Effects of Claroideoglomus etunicatum Fungi Inoculation on Arsenic Uptake by Maize and Pteris vittata L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. AMF Inoculants and Inoculation Methods
2.3. Experimental Setup
2.3.1. Effect of Ce Infection on the Secretion of Organic Acids from Maize/P. vittate Root
2.3.2. Effect of Ce Infection on the Rhizosphere pH of Maize and P. vittata
2.3.3. Method of Collection of the Organic Acids
2.3.4. Plant Sample Collection Method
2.4. Sample Analysis
2.4.1. Mycorrhizal Colonization
2.4.2. Determination of Organic Acid Content
2.4.3. Determination of pH and Proton Secretion in the Rhizosphere
2.4.4. Content of As and P in the Plants
2.4.5. Determination of the Morphology of As in the Plant
2.5. Relevant Parameters
- (1)
- Transfer coefficient (TF): (Heavy metal content in plant of shoot(mg/kg))/(Heavy metal content in plant root (mg/kg)).
- (2)
- Effective transport coefficient (ETF): (Heavy metal content in plant of shoot (mg/kg) × Shoot biomass (g))/(Heavy metal content in plant root (mg/kg) × Root biomass (g)).
- (3)
- Phosphorus arsenic ratio (P/As): (P content of plants (mg/kg)/(As content of plants (mg/kg)).
- (4)
- As(III)/As(V): As(III) content in plant/As(V) content in plant.
2.6. Statistical Analysis
3. Results
3.1. Effect of Inoculation with Ce on the Secretion of Organic Acids from Maize and P. vittata at Different Growth Stages
3.1.1. Rates of Maize and P. vittata Mycorrhizal Colonization at Different Growth Stages
3.1.2. Biomass of Maize and P. vittata at Different Growth Stages
3.1.3. The Content and Accumulation of As in Maize and P. vittata at Different Growth Stages
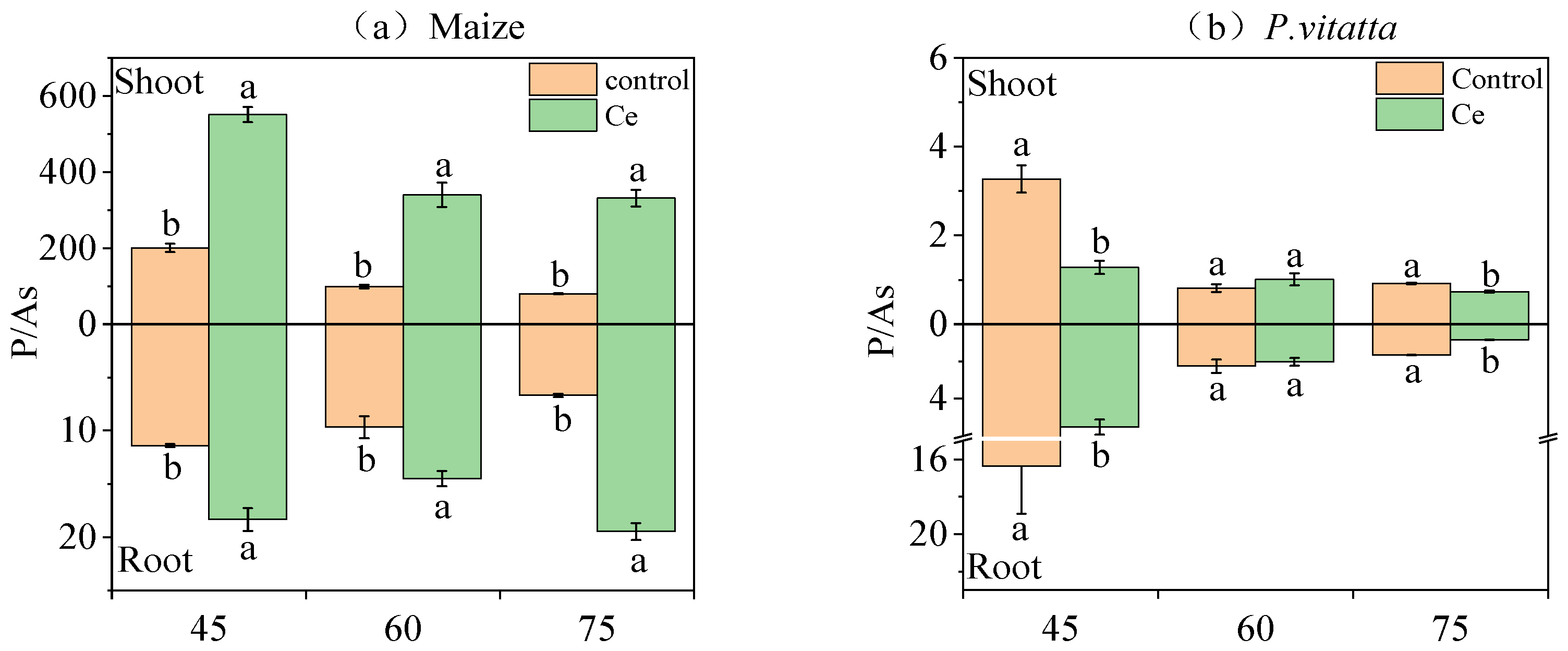
3.1.4. P/As of Maize and P. vittata at Different Growth Stages
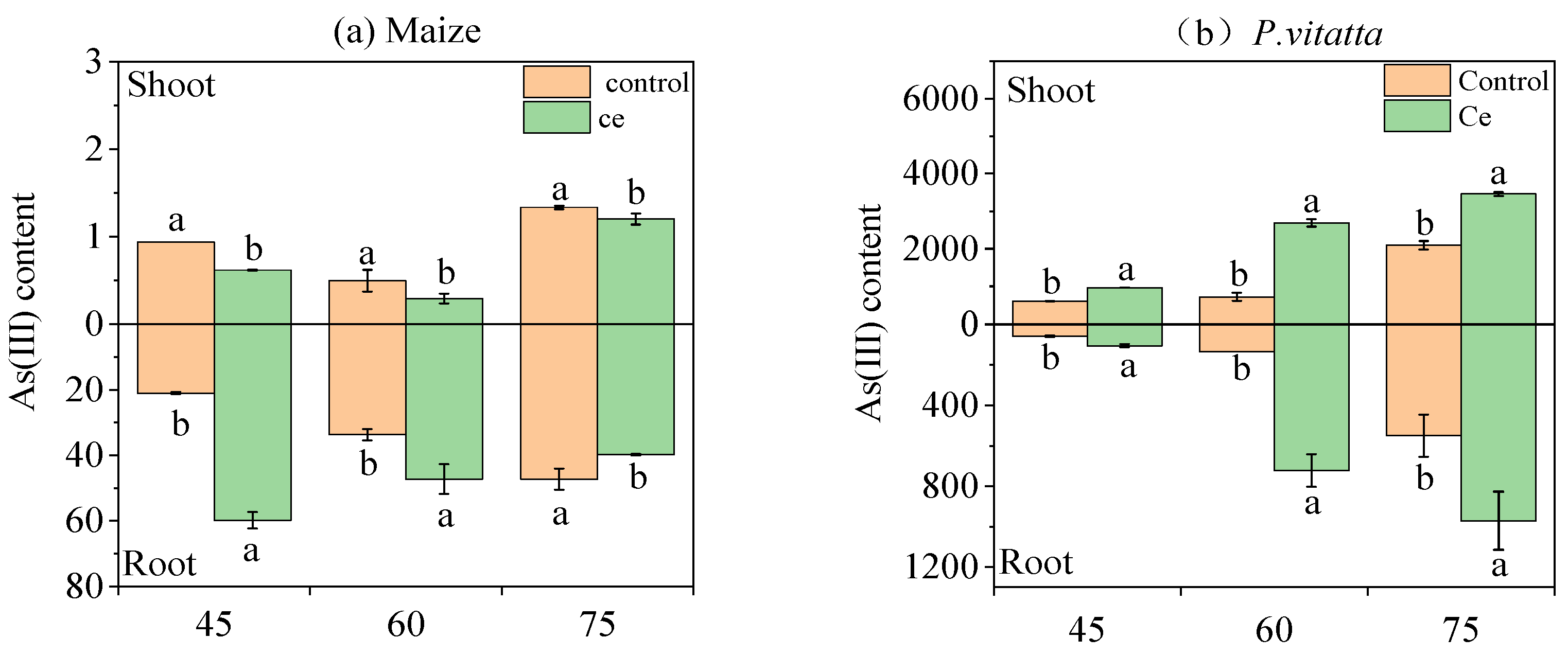
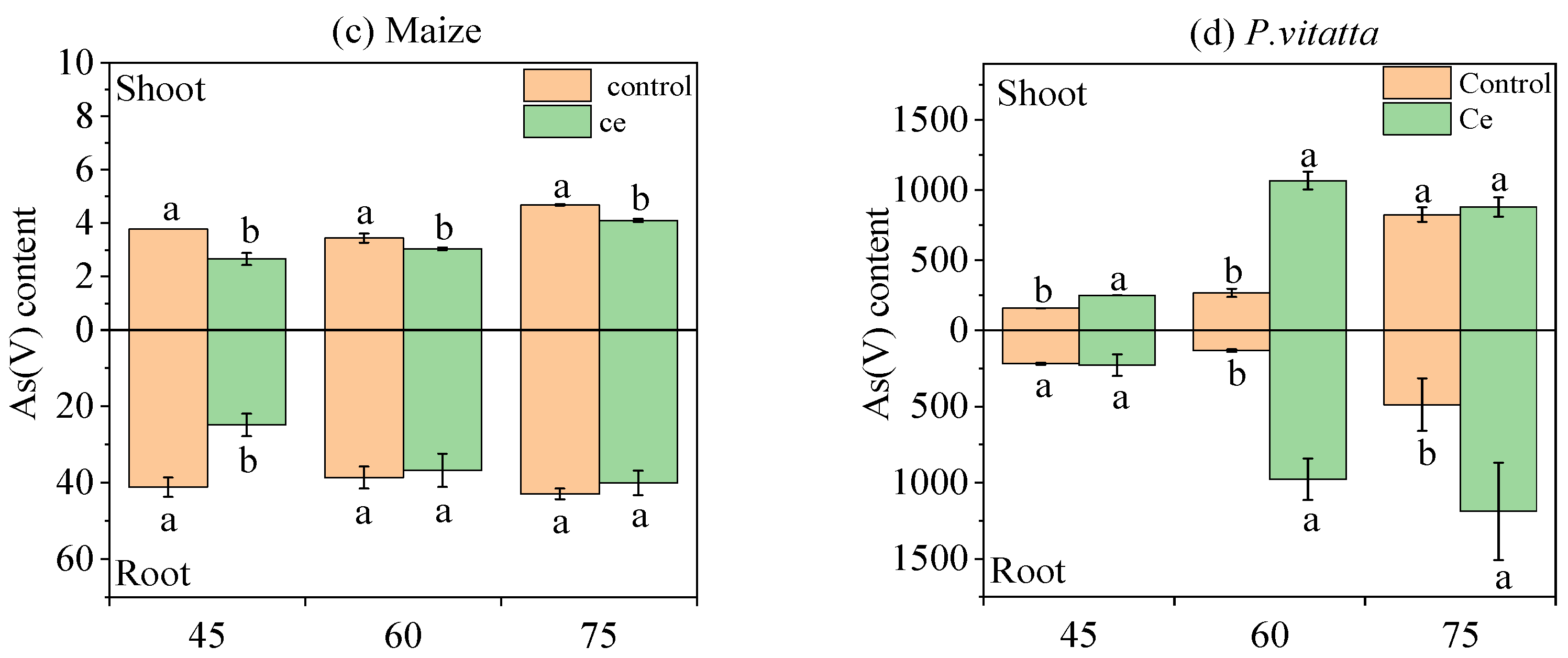
3.1.5. Effects of Ce Infection on the Content of As Species in Maize and P. vittata at Different Growth Stages
3.1.6. Translocation Factors of As in Maize and P. vittata at Different Growth Stages
3.1.7. Effects of Ce Infection on the Secretion of Organic Acids from Maize and P. vittata Roots at Different Growth Stages
3.2. Effects of Infection of Maize and P. vittata by Ce on the Rhizosphere pH
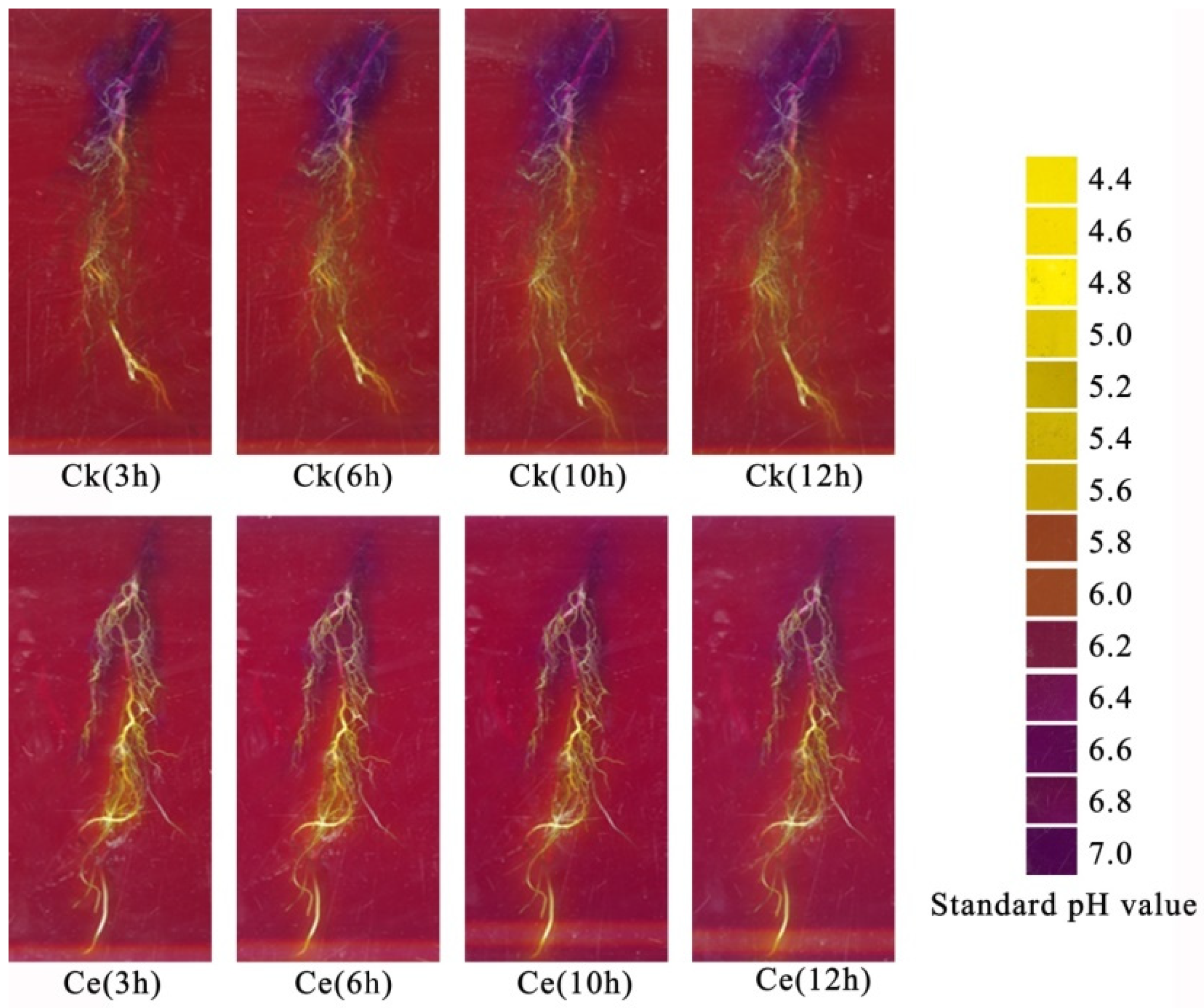
3.2.1. In Situ Chromogenic Characteristics of Rhizosphere pH in Maize and P. vittata
3.2.2. Quantitative Estimation of the Maize and P. vittata Rhizosphere pH
