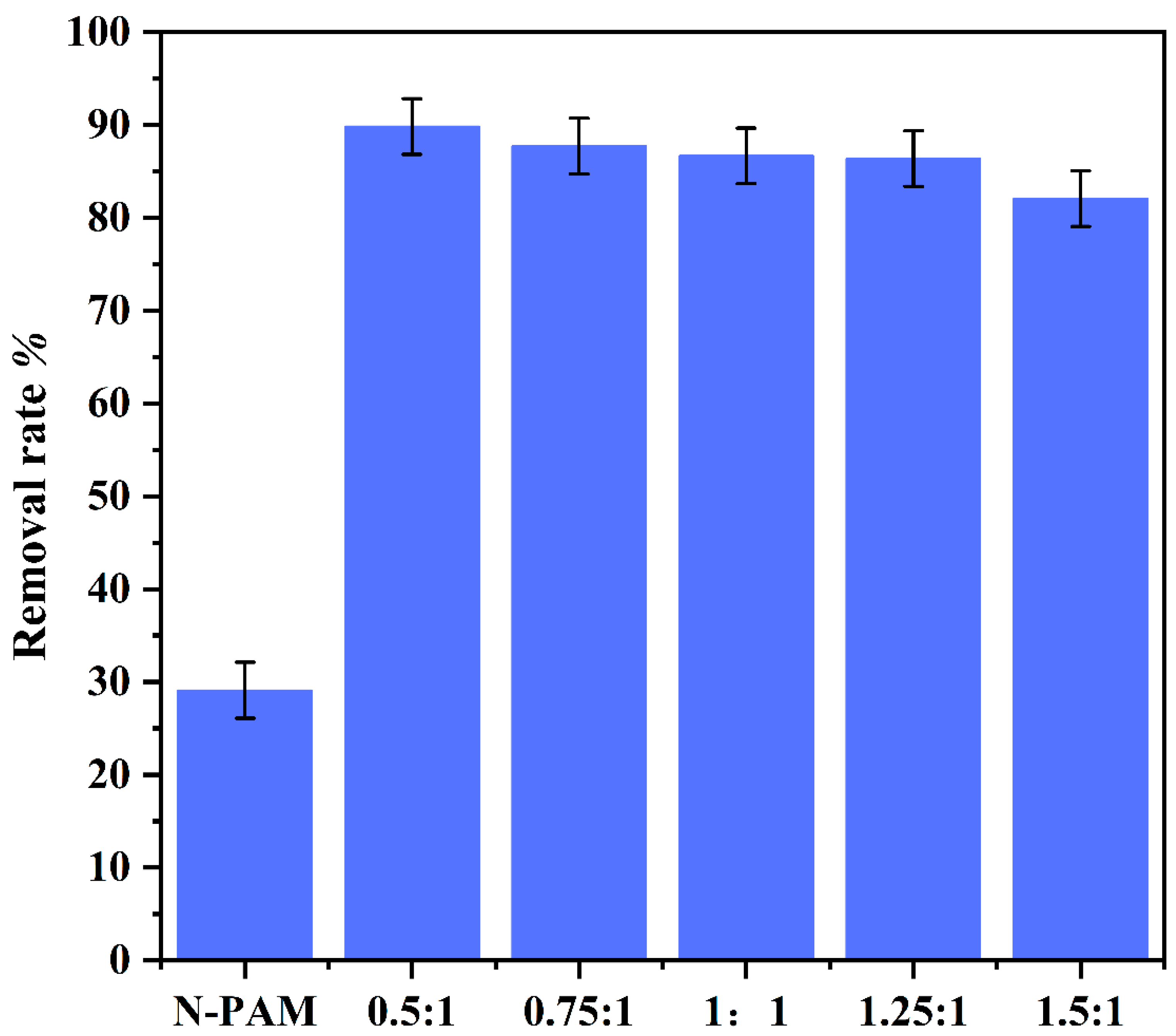
3.2.1. Influence of Different Molar Reaction Ratios
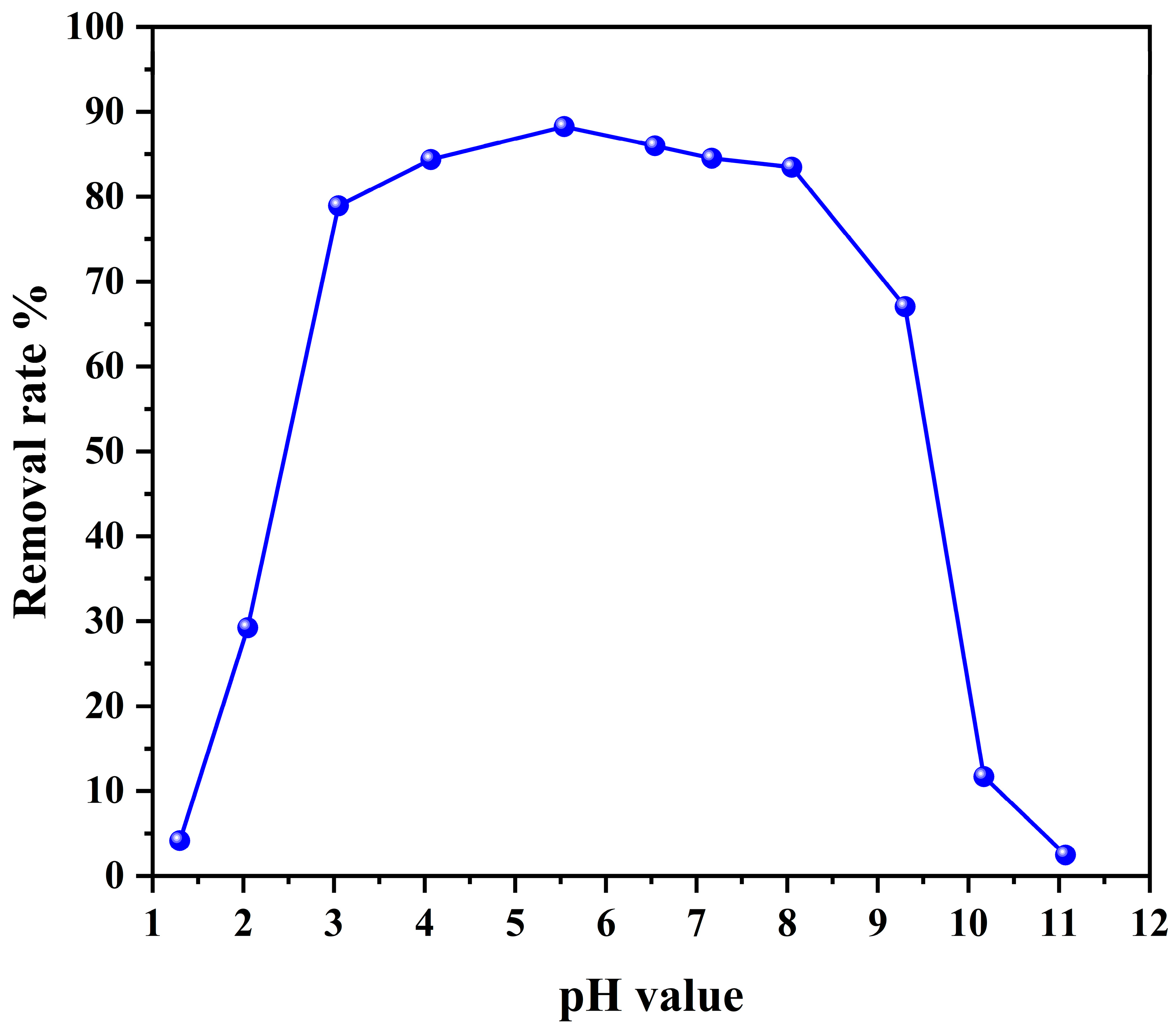
3.2.2. Effect of Initial pH
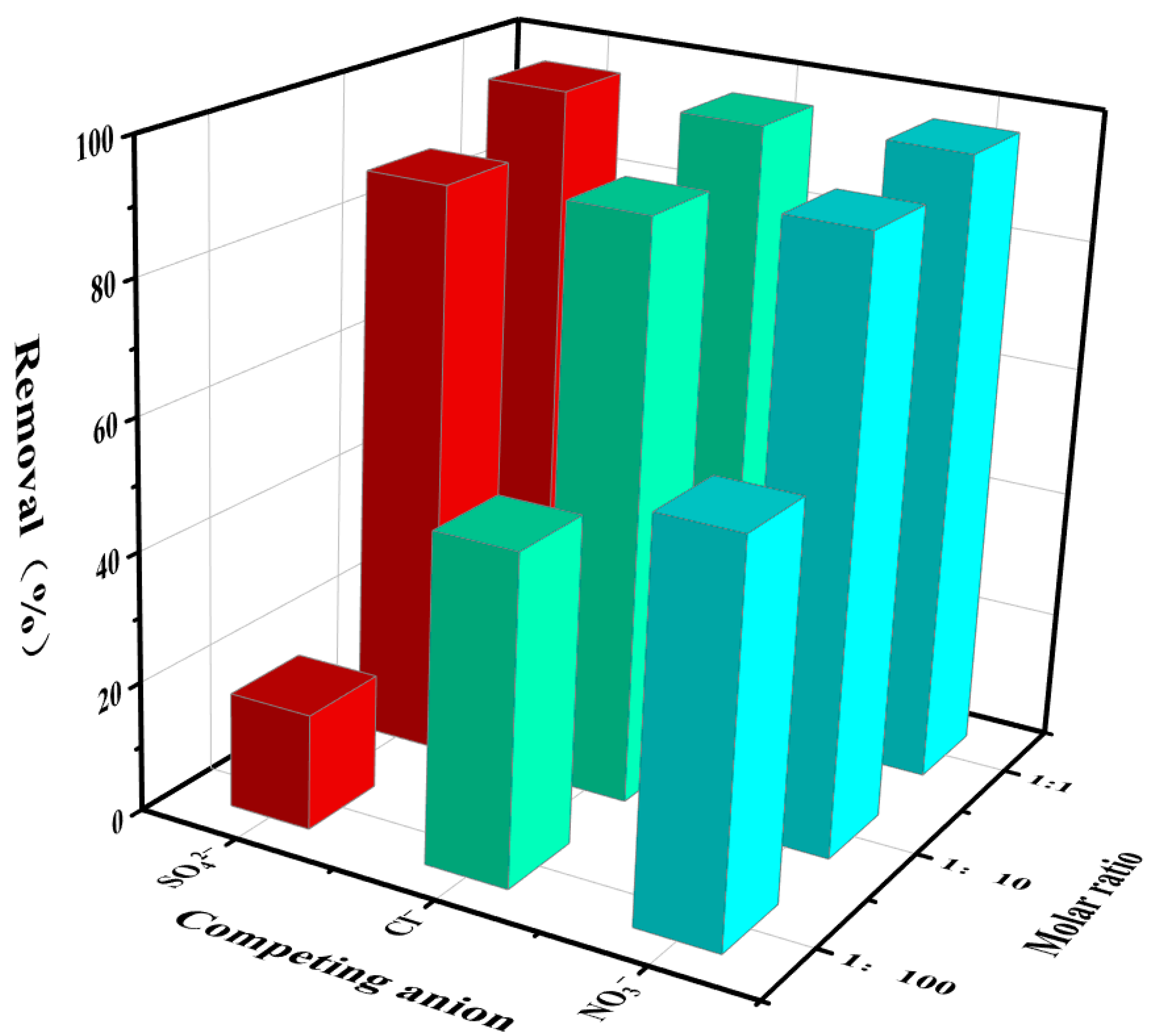
3.2.3. Influence of Competitive Anions
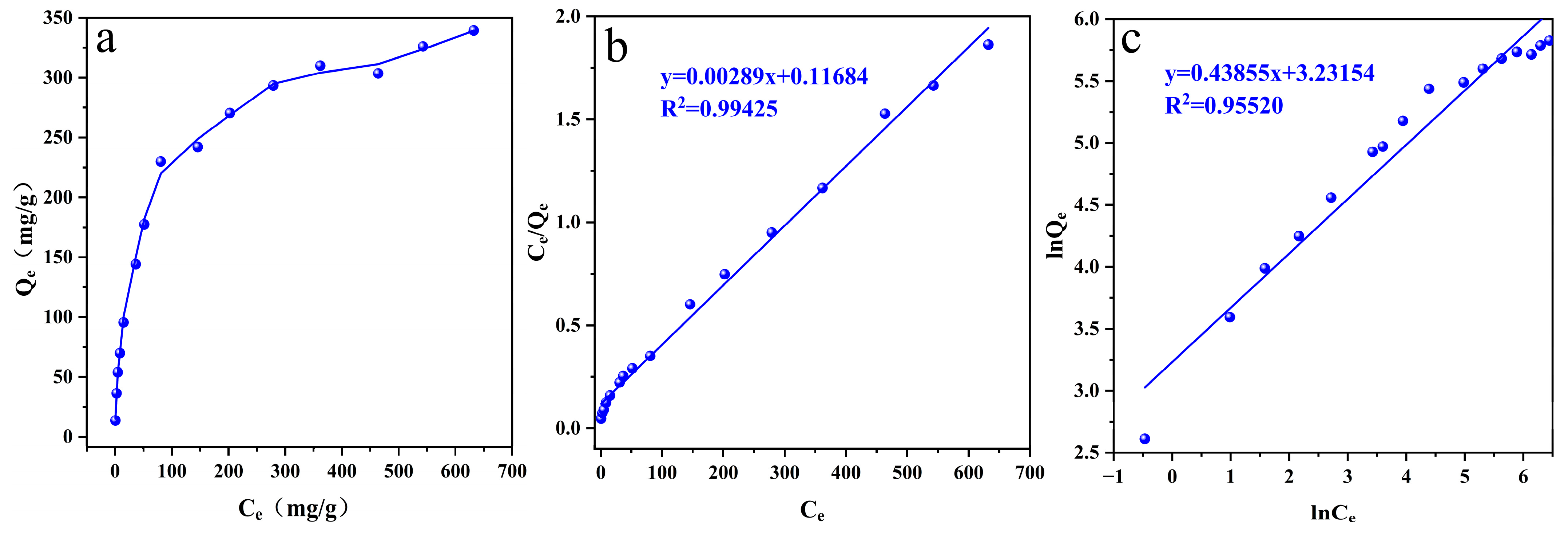
3.2.4. Adsorption Isotherm
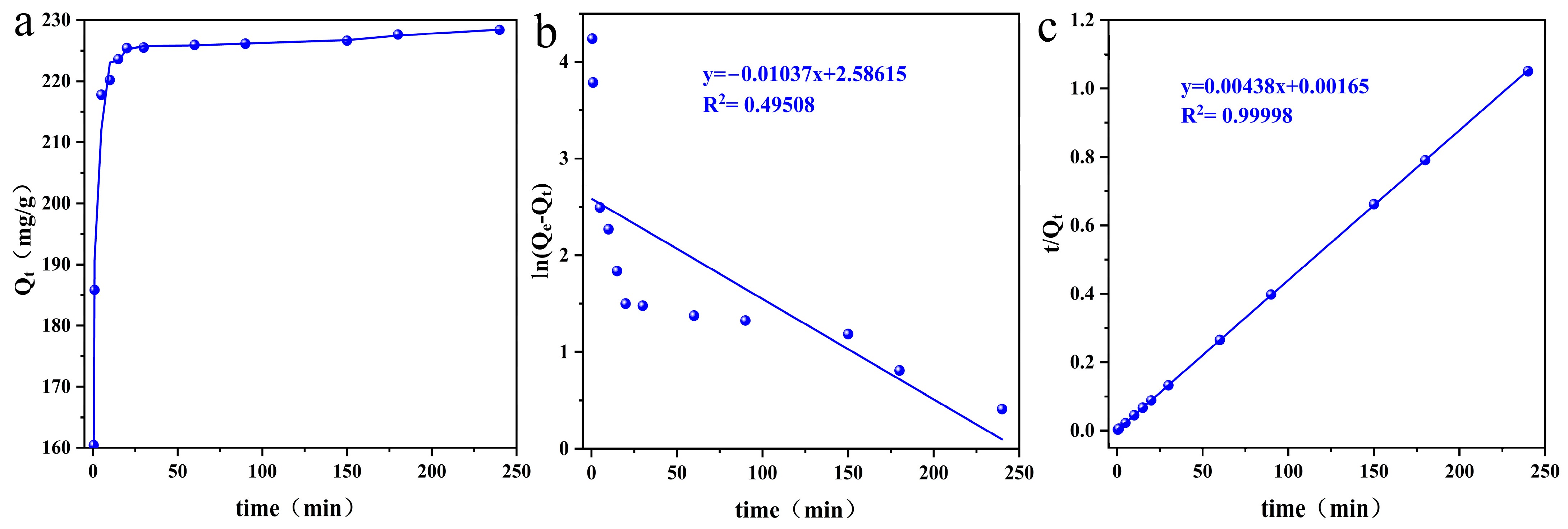
3.2.5. Adsorption Kinetics
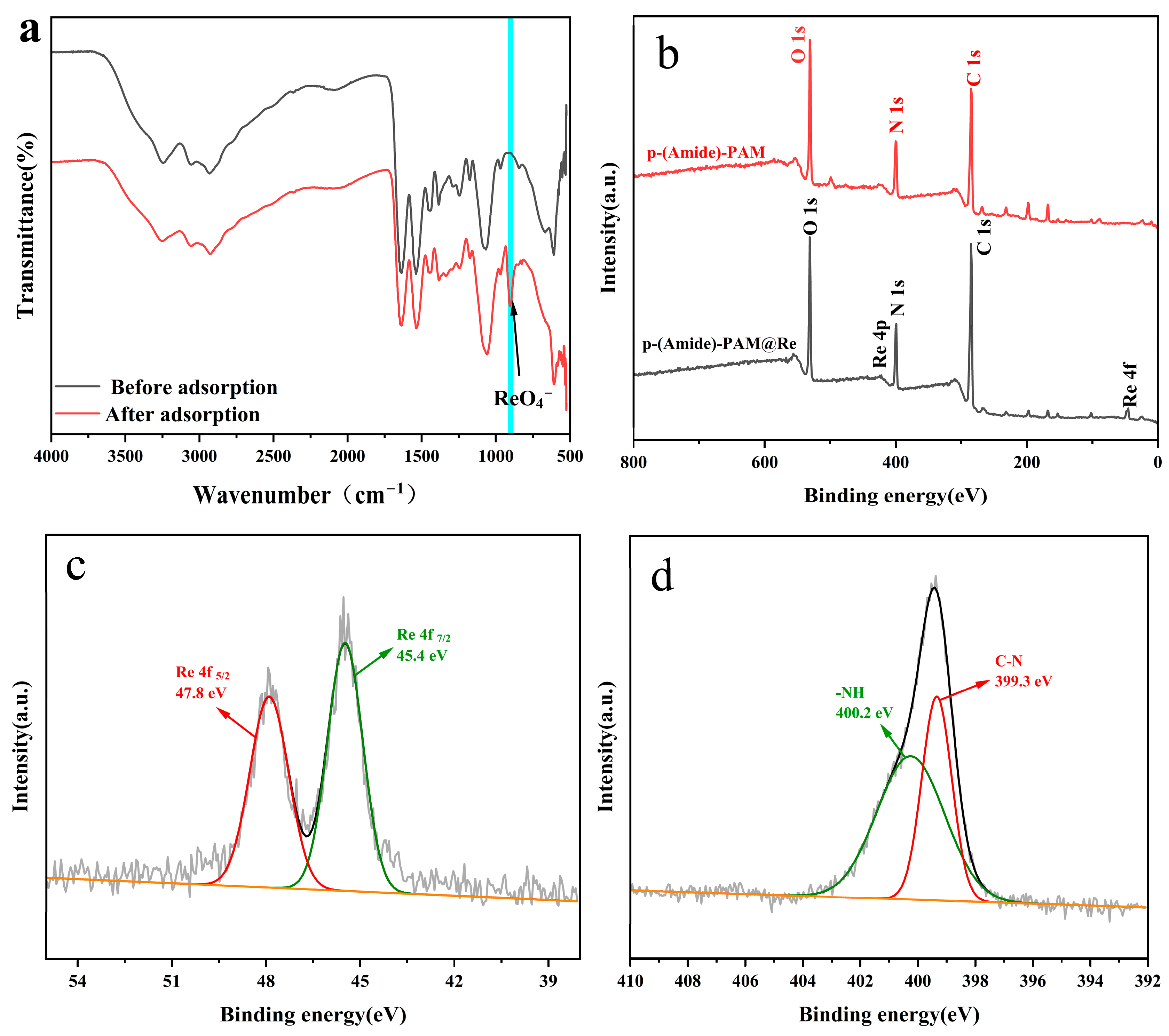
3.3. Sorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, K.; Zhang, F.; Xu, Z.; Feng, J.; Chen, X. Research on the Prospect of Nuclear Power Development in Mid-and-Long Term. IOP Conf. Ser. Earth Environ. Sci. 2020, 546, 022028. [Google Scholar] [CrossRef]
- Zhan, L.; Bo, Y.; Lin, T.; Fan, Z. Development and outlook of advanced nuclear energy technology. Energy Strategy Rev. 2021, 34, 100630. [Google Scholar] [CrossRef]
- Zhang, X.; Ping, G.; Liu, Y. Decontamination of radioactive wastewater: State of the art and challenges forward. Chemosphere 2018, 215, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Gephart, R.E. A short history of waste management at the Hanford Site. Phys. Chem. Earth Parts A/B/C 2010, 35, 298–306. [Google Scholar] [CrossRef]
- Smirnov, I.V.; Karavan, M.D.; Logunov, M.V.; Tananaev, I.G.; Myasoedov, B.F. Extraction of Radionuclides from Alkaline and Carbonate Media. Radiochemistry 2018, 60, 470–487. [Google Scholar] [CrossRef]
- Zhou, X.; Ye, G.-A.; Zhang, H.; Li, L.; Luo, F.; Meng, Z. Chemical behavior of neptunium in the presence of technetium in nitric acid media. Radiochim. Acta 2014, 102, 111–116. [Google Scholar] [CrossRef]
- Lee, M.S.; Um, W.; Wang, G.; Kruger, A.A.; Lukens, W.W.; Rousseau, R.; Glezakou, V.A. Impeding 99Tc(IV) mobility in novel waste forms. Nat. Commun. 2016, 7, 12067. [Google Scholar] [CrossRef]
