Plant Resistance to Fungal Pathogens: Bibliometric Analysis and Visualization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
3. Results
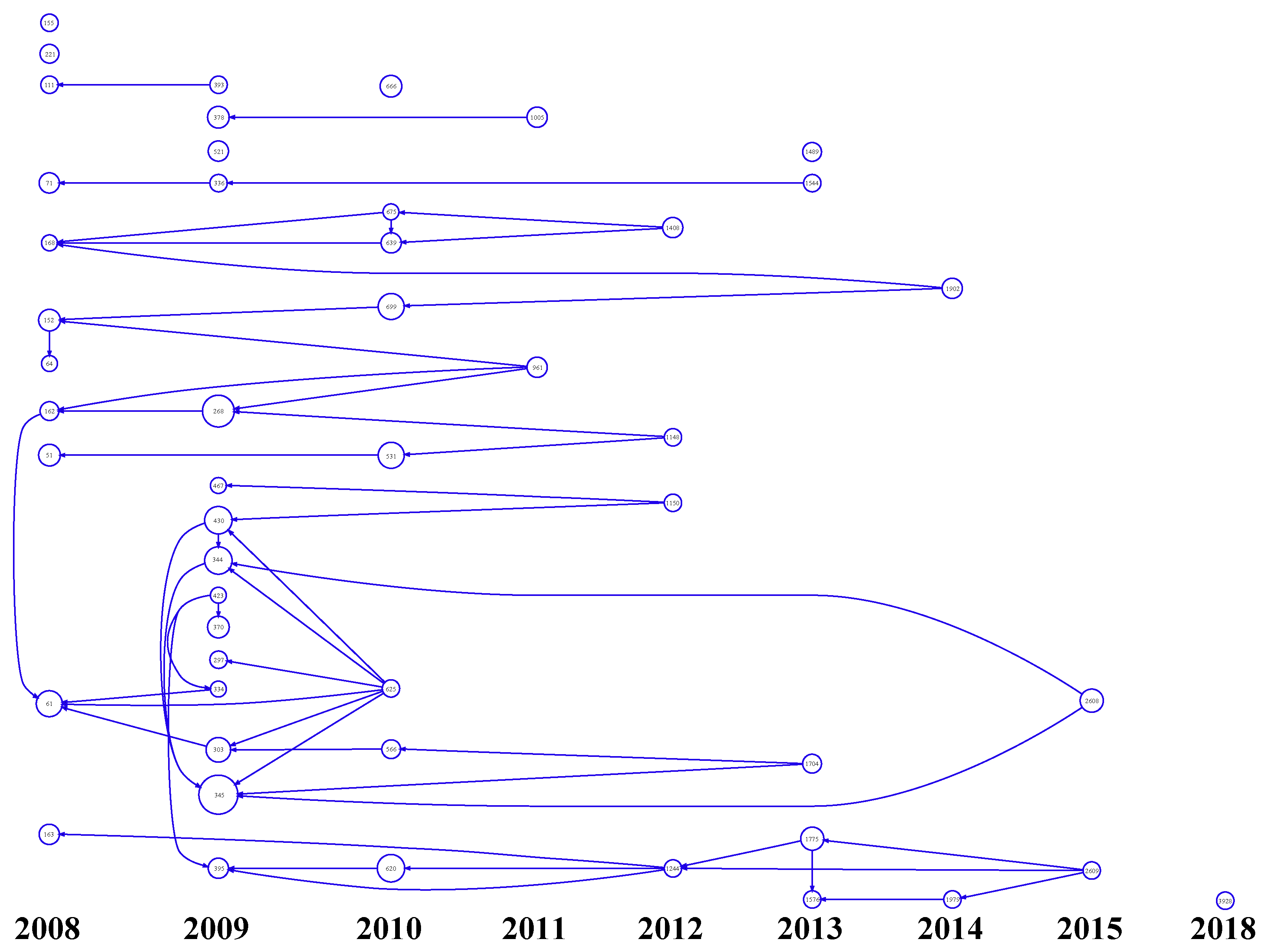
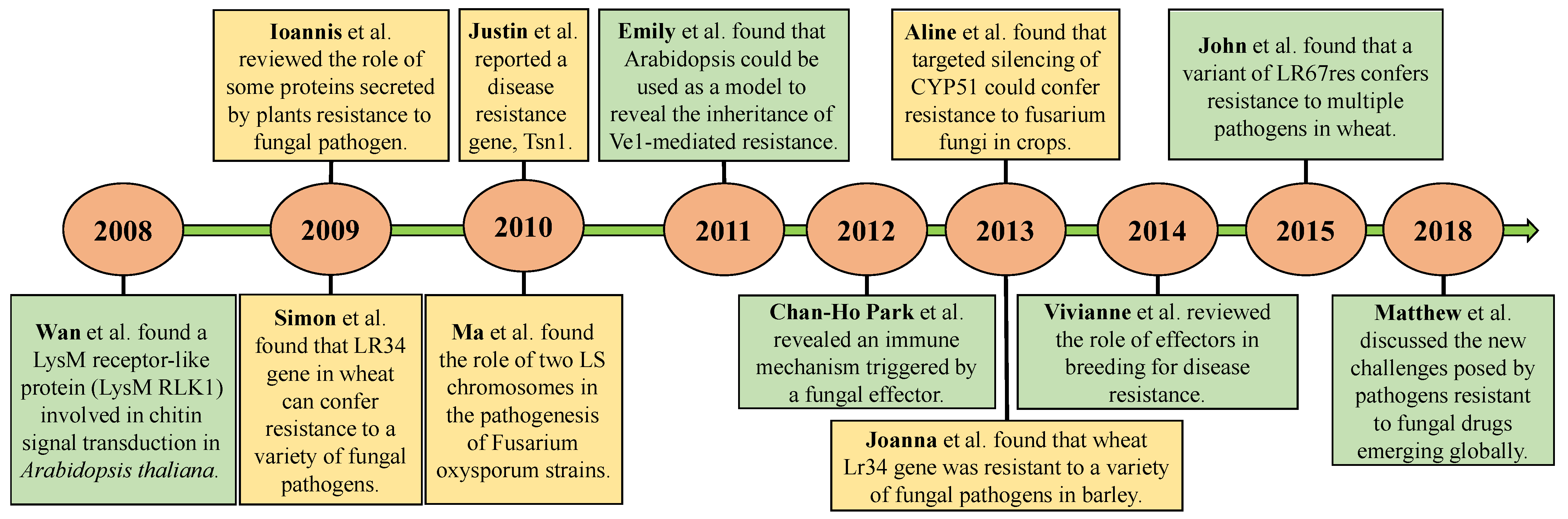
3.1. Citation Analysis
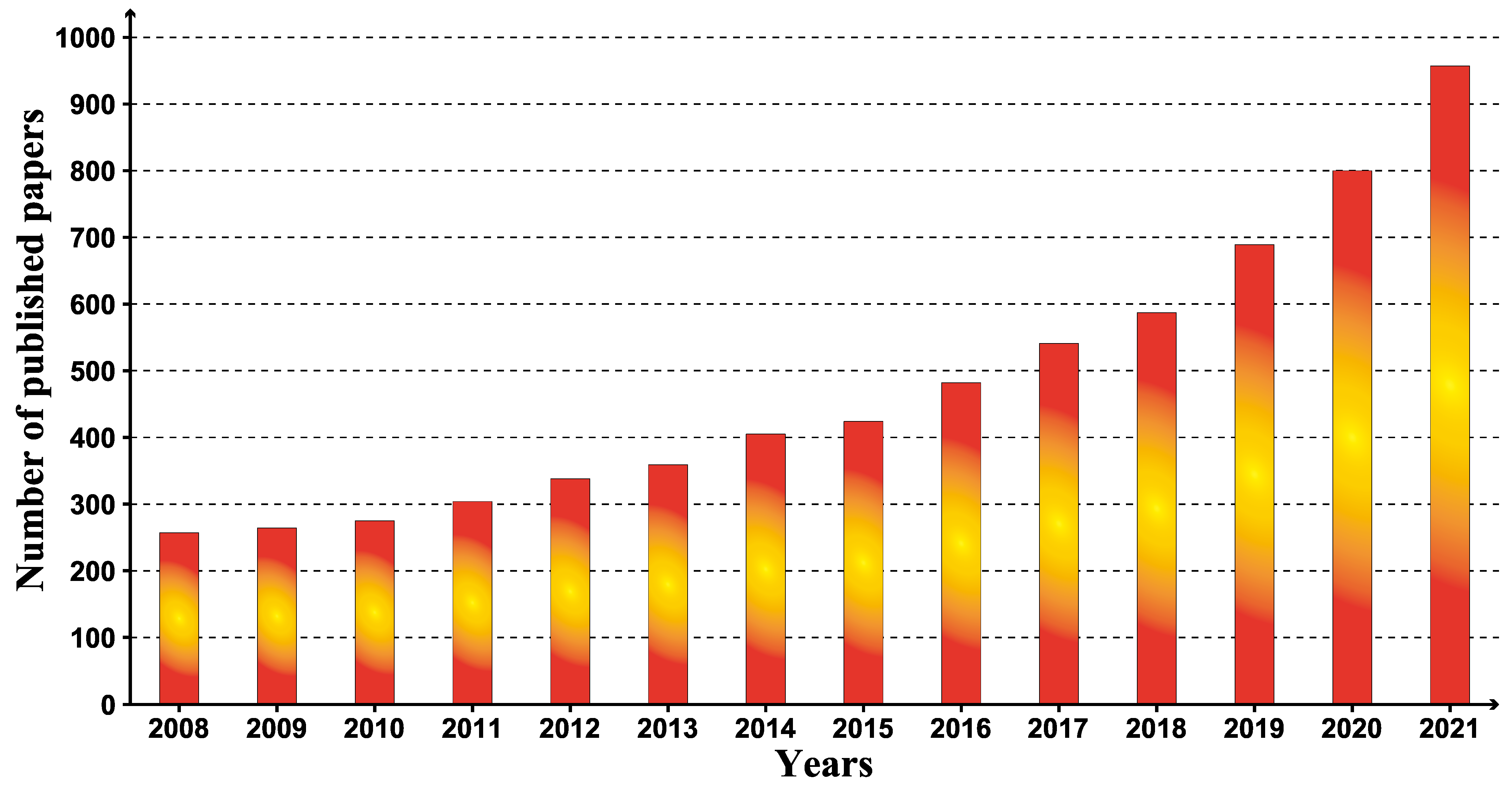
3.2. Annual Research Analysis
3.3. Research Analysis
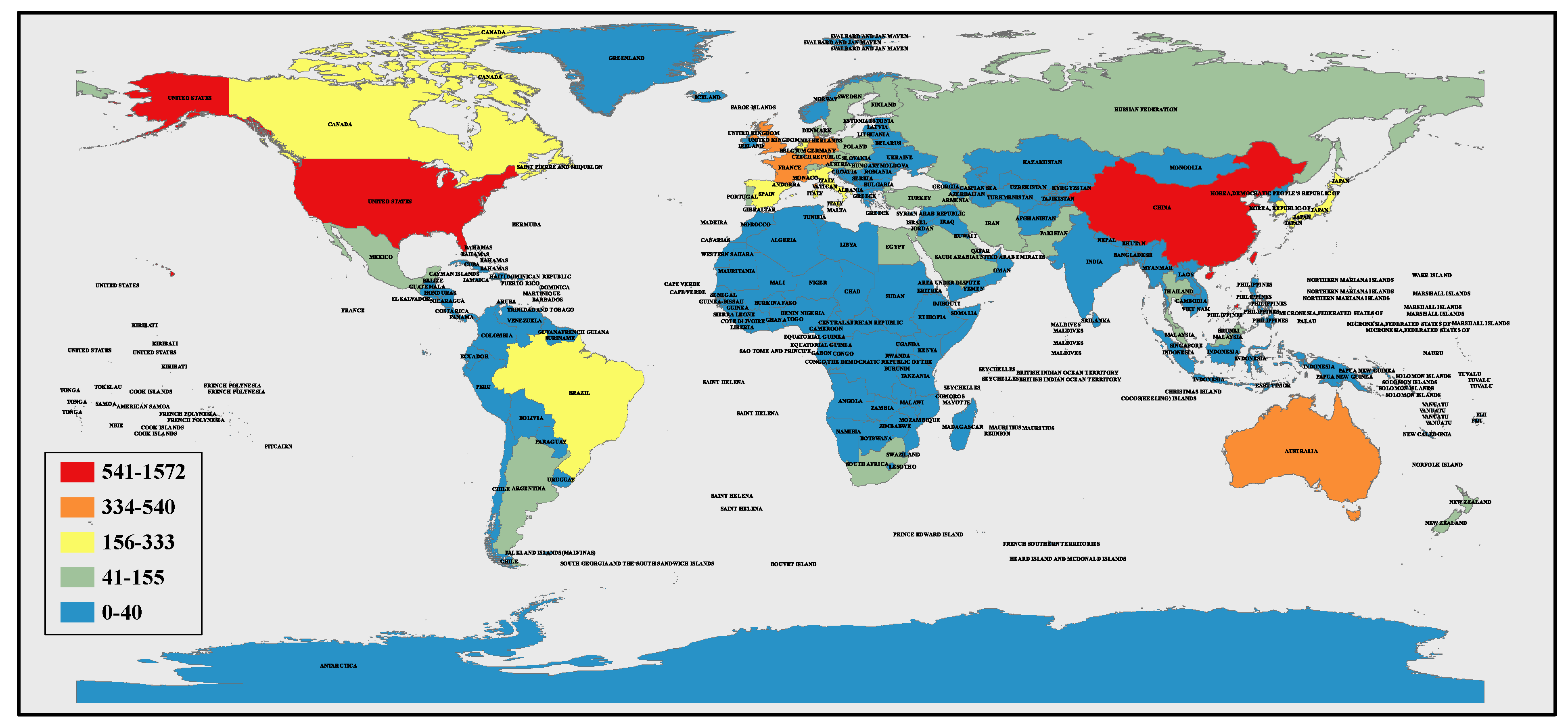
3.3.1. Countries Assessment
3.3.2. Country Collaboration
3.3.3. Institutions Analysis
3.3.4. Author Analysis
3.4. Journal Analysis
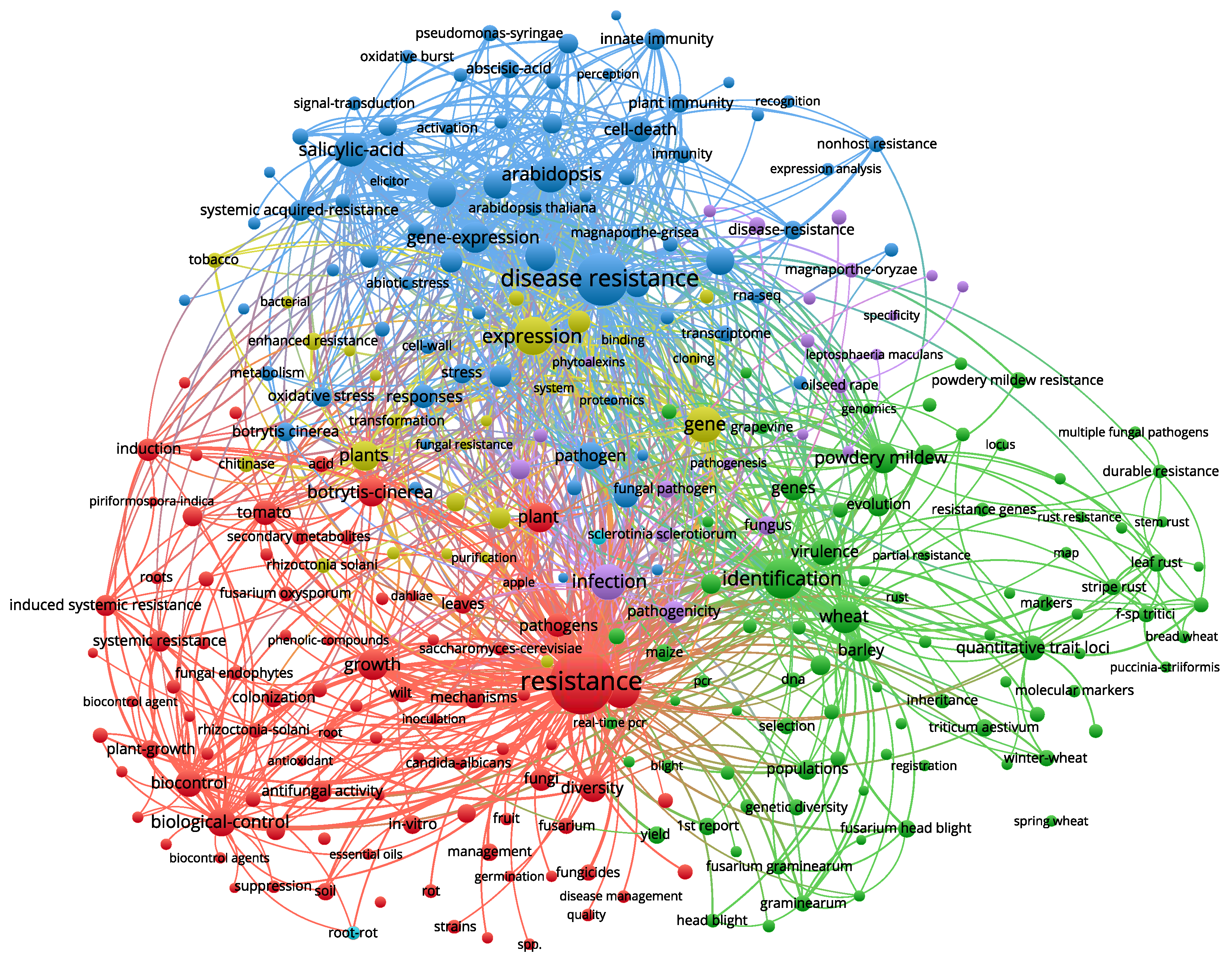
3.5. Keyword Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hollomon, D.W.; Brent, K.J. Combating plant diseases—The Darwin connection. Pest Manag. Sci. 2009, 65, 1156–1163. [Google Scholar] [CrossRef]
- Babu, B.; Kefialew, Y.W.; Li, P.F.; Yang, X.P.; George, S.; Newberry, E.; Dufault, N.; Abate, D.; Ayalew, A.; Marois, J.; et al. Genetic Characterization of Didymella bryoniae Isolates Infecting Watermelon and Other Cucurbits in Florida and Georgia. Plant Dis. 2015, 99, 1488–1499. [Google Scholar] [CrossRef] [Green Version]
- de Gruyter, J.; Woudenberg, J.H.; Aveskamp, M.M.; Verkley, G.J.; Groenewald, J.Z.; Crous, P.W. Redisposition of phoma-like anamorphs in Pleosporales. Stud. Mycol. 2013, 75, 1–36. [Google Scholar] [CrossRef] [Green Version]
- França-Silva, F.; Rego, C.; Gomes-Junior, F.G.; Moraes, M.; Medeiros, A.D.; Silva, C. Detection of Drechslera avenae (Eidam) Sharif [Helminthosporium avenae (Eidam)] in Black Oat Seeds (Avena strigosa Schreb) Using Multispectral Imaging. Sensors 2020, 20, 3343. [Google Scholar] [CrossRef]