4. Discussion
4.1. Effects of Ce on the Secretion of Organic Acids from Maize and P. vittata
4.2. Effects of Ce on As Uptake of by Maize and P. vittata
4.3. Effect of Ce Inoculation on the Accumulation of Arsenic in Maize and P. vittata
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.S.; Lee, D.S.; Hong, B.D.; Seo, I.S.; Lim, C.S.; Jung, H.K.; Chung, D.Y. Status and future perspective for soil contamination of arable land in China. Korean J. Agric. Sci. 2019, 46, 869–883. [Google Scholar]
- Chen, H.; Tang, Z.; Wang, P.; Zhao, F.J. Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice. Environ. Pollut. 2018, 238, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Hartley-Whitaker, J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 2002, 154, 29–43. [Google Scholar] [CrossRef]
- Han, L.; Gao, B.; Hao, H.; Lu, J.; Xu, D. Arsenic pollution of sediments in China, An assessment by geochemical baseline. Sci. Total Environ. 2019, 651, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, F.J.; Meharg, A.A.; Raab, A.; Feldmann, J.; McGrath, S.P. Mechanisms of arsenic hyperaccumulation in Pterisvittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol. 2002, 130, 1552–1561. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, W.; Qin, J.; Li, S.; Yang, X.; Wei, X.; Li, H. Yield advantage and cadmium decreasing of rice in intercropping with water spinach under moisture management. Ecotoxicol. Environ. Saf. 2020, 190, 110102. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.M.; Suchismita, D.; Gress, J.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Arsenic uptake by lettuce from As-contaminated soil remediated with Pterisvittata and organic amendment. Chemosphere 2017, 176, 249–254. [Google Scholar] [CrossRef]
- Ma, J.; Lei, E.; Lei, M.; Liu, Y.; Chen, T. Remediation of Arsenic contaminated soil using malposed intercropping of Pterisvittata L. and maize. Chemosphere 2018, 194, 737–744. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Rinklebe, J. Trace elements in the soil-plant interface, Phytoavailability, translocation, and phytoremediation—A review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Cantamessa, S.; Massa, N.; Gamalero, E.; Berta, G. Phytoremediation of a highly arsenic polluted site, using Pterisvittata L. and Arbuscular Mycorrhizal fungi. Plants 2020, 9, 1211. [Google Scholar] [CrossRef]