- Li, J.; Dai, X.; Zhu, L.; Xu, C.; Zhang, D.; Silver, M.A.; Li, P.; Chen, L.; Li, Y.; Zuo, D.; et al. 99TcO4− remediation by a cationic polymeric network. Nat. Commun. 2018, 9, 3007. [Google Scholar] [CrossRef]
- Shu, X.; Xu, Y.; Liu, L.; Fan, Y.; Zhuang, X.; Huang, C.; Chen, S.; Zheng, C.; Jin, Y.; Xia, C. Synthesis of new carbazole derivative extractants and study on extraction of perrhenate/pertechnetate. Polyhedron 2022, 214, 115641. [Google Scholar] [CrossRef]
- Patra, K.; Sengupta, A.; Boda, A.; Ali, M.; Mittal, V.K.; Valsala, T.P.; Kaushik, C.P. Mechanism unravelling for highly efficient and selective 99TcO4− sequestration utilising crown ether based solvent system from nuclear liquid waste: Experimental and computational investigations. RSC Adv. 2022, 12, 3216–3226. [Google Scholar] [CrossRef]
- Wang, X.; Ding, S.; Wang, Z.; Song, L.; Yang, X.; Xiao, Q.; Xu, H.; Wang, J.; Shen, Z.; Wang, H. A H-Bonding and Electrostatic Interaction Combined Strategy for TcO4− Separation by a Nitrotriacetate-Derived Amine–Amide Extractant. Inorg. Chem. 2021, 60, 10899–10908. [Google Scholar] [CrossRef] [PubMed]
- Fiskum, S.K.; Thompson, C.J.; Riley, R.G. Preconcentration and analysis of strontium-90 and technetium-99 from Hanford groundwater using solid phase extraction. J. Radioanal. Nucl. Chem. 2000, 245, 261–272. [Google Scholar] [CrossRef]
- Kameo, Y.; Katayama, A.; Hoshi, A.; Haraga, T.; Nakashima, M. Simple determination of 99Tc in radioactive waste using Tc extraction disk and imaging plates. Appl. Radiat. Isot. 2010, 68, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lan, J.; Yang, X.; Zhong, S.; Yuan, L.; Li, J.; Peng, J.; Chai, Z.; Gibson, J.K.; Zhai, M.; et al. Superhydrophobic Phosphonium Modified Robust 3D Covalent Organic Framework for Preferential Trapping of Charge Dispersed Oxoanionic Pollutants. Adv. Funct. Mater. 2022, 32, 2205222. [Google Scholar] [CrossRef]