- Shi, Y.L.; Sheng, Y.Y.; Cai, Z.Y.; Yang, R.; Li, Q.S.; Li, X.M.; Li, D.; Guo, X.Y.; Lu, J.L.; Ye, J.H.; et al. Involvement of Salicylic Acid in Anthracnose Infection in Tea Plants Revealed by Transcriptome Profiling. Int. J. Mol. Sci. 2019, 20, 2439. [Google Scholar] [CrossRef] [Green Version]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Zhang, B.; Shao, L.; Wang, J.; Zhang, Y.; Guo, X.; Peng, Y.; Cao, Y.; Lai, Z. Phosphorylation of ATG18a by BAK1 suppresses autophagy and attenuates plant resistance against necrotrophic pathogens. Autophagy 2021, 17, 2093–2110. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ahammed, G.J.; Wu, C.; Fan, S.Y.; Zhou, Y.H. Crosstalk among Jasmonate, Salicylate and Ethylene Signaling Pathways in Plant Disease and Immune Responses. Curr. Protein Pept. Sci. 2015, 16, 450–461. [Google Scholar] [CrossRef]
- Osbourn, A.E. Antimicrobial phytoprotectants and fungal pathogens: A commentary. Fungal Genet. Biol. 1999, 26, 163–168. [Google Scholar] [CrossRef]
- Perez-Nadales, E.; Nogueira, M.F.; Baldin, C.; Castanheira, S.; El, G.M.; Grund, E.; Lengeler, K.; Marchegiani, E.; Mehrotra, P.V.; Moretti, M.; et al. Fungal model systems and the elucidation of pathogenicity determinants. Fungal Genet. Biol. 2014, 70, 42–67. [Google Scholar] [CrossRef]
- Kroon, L.P.; Brouwer, H.; de Cock, A.W.; Govers, F. The genus Phytophthora anno 2012. Phytopathology 2012, 102, 348–364. [Google Scholar] [CrossRef] [Green Version]