- De Boulois, H.D.; Voets, L.; Delvaux, B.; Jakobsen, I.; Declerck, S. Transport of radiocaesium by arbuscular mycorrhizal fungi to Medicagotruncatula under in vitro conditions. Environ. Microbiol. 2006, 8, 1926–1934. [Google Scholar] [CrossRef]
- Leung, H.M.; Leung, A.O.W.; Ye, Z.H.; Cheung, K.C.; Yung, K.K.L. Mixed arbuscular mycorrhizal (AM) fungal application to improve growth and arsenic accumulation of Pterisvittata (As hyperaccumulator) grown in As-contaminated soil. Chemosphere 2013, 92, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qin, J.; Li, J.; Lai, Z.; Li, H. Upland rice intercropping with Solanum nigrum inoculated with arbuscular mycorrhizal fungi reduces grain Cd while promoting phytoremediation of Cd-contaminated soil. J. Hazard. Mater. 2021, 406, 124325. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W. Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil 2009, 321, 385–408. [Google Scholar] [CrossRef]
- Fitz, W.J.; Wenzel, W.W. Arsenic transformations in the soil–rhizosphere–plant system: Fundamentals and potential application to phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Effects of three low-molecular-weight organic acids (LMWOAs) and pH on the mobilization of arsenic and heavy metals (Cu, Pb, and Zn) from mine tailings. Environ. Geochem. Health 2013, 35, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, S.; Farahbakhsh, M.; Heydarpoor, G.; Besalatpour, A.A. Mitigation in availability and toxicity of multi-metal contaminated soil by combining soil washing and organic amendments stabilization. Ecotoxicol. Environ. Saf. 2020, 201, 110807. [Google Scholar] [CrossRef]
- Rao, T.P.; Yano, K.; Iijima, M.; Yamauchi, A.; Tatsumi, J. Regulation of rhizosphere acidification by photosynthetic activity in cowpea (Vigna unguiculata L. Walp.) seedlings. Ann. Bot. 2002, 89, 213–220. [Google Scholar] [CrossRef]
- Rao, T.P.; Yano, K.; Yamauchi, A.; Tatsumi, J. A simple method for quantitative estimation of rhizosphere pH along root axes through visualization. Plant Prod. Sci. 2000, 3, 94–100. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Ma, F.; You, Y.; Wang, Y.; Yang, D. Integration of earthworms and arbuscular mycorrhizal fungi into phytoremediation of cadmium-contaminated soil by Solanum nigrum L. J. Hazard. Mater. 2020, 389, 121873. [Google Scholar] [CrossRef]