- Sheng, D.; Zhu, L.; Xu, C.; Xiao, C.; Wang, Y.; Wang, Y.; Chen, L.; Diwu, J.; Chen, J.; Chai, Z.; et al. Efficient and Selective Uptake of TcO4− by a Cationic Metal-Organic Framework Material with Open Ag+ Sites. Environ. Sci. Technol. 2017, 51, 3471–3479. [Google Scholar] [CrossRef]
- Hao, M.; Chen, Z.; Yang, H.; Waterhouse, G.I.N.; Ma, S.; Wang, X. Pyridinium salt-based covalent organic framework with well-defined nanochannels for efficient and selective capture of aqueous 99TcO4−. Sci. Bull. 2022, 67, 924–932. [Google Scholar] [CrossRef]
- Sheng, D.; Zhu, L.; Dai, X.; Xu, C.; Li, P.; Pearce, C.I.; Xiao, C.; Chen, J.; Zhou, R.; Duan, T.; et al. Successful Decontamination of 99TcO4 − in Groundwater at Legacy Nuclear Sites by a Cationic Metal-Organic Framework with Hydrophobic Pockets. Angew. Chem. Int. Ed. Engl. 2019, 58, 4968–4972. [Google Scholar] [CrossRef]
- Kang, K.; Shen, N.; Wang, Y.; Li, L.; Zhang, M.; Zhang, X.; Lei, L.; Miao, X.; Wang, S.; Xiao, C. Efficient sequestration of radioactive 99TcO4− by a rare 3-fold interlocking cationic metal-organic framework: A combined batch experiments, pair distribution function, and crystallographic investigation. Chem. Eng. J. 2022, 427, 130942. [Google Scholar] [CrossRef]
- Kang, K.; Liu, S.; Zhang, M.; Li, L.; Liu, C.; Lei, L.; Dai, X.; Xu, C.; Xiao, C. Fast Room-Temperature Synthesis of an Extremely Alkaline-Resistant Cationic Metal-Organic Framework for Sequestering TcO4− with Exceptional Selectivity. Adv. Funct. Mater. 2022, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Xiao, C.; Chen, L.; Chai, Z.; Wang, S. Efficient uptake of perrhenate/pertechnenate from aqueous solutions by the bifunctional anion-exchange resin. Radiochim. Acta 2018, 106, 581–591. [Google Scholar] [CrossRef]