- Pautasso, M.; Aas, G.; Queloz, V.; Holdenrieder, O. European ash (Fraxinus excelsior) dieback—A conservation biology challenge. Biol. Conserv. 2013, 158, 37–49. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Ninkov, A.; Frank, J.R.; Maggio, L.A. Bibliometrics: Methods for studying academic publishing. Perspect. Med. Educ. 2022, 11, 173–176. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-Espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef] [Green Version]
- Stergiopoulos, I.; de Wit, P.J. Fungal effector proteins. Annu. Rev. Phytopathol. 2009, 47, 233–263. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Zhang, X.C.; Neece, D.; Ramonell, K.M.; Clough, S.; Kim, S.Y.; Stacey, M.G.; Stacey, G. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 2008, 20, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Fradin, E.F.; Abd-El-Haliem, A.; Masini, L.; van den Berg, G.C.; Joosten, M.H.; Thomma, B.P. Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol. 2011, 156, 2255–2265. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Faris, J.D.; Zhang, Z.; Lu, H.; Lu, S.; Reddy, L.; Cloutier, S.; Fellers, J.P.; Meinhardt, S.W.; Rasmussen, J.B.; Xu, S.S.; et al. A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proc. Natl. Acad. Sci. USA 2010, 107, 13544–13549. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Chen, S.; Shirsekar, G.; Zhou, B.; Khang, C.H.; Songkumarn, P.; Afzal, A.J.; Ning, Y.; Wang, R.; Bellizzi, M.; et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell 2012, 24, 4748–4762. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.; Kumar, N.; Weber, L.; Keller, H.; Imani, J.; Kogel, K.H. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase-encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA 2013, 110, 19324–19329. [Google Scholar] [CrossRef] [Green Version]