- Marques, A.P.G.C.; Oliveira, R.S.; Rangel, A.O.S.S.; Castro, P.M. Application of manure and compost to contaminated soils and its effect on zinc accumulation by Solanum nigrum inoculated with arbuscular mycorrhizal fungi. Environ. Pollut. 2008, 151, 608–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B. Biol. 2018, 180, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Pearse, S.J.; Veneklaas, E.J.; Cawthray, G.R.; Bolland, M.D.; Lambers, H. Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 2006, 288, 127–139. [Google Scholar] [CrossRef]
- Das, S.; Chou, M.L.; Jean, J.S.; Yang, H.J.; Kim, P.J. Arsenic-enrichment enhanced root exudates and altered rhizosphere microbial communities and activities in hyperaccumulator Pterisvittata. J. Hazard. Mater. 2017, 325, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhang, X.X.; Wang, P.; Gao, Y.; Chen, N.C.; Leng, X.Y. Changes of enzyme activities in rhizosphere soil of different varieties of rice in polymetallic sulfide mining area. J. Ecol. Environ. 2013, 22, 1830–1836. [Google Scholar]
- Visioli, G.; D’Egidio, S.; Sanangelantoni, A.M. The bacterial rhizobiome of hyperaccumulators: Future perspectives based on omics analysis and advanced microscopy. Front. Plant Sci. 2015, 5, 752. [Google Scholar] [CrossRef]
- Chen, J.; Shafi, M.; Wang, Y.; Wu, J.S.; Ye, Z.Q.; Liu, C.; Zhong, B.; Guo, H.; He, L.Z.; Liu, D. Organic acid compounds in root exudation of Moso Bamboo (Phyllostachyspubescens) and its bioactivity as affected by heavy metals. Environ. Sci. Pollut. Res. 2016, 23, 20977–20984. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Wang, Z.; Liu, Y.; Ma, M.; Wu, J. Impact of a long-term cultivation on low molecular weight organic acids in greenhouse soil and their influence on vegetable uptake heavy metals. Soil Sediment Contam. Int. J. 2021, 30, 1–11. [Google Scholar] [CrossRef]
- Lu, H.L.; Yan, C.L.; Liu, J.C. Low-molecular-weight organic acids exuded by Mangrove (Kandeliacandel (L.) Druce) roots and their effect on cadmium species change in the rhizosphere. Environ. Exp. Bot. 2007, 61, 159–166. [Google Scholar]
- Su, S.W. Soil pH and free Fe/Al oxides control As availability and fractionation in representative Taiwan soils contaminated by As. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 231–234. [Google Scholar]
- Wang, S.L.; Wang, P.; Wang, C.Y. Changes in Rhizosphere pH and Exudation of Organic Acids of Masson Pine (Pinusmassoniana Lamb.) Seedlings under Aluminum Stress. J. Ecol. Rural. Environ. 2010, 26, 87–91. [Google Scholar]