- Fu, L.; Zu, J.; He, L.; Gu, E.; Wang, H. An adsorption study of 99Tc using nanoscale zero-valent iron supported on D001 resin. Front. Energy 2019, 14, 11–17. [Google Scholar] [CrossRef]
- Milićević, S.; Matović, L.; Petrović, Đ.; Đukić, A.; Milošević, V.; Đokić, D.; Kumrić, K. Surfactant modification and adsorption properties of clinoptilolite for the removal of pertechnetate from aqueous solutions. J. Radioanal. Nucl. Chem. 2016, 310, 805–815. [Google Scholar] [CrossRef]
- Dickson, J.; Conroy, N.A.; Xie, Y.; Powell, B.A.; Seaman, J.C.; Boyanov, M.I.; Kemner, K.M.; Kaplan, D.I. Surfactant-modified siliceous zeolite Y for pertechnetate remediation. Chem. Eng. J. 2020, 402, 126268. [Google Scholar] [CrossRef]
- Xiong, Y.; Song, Y.; Tong, Q.; Zhang, P.; Wang, Y.; Lou, Z.; Zhang, F.; Shan, W. Adsorption-controlled preparation of anionic imprinted amino-functionalization chitosan for recognizing rhenium(VII). Sep. Purif. Technol. 2017, 177, 142–151. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, F.; Lu, C.; Du, C.; Bai, R.; Zeng, X.; Zhao, Z.; Cai, C.; Li, J. Effective decontamination of 99TcO4−/ReO4− from Hanford low-activity waste by functionalized graphene oxide-chitosan sponges. Environ. Chem. Lett. 2020, 18, 1379–1388. [Google Scholar] [CrossRef]
- Banerjee, D.; Kim, D.; Schweiger, M.J.; Kruger, A.A.; Thallapally, P.K. Removal of TcO4− ions from solution: Materials and future outlook. Chem. Soc. Rev. 2016, 45, 2724–2739. [Google Scholar] [CrossRef]
- Hu, Q.-H.; Jiang, W.; Liang, R.-P.; Lin, S.; Qiu, J.-D. Synthesis of imidazolium-based cationic organic polymer for highly efficient and selective removal of ReO4−/TcO4−. Chem. Eng. J. 2021, 419, 129546. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, M.; Ding, J.; Kang, J.; Yan, Z.; Zhao, P.; Zhou, X.; Jin, Y.; Chen, S.; Xia, C. Targeted synthesis of a high-stability cationic porous aromatic framework for highly efficient remediation of 99TcO4−. Chem. Eng. J. 2022, 435, 134785. [Google Scholar] [CrossRef]