- Risk, J.M.; Selter, L.L.; Chauhan, H.; Krattinger, S.G.; Kumlehn, J.; Hensel, G.; Viccars, L.A.; Richardson, T.M.; Buesing, G.; Troller, A.; et al. The wheat Lr34 gene provides resistance against multiple fungal pathogens in barley. Plant Biotechnol. J. 2013, 11, 847–854. [Google Scholar] [CrossRef]
- Vleeshouwers, V.; Oliver, R. Effectors as Tools in Disease Resistance Breeding Against Biotrophic, Hemibiotrophic, and Necrotrophic Plant Pathogens. Mol. Plant-Microbe Interact. MPMI 2014, 27. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.W.; Herrera-Foessel, S.; Lan, C.; Schnippenkoetter, W.; Ayliffe, M.; Huerta-Espino, J.; Lillemo, M.; Viccars, L.; Milne, R.; Periyannan, S.; et al. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015, 47, 1494–1498. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Zaharia, I.L.; Gai, Y.; Smith, K.C.; Ward, D.E. Metabolism of the Host-Selective Toxins Destruxin B and Homodestruxin B: Probing a Plant Disease Resistance Trait. Org. Lett. 1999, 1, 1655–1658. [Google Scholar] [CrossRef]
- Pedras, M.S.; Chumala, P.B.; Suchy, M. Phytoalexins from Thlaspi arvense, a wild crucifer resistant to virulent Leptosphaeria maculans: Structures, syntheses and antifungal activity. Phytochemistry 2003, 64, 949–956. [Google Scholar] [CrossRef]
- Kwon, C.; Neu, C.; Pajonk, S.; Yun, H.S.; Lipka, U.; Humphry, M.; Bau, S.; Straus, M.; Kwaaitaal, M.; Rampelt, H.; et al. Co-option of a default secretory pathway for plant immune responses. Nature 2008, 451, 835–840. [Google Scholar] [CrossRef]