- Huang, G.; Guo, G.; Yao, S.; Zhang, N.; Hu, H. Organic acids, amino acids compositions in the root exudates and Cu-accumulation in castor (Ricinus communis L.) under Cu stress. Int. J. Phytoremediation 2016, 18, 33–40. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Chaffai, R.; Nouhou Seybou, T.; Marzouk, B.; EI Ferjani, E. Changes induced by aluminum stress in the organic acid content of maize seedlings: The crucial role of exogenous citrate in enhancing seedling growth. Biologia 2009, 64, 1129–1135. [Google Scholar] [CrossRef]
- Pellet, D.M.; Grunes, D.L.; Kochian, L.V. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.). Planta 1995, 196, 788–795. [Google Scholar] [CrossRef]
- Fu, J.W.; Liu, X.; Han, Y.H.; Mei, H.; Cao, Y.; de Oliveira, L.M.; Liu, Y.G.; Rathinasabapathi, B.; Chen, Y.S.; Ma, L.Q. Arsenic-hyperaccumulator Pterisvittata efficiently solubilized phosphate rock to sustain plant growth and As uptake. J. Hazard. Mater. 2017, 330, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, J.W.; Da Silva, E.; Shi, X.X.; Cao, Y.; Rathinasabapathi, B.; Chen, Y.S.; Ma, L.Q. Microbial siderophores and root exudates enhanced goethite dissolution and Fe/As uptake by As-hyperaccumulator Pterisvittata. Environ. Pollut. 2017, 223, 230–237. [Google Scholar] [CrossRef]
- Tu, S.; Ma, L.; Luongo, T. Root exudates and arsenic accumulation in arsenic hyperaccumulating Pterisvittata and non-hyperaccumulating Nephrolepisexaltata. Plant Soil 2004, 258, 9–19. [Google Scholar] [CrossRef]
- Yang, C.; Chien, M.F.; Ho, Y.N.; Inoue, C. Phosphorus-and Iron-Deficiency Stresses Affect Arsenic Accumulation and Root Exudates in Pterisvittata. Int. J. Environ. Sci. Dev. 2019, 10, 430–434. [Google Scholar] [CrossRef]
- Wu, F.; Xu, F.; Ma, X.; Luo, W.; Lou, L.; Wong, M.H. Do arsenate reductase activities and oxalate exudation contribute to variations of arsenic accumulation in populations of Pterisvittata? J. Soils Sediments 2018, 18, 3177–3185. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Wang, X.H.; Guo, Z.; Xiao, X.; Peng, C.; Yang, J.; Zhou, C.; Zeng, P. Chelator-assisted phytoextraction of arsenic, cadmium and lead by Pterisvittata L. and soil microbial community structure response. Int. J. Phytoremediation 2019, 21, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, J.W.; Guan, D.X.; Cao, Y.; Luo, J.; Rathinasabapathi, B.; Chen, Y.S.; Ma, L.Q. Arsenic induced phytate exudation, and promoted FeAsO4 dissolution and plant growth in As-hyperaccumulator Pterisvittata. Environ. Sci. Technol. 2016, 50, 9070–9077. [Google Scholar] [CrossRef] [PubMed]