- Han, D.; Li, X.; Cui, Y.; Yang, X.; Chen, X.; Xu, L.; Peng, J.; Li, J.; Zhai, M. Polymeric ionic liquid gels composed of hydrophilic and hydrophobic units for high adsorption selectivity of perrhenate. RSC Adv. 2018, 8, 9311–9319. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Shen, N.; Chen, L.; Guo, Q.; Chen, L.; He, L.; Dai, X.; Chai, Z.; Wang, S. Task-Specific Tailored Cationic Polymeric Network with High Base-Resistance for Unprecedented 99TcO4− Cleanup from Alkaline Nuclear Waste. ACS Cent. Sci. 2021, 7, 1441–1450. [Google Scholar] [CrossRef]
- Sarri, S.; Misaelides, P.; Zamboulis, D.; Gaona, X.; Altmaier, M.; Geckeis, H. Rhenium(VII) and technetium(VII) separation from aqueous solutions using a polyethylenimine-epichlorohydrin resin. J. Radioanal. Nucl. Chem. 2015, 307, 681–689. [Google Scholar] [CrossRef]
- Xiong, Y.; Cui, X.; Zhang, P.; Wang, Y.; Lou, Z.; Shan, W. Improving Re(VII) Adsorption on Diisobutylamine-Functionalized Graphene Oxide. ACS Sustain. Chem. Eng. 2016, 5, 1010–1018. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, S.; Qi, F.; Huang, A. Highly stable cellulose nanofiber/polyacrylamide aerogel via in-situ physical/chemical double crosslinking for highly efficient Cu(II) ions removal. Int. J. Biol. Macromol. 2022, 209, 1922–1932. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. Npj Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Tuzikov, A.; Chinarev, A.; Shilova, N.; Gordeeva, E.; Galanina, O.; Ovchinnikova, T.; Schaefer, M.; Bovin, N. 40 years of glyco-polyacrylamide in glycobiology. Glycoconj. J. 2021, 38, 89–100. [Google Scholar] [CrossRef]
- Farrell, D.; Gloe, K.; Gloe, K.; Goretzki, G.; Mckee, V.; Nelson, J.; Nieuwenhuyzen, M.; Pál, I.; Stephan, H.; Town, R.M.; et al. Towards promising oxoanion extractants: Azacages and open-chain counterparts. Dalton Trans. 2003, 2003, 1961–1968. [Google Scholar] [CrossRef]