- Fukuoka, S.; Saka, N.; Koga, H.; Ono, K.; Shimizu, T.; Ebana, K.; Hayashi, N.; Takahashi, A.; Hirochika, H.; Okuno, K.; et al. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 2009, 325, 998–1001. [Google Scholar] [CrossRef]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; et al. Metabolic priming by a secreted fungal effector. Nature 2011, 478, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Wang, M.; Weiberg, A.; Lin, F.M.; Thomma, B.P.; Huang, H.D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bai, Y.; Wu, G.; Zou, S.; Chen, Y.; Gao, C.; Tang, D. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 2017, 91, 714–724. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Luo, M.; Xie, L.; Chakraborty, S.; Wang, A.; Matny, O.; Jugovich, M.; Kolmer, J.A.; Richardson, T.; Bhatt, D.; Hoque, M.; et al. A five-transgene cassette confers broad-spectrum resistance to a fungal rust pathogen in wheat. Nat. Biotechnol. 2021, 39, 561–566. [Google Scholar] [CrossRef]
- Chen, J.J.; Bai, W.; Lu, Y.B.; Feng, Z.Y.; Gao, K.; Yue, J.M. Quassinoids with Inhibitory Activities against Plant Fungal Pathogens from Picrasma javanica. J. Nat. Prod. 2021, 84, 2111–2120. [Google Scholar] [CrossRef]

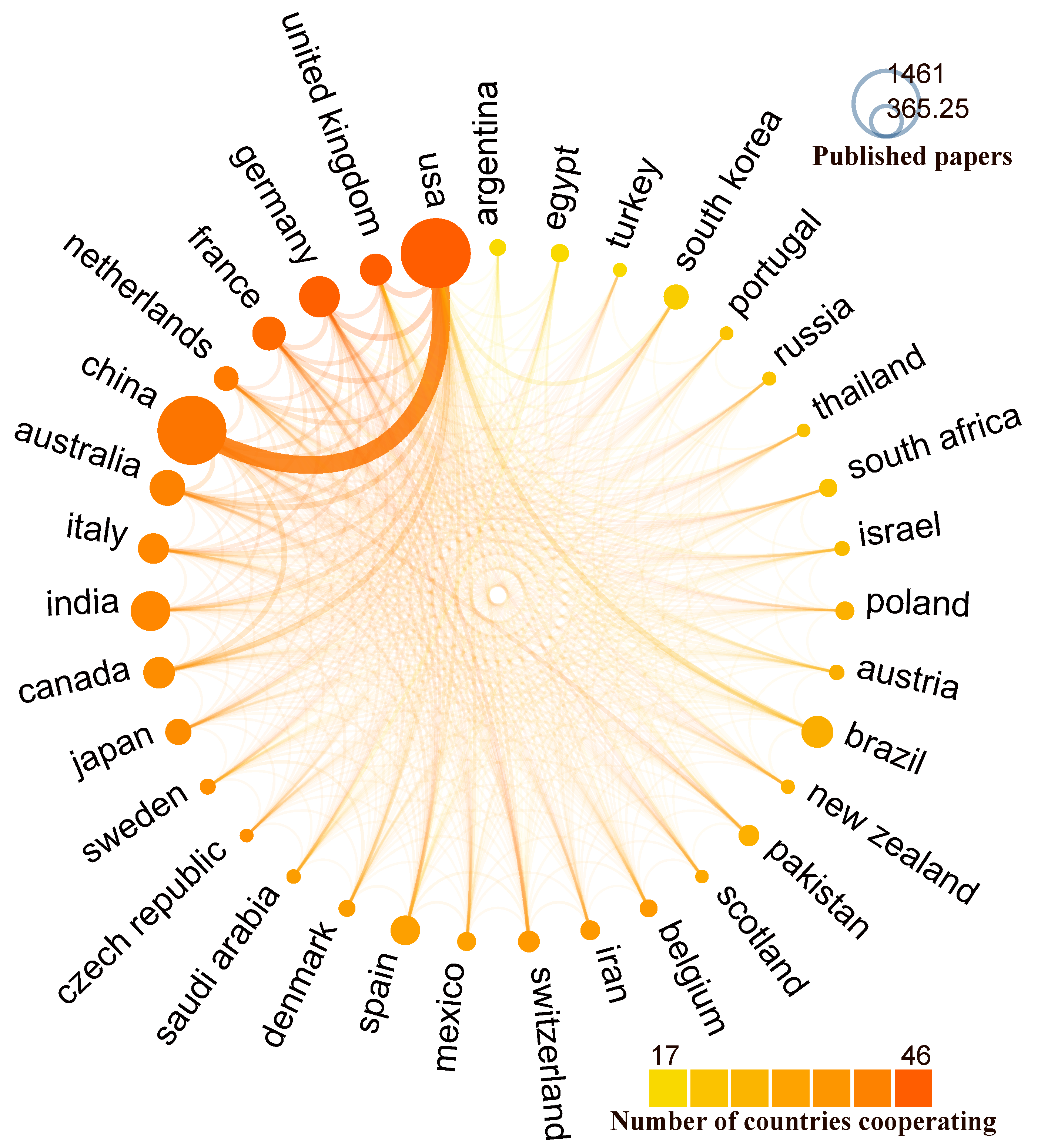



| Country | Documents | Proportion (%) | TLCS | TGCS |
|---|---|---|---|---|
| USA | 1571 | 23.493 | 4263 | 56,964 |
| China | 1516 | 22.671 | 2749 | 34,830 |
| Germany | 541 | 8.090 | 1849 | 21,892 |
| India | 517 | 7.731 | 852 | 12,487 |
| Australia | 421 | 6.296 | 2093 | 17,568 |
| UK | 374 | 5.593 | 1346 | 18,974 |
| France | 372 | 5.563 | 1128 | 14,456 |
| Canada | 334 | 4.995 | 811 | 10,901 |
| Brazil | 315 | 4.711 | 339 | 6714 |