- Lessl, J.T.; Ma, L.Q.; Rathinasabapathi, B.; Guy, C. Novel phytase from Pterisvittata resistant to arsenate, high temperature, and soil deactivation. Environ. Sci. Technol. 2013, 47, 2204–2211. [Google Scholar] [CrossRef]
- Liu, X.; Feng, H.Y.; Fu, J.W.; Sun, D.; Cao, Y.; Chen, Y.; Xiang, P.; Liu, Y.G.; Ma, L.Q. Phytate promoted arsenic uptake and growth in arsenic-hyperaccumulator Pterisvittata by upregulating phosphorus transporters. Environ. Pollut. 2018, 241, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Zhao, J.; Li, J.; Liu, Y.; Luo, J.; Yuan, S.; Li, B.; Li, Q.Q.; Xu, Q.; Yu, X.F.; et al. Unique root exudate tartaric acid enhanced cadmium mobilization and uptake in Cd-hyperaccumulator Sedum alfredii. J. Hazard. Mater. 2020, 383, 121177. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, S.; Huang, H.; Luo, L.; Wen, B. Arsenic accumulation and speciation in maize as affected by inoculation with arbuscular mycorrhizal fungus Glomus mosseae. J. Agric. Food Chem. 2009, 57, 3695–3701. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.G.; Chen, B.D.; Christie, P.; Li, X.L. Yield and arsenate uptake of arbuscular mycorrhizal tomato colonized by Glomus mosseae BEG167 in As spiked soil under glasshouse conditions. Environ. Int. 2005, 31, 867–873. [Google Scholar] [CrossRef]
- Xu, P.; Christie, P.; Liu, Y.; Zhang, J.; Li, X. The arbuscular mycorrhizal fungus Glomus mosseae can enhance arsenic tolerance in Medicagotruncatula by increasing plant phosphorus status and restricting arsenate uptake. Environ. Pollut. 2008, 156, 215–220. [Google Scholar] [CrossRef]
- Garbinski, L.D.; Rosen, B.P.; Chen, J. Pathways of arsenic uptake and efflux. Environ. Int. 2019, 126, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, C.; Chen, F.; Cao, X.; Ma, C.; Chen, F.; Luo, X.; Musante, C.; White, J.C.; Zhao, X.L.; et al. New insight into the mechanism of graphene oxide-enhanced phytotoxicity of arsenic species. J. Hazard. Mater. 2021, 410, 124959. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Zhang, S.; Sharifan, H.; Ma, X. Elucidating the effects of cerium oxide nanoparticles and zinc oxide nanoparticles on arsenic uptake and speciation in rice (Oryza sativa) in a hydroponic system. Environ. Sci. Technol. 2018, 52, 10040–10047. [Google Scholar] [CrossRef]