- Tamami, B.; Fadavi, A. Amino group immobilized on polyacrylamide: An efficient heterogeneous catalyst for the Knoevenagel reaction in solvent-free and aqueous media. Catal. Commun. 2005, 6, 747–751. [Google Scholar] [CrossRef]
- Zare, M.A.; Husain, S.W.; Tehrani, M.S.; Azar, P.A. Pentaazatetraethylene supported polyacrylamide (PAA-N5) as a novel adsorbent for the efficient removal of industrial dyes from aqueous solutions: Adsorption isotherms and kinetics. Mon. Fur Chem. Chem. Mon. 2016, 148, 191–197. [Google Scholar] [CrossRef]
- Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Development of Novel Extractants with Amino Acid Structure for Efficient Separation of Nickel and Cobalt from Manganese Ions. Ind. Eng. Chem. Res. 2014, 53, 812–818. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Jiao, Z.; Zhan, W.; Ge, S.; Ning, S.; Fang, S.; Ruan, X. Simultaneous Removal of Cu2+, Cd2+ and Pb2+ by Modified Wheat Straw Biochar from Aqueous Solution: Preparation, Characterization and Adsorption Mechanism. Toxics 2022, 10, 316. [Google Scholar] [CrossRef]
- Lin, W.; Zhao, Z.; Yang, F.; Liu, Z.; Tan, F.; Xie, M.; Ma, Y.; Meng, L. Promising priority separation of europium from lanthanide by novel DGA-functionalized metal organic frameworks. Miner. Eng. 2021, 164, 106831. [Google Scholar] [CrossRef]
- Wen, D.; Hua, R.; Dong, Z.; Xie, K.; Qi, W.; Zhao, L. Efficient separation and recovery of Re(VII) from Re/U bearing acidic solutions using aminotriazole modified cellulose microsphere adsorbents. Environ. Sci. Pollut. Res. Int. 2021, 28, 52225–52235. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Ji, J.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Primary and tertiary amines bifunctional graphene oxide for cooperative catalysis. Nanoscale 2013, 5, 6030–6033. [Google Scholar] [CrossRef]