| Italy | 290 | 4.337 | 698 | 8957 |
| Institution | Country | Documents | Proportion (%) | TLCS | TGCS |
|---|---|---|---|---|---|
| USDA | USA | 251 | 3.754 | 723 | 8349 |
| Chinese Acad Agr Sci | China | 231 | 3.454 | 544 | 5573 |
| ARS | USA | 149 | 2.228 | 464 | 5007 |
| Chinese Acad Sci | China | 140 | 2.094 | 328 | 5033 |
| Nanjing Agr Univ | China | 120 | 1.795 | 194 | 2915 |
| Northwest A&F Univ | China | 115 | 1.720 | 125 | 2154 |
| Agr and Agri Food Canada | Canada | 113 | 1.690 | 420 | 4614 |
| Univ Calif Davis | USA | 113 | 1.690 | 381 | 5469 |
| Huazhong Agr Univ | China | 110 | 1.645 | 381 | 4165 |
| China Agr Univ | China | 100 | 1.495 | 215 | 2674 |
| Journal | Country | Documents | Citations | IF (2021) |
|---|---|---|---|---|
| Frontiers in Plant Science | Switzerland | 300 | 6146 | 5.75 |
| European Journal of Plant Pathology | Netherlands | 205 | 3577 | 1.91 |
| Plant Disease | USA | 175 | 2018 | 4.44 |
| Plos One | USA | 164 | 5024 | 3.24 |
| Molecular Plant Pathology | Britain | 144 | 5381 | 5.66 |
| Phytopathology | USA | 143 | 2780 | 4.03 |
| Plant Pathology | Britain | 137 | 2390 | 5.59 |
| Molecular Plant-microbe Interactions | USA | 127 | 5101 | 4.17 |
| Scientific Reports | Britain | 116 | 2571 | 4.38 |
| Theoretical and Applied Genetics | Germany | 110 | 2883 | 5.70 |
| NO. | Keyword | Occurrences | NO. | Keyword | Occurrences |
|---|---|---|---|---|---|
| 1 | Resistance | 1888 | 11 | Defense | 416 |
| 2 | Disease Resistance | 1308 | 12 | Gene-expression | 405 |
| 3 | Identification | 724 | 13 | Growth | 388 |
| 4 | Expression | 637 | 14 | Plants | 371 |
| 5 | Disease | 547 | 15 | Powdery-mildew | 370 |