- Tu, C.; Ma, L.Q.; Zhang, W.; TCai, Y.; Harris, W.G. Arsenic species and leachability in the fronds of the hyperaccumulator Chinese brake (Pterisvittata L.). Environ. Pollut. 2003, 124, 223–230. [Google Scholar] [CrossRef]
- Hong, S.H.; Choi, S.A.; Lee, M.H.; Min, B.R.; Yoon, C.; Yoon, H.; Cho, K.S. Effect of arsenic species on the growth and arsenic accumulation in Cucumis sativus. Environ. Geochem. Health 2011, 33, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.N.; Qiu, D.; Meng, D.K.; Gu, M.; Wang, X. Effects of arbuscular mycorrhizal fungi on arsenic fractionation in rhizosphere soil and arsenic accumulation by Pterisvittata. Mycosystema 2017, 36, 1048–1055. [Google Scholar]
- Wan, X.M.; Liu, Y.R.; Lei, M.; Huang, Z.H.; Chen, T.B. X-ray absorption spectroscopic study on arsenic forms in centipede grass of different populations. Spectrosc. Spectr. Anal. 2015, 35, 2329–2332. [Google Scholar]
- Zhang, T.; Yan, H.L.; He, Z.Y. Research Progress on the molecular mechanism of arsenic hyperaccumulation in centipede. J. Bioeng. 2020, 36, 397–406. [Google Scholar]
- Marin, A.R.; Masscheleyn, P.H.; Patrick, W.H. The influence of chemical form and concentration of arsenic on rice growth and tissue arsenic concentration. Plant Soil 1992, 139, 175–183. [Google Scholar] [CrossRef]
- Lei, M.; Wan, X.M.; Huang, Z.C.; Chen, T.B.; Li, X.W.; Liu, Y.R. First evidence on different transportation modes of arsenic and phosphorus in arsenic hyperaccumulator Pterisvittata. Environ. Pollut. 2012, 161, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, L.Q.; Rathinasabapathi, B.; Liu, Y.; Zeng, G. Uptake and translocation of arsenite and arsenate by Pterisvittata L.: Effects of silicon, boron and mercury. Environ. Exp. Bot. 2010, 68, 222–229. [Google Scholar] [CrossRef]
- Trotta, A.; Falaschi, P.; Cornara, L.; Trotta, A.; Falaschi, P.; Cornara, L.; Minganti, V.; Fusconi, A.; Drava, G.; Berta, G. Arbuscular mycorrhizae increase the arsenic translocation factor in the Ashyperaccumulating fern Pterisvittata L. Chemosphere 2006, 65, 74–81. [Google Scholar] [CrossRef]





| Treatments | Colonization% | ||
|---|---|---|---|
| Maize | 45 days | Control | 0.00 ± 0.00 b |
| Ce | 38.26 ± 0.34 a | ||
| 60 days | Control | 0.00 ± 0.00 b | |
| Ce | 39.01 ± 0.32 a | ||
| 75 days | Control | 0.00 ± 0.00 b | |
| Ce | 40.53 ± 1.38 a | ||
| P. vittata | 45 days | Control | 0.00 ± 0.00 b |
| Ce | 25.32 ± 1.69 a | ||