- Ding, M.; Chen, L.; Xu, Y.; Chen, B.; Ding, J.; Wu, R.; Huang, C.; He, Y.; Jin, Y.; Xia, C. Efficient capture of Tc/Re(VII, IV) by a viologen-based organic polymer containing tetraaza macrocycles. Chem. Eng. J. 2020, 380, 122581. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Wu, F.; Yang, S.; Dong, F.; Zhi, X.; Chen, X.; Tian, G.; Shen, Y. A Novel Functionalized Ionic Liquid for Highly Selective Extraction of TcO4−. Inorg. Chem. 2022, 61, 10609–10617. [Google Scholar] [CrossRef]
- Zhu, L.; Sheng, D.; Xu, C.; Dai, X.; Silver, M.A.; Li, J.; Li, P.; Wang, Y.; Wang, Y.; Chen, L.; et al. Identifying the Recognition Site for Selective Trapping of 99TcO4− in a Hydrolytically Stable and Radiation Resistant Cationic Metal-Organic Framework. J. Am. Chem. Soc. 2017, 139, 14873–14876. [Google Scholar] [CrossRef]
- Shen, N.; Yang, Z.; Liu, S.; Dai, X.; Xiao, C.; Taylor-Pashow, K.; Li, D.; Yang, C.; Li, J.; Zhang, Y.; et al. 99TcO4− removal from legacy defense nuclear waste by an alkaline-stable 2D cationic metal organic framework. Nat. Commun. 2020, 11, 5571. [Google Scholar] [CrossRef]
- Li, W.; Dong, X.; Zhu, L.; Tang, H. Highly selective separation of Re(VII) from Mo(VI) by using biomaterial-based ionic gel adsorbents: Extractive adsorption enrichment of Re and surface blocking of Mo. Chem. Eng. J. 2020, 387, 124078. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Xu, Y. Adsorption of Pb(II) and Cr(VI) from Aqueous Solution by Synthetic Allophane Suspension: Isotherm, Kinetics, and Mechanisms. Toxics 2022, 10, 291. [Google Scholar] [CrossRef]
| Elemental | PAM | N-PAM | p-(Amide)-PAM |
|---|---|---|---|
| C [%] | 45.140 | 42.667 | 39.926 |
| H [%] | 7.580 | 8.670 | 7.559 |
| O [%] | 30.577 | 29.696 | 36.562 |