| 6 | Arabidopsis | 538 | 16 | Plant | 363 |
| 7 | Gene | 537 | 17 | Biological-control | 349 |
| 8 | Infection | 533 | 18 | Defense-Responses | 338 |
| 9 | Salicylic-acid | 480 | 19 | Arabidopsis-thaliana | 336 |
| 10 | Wheat | 430 | 20 | Botrytis-cinerea | 329 |
| Outbreak Word | Year | Score | Start | End | 2021–2003 |
|---|---|---|---|---|---|
| Magnaporthe grisea | 2003 | 15.70 | 2008 | 2012 | ▂▂▂▂▂▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Systemic acquired resistance | 2003 | 12.88 | 2008 | 2012 | ▂▂▂▂▂▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Induction | 2003 | 9.83 | 2008 | 2011 | ▂▂▂▂▂▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Salicylic acid | 2003 | 8.93 | 2008 | 2009 | ▂▂▂▂▂▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Chitinase | 2003 | 8.23 | 2008 | 2014 | ▂▂▂▂▂▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂ |
| Hypersensitive response | 2003 | 12.41 | 2009 | 2014 | ▂▂▂▂▂▂▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂ |
| Magnaporthe oryzae | 2003 | 9.33 | 2009 | 2013 | ▂▂▂▂▂▂▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Maize | 2003 | 9.86 | 2010 | 2014 | ▂▂▂▂▂▂▂▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂ |
| Antifungal protein | 2003 | 9.16 | 2010 | 2014 | ▂▂▂▂▂▂▂▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂ |
| Tobacco | 2003 | 10.70 | 2011 | 2014 | ▂▂▂▂▂▂▂▂▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂ |
| Flax rust | 2003 | 8.94 | 2011 | 2014 | ▂▂▂▂▂▂▂▂▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂ |
| Abscisic acid | 2003 | 8.12 | 2011 | 2013 | ▂▂▂▂▂▂▂▂▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Multiple fungal pathogen | 2003 | 8.43 | 2014 | 2018 | ▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃▂▂▂▂▂▂▂▂ |
| Linkage map | 2003 | 8.02 | 2015 | 2017 | ▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▂▂▂▂▂▂▂▂▂ |
| Immunity | 2003 | 9.89 | 2019 | 2021 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃ |
| Influence | 2003 | 9.50 | 2019 | 2021 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃ |
| Rhizosphere microorganism | 2003 | 8.58 | 2019 | 2021 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃ |
| Bacterial community | 2003 | 8.58 | 2019 | 2021 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃ |
| Spp. | 2003 | 8.27 | 2019 | 2021 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃ |
| Quantitative | 2003 | 8.07 | 2019 | 2021 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; He, G.; He, Y.; He, T. Plant Resistance to Fungal Pathogens: Bibliometric Analysis and Visualization. Toxics 2022, 10, 624. https://doi.org/10.3390/toxics10100624
Tang Y, He G, He Y, He T. Plant Resistance to Fungal Pathogens: Bibliometric Analysis and Visualization. Toxics. 2022; 10(10):624. https://doi.org/10.3390/toxics10100624
Chicago/Turabian StyleTang, Yueyue, Guandi He, Yeqing He, and Tengbing He. 2022. "Plant Resistance to Fungal Pathogens: Bibliometric Analysis and Visualization" Toxics 10, no. 10: 624. https://doi.org/10.3390/toxics10100624
APA StyleTang, Y., He, G., He, Y., & He, T. (2022). Plant Resistance to Fungal Pathogens: Bibliometric Analysis and Visualization. Toxics, 10(10), 624. https://doi.org/10.3390/toxics10100624