| N [%] | 16.517 | 18.994 | 15.508 |
| Models | Parameters | p-(Amide)-PAM |
|---|---|---|
| Langmuir | KL | 0.0247 |
| Qmax (mg/g) | 346.02 | |
| R2 | 0.99452 | |
| Freundlich | KF | 25.319 |
| n | 2.28 | |
| R2 | 0.95520 |
| Adsorbent | Adsorption Capacity (mg/g) | References |
|---|---|---|
| p-(Amide)-PAM | 346.02 | This work |
| GO-DEADIBA | 140.82 | [32] |
| SCU-100 | 541 | [15] |
| SCU-101 | 217 | [46] |
| SCU-102 | 291 | [17] |
| SCU-103 | 318 | [47] |
| Ag-TPPE | 251 | [19] |
| ZJU-X6 | 507 | [18] |
| 3-ATAR | 146.4 | [42] |
| DNOA–GO–CS | 90.33 | [25] |
| CSN | 222 | [48] |
| SCU-CPN-4 | 437 | [30] |
| Models | Parameters | p-(Amide)-PAM |
|---|---|---|
| Pseudo-first-order | k1 | 0.01037 |
| Qe1(mg/g) | 13.279 | |
| R2 | 0.49508 | |
| Pseudo-second-order | k2 | 0.0116 |
| Qe2(mg/g) | 228.311 | |
| R2 | 0.99998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, B.; Hu, Y.; Xie, M.; Xue, L.; Liao, C.; Yang, F. Highly Selective Adsorption of 99TcO4−/ReO4− by a Novel Polyamide-Functionalized Polyacrylamide Polymer Material. Toxics 2022, 10, 630. https://doi.org/10.3390/toxics10100630
Qin B, Hu Y, Xie M, Xue L, Liao C, Yang F. Highly Selective Adsorption of 99TcO4−/ReO4− by a Novel Polyamide-Functionalized Polyacrylamide Polymer Material. Toxics. 2022; 10(10):630. https://doi.org/10.3390/toxics10100630
Chicago/Turabian StyleQin, Ben, Yanqin Hu, Meiying Xie, Liyan Xue, Chunfa Liao, and Fan Yang. 2022. "Highly Selective Adsorption of 99TcO4−/ReO4− by a Novel Polyamide-Functionalized Polyacrylamide Polymer Material" Toxics 10, no. 10: 630. https://doi.org/10.3390/toxics10100630
APA StyleQin, B., Hu, Y., Xie, M., Xue, L., Liao, C., & Yang, F. (2022). Highly Selective Adsorption of 99TcO4−/ReO4− by a Novel Polyamide-Functionalized Polyacrylamide Polymer Material. Toxics, 10(10), 630. https://doi.org/10.3390/toxics10100630