| 60 days | Control | 0.00 ± 0.00 b | |
| Ce | 27.32 ± 0.69 a | ||
| 75 days | Control | 0.00 ± 0.00 b | |
| Ce | 29.35 ± 1.63 a |
| Treatments | Dry Weight (g) | |||
|---|---|---|---|---|
| Root | Shoot | |||
| Maize | 45 days | Control | 3.60 ± 0.08 a | 21.85 ± 0.03 b |
| Ce | 3.64 ± 0.05 a | 23.47 ± 0.37 a | ||
| 60 days | Control | 5.95 ± 0.07 b | 29.64 ± 0.48 b | |
| Ce | 7.12 ± 0.17 a | 35.58 ± 1.07 a | ||
| 75 days | Control | 7.51 ± 0.11 b | 32.20 ± 0.26 b | |
| Ce | 8.89 ± 0.03 a | 38.10 ± 0.28 a | ||
| P. vittata | 45 days | Control | 1.07 ± 0.05 b | 1.47 ± 0.05 b |
| Ce | 1.49 ± 0.04 a | 3.68 ± 0.36 a | ||
| 60 days | Control | 5.70 ± 0.33 b | 15.22 ± 0.95 b | |
| Ce | 10.89 ± 0.73 a | 19.15 ± 0.99 a | ||
| 75 days | Control | 8.37 ± 0.16 b | 16.83 ± 0.38 b | |
| Ce | 11.30 ± 0.36 a | 27.79 ± 1.42 a | ||
| Treatments | Parts | As(III)/As(V) | ||
|---|---|---|---|---|
| Maize | 45 days | Control | Root | 0.51 |
| Shoot | 0.25 | |||
| Ce | Root | 2.40 | ||
| Shoot | 0.23 | |||
| 60 days | Control | Root | 0.87 | |
| Shoot | 0.14 | |||
| Ce | Root | 1.28 | ||
| Shoot | 0.10 | |||
| 75 days | Control | Root | 1.10 | |
| Shoot | 0.28 | |||
| Ce | Root | 0.99 | ||
| Shoot | 0.29 | |||
| P. vittata | 45 days | Control | Root | 0.27 |
| Shoot | 3.87 | |||
| Ce | Root | 0.46 | ||
| Shoot | 3.88 | |||
| 60 days | Control | Root | 1.01 | |
| Shoot | 2.71 | |||
| Ce | Root | 0.74 | ||
| Shoot | 2.53 | |||
| 75 days | Control | Root | 1.13 | |
| Shoot | 2.54 | |||
| Ce | Root | 0.82 | ||
| Shoot | 3.94 |
| Treatments | Translocation Factor (TF) | Effective Translocation Factor (ETF) | ||
|---|---|---|---|---|
| Root–Shoot | Root–Shoot | |||
| Maize | 45 days | Control | 0.07 ± 0.004 a | 0.43 ± 0.03 a |
| Ce | 0.04 ± 0.001 b | 0.25 ± 0.02 b | ||
| 60 days | Control | 0.10 ± 0.010 a | 0.51 ± 0.07 a | |
| Ce | 0.04 ± 0.002 b | 0.19 ± 0.02 b | ||
| 75 days | Control | 0.09 ± 0.002 a | 0.38 ± 0.01 a | |
| Ce | 0.06 ± 0.003 b | 0.28 ± 0.02 b | ||
| P. vittata | 45 days | Control | 1.39 ± 0.08 b | 2.13 ± 0.27 b |
| Ce | 1.68 ± 0.03 a | 4.11 ± 0.25 a | ||
| 60 days | Control | 5.30 ± 0.53 b | 13.61 ± 0.02 b | |
| Ce | 8.01 ± 0.57 a | 15.43 ± 0.49 a | ||
| 75 days | Control | 1.85 ± 0.08 b | 3.72 ± 0.07 b | |
| Ce | 2.23 ± 0.05 a | 5.50 ± 0.33 a | ||
| Treatments | Parts | Time (h) | Initial pH | pH Value | Proton Secretion (nmol/h) |
|---|---|---|---|---|---|
| Control | Root tip | 3 | 6.00 | 5.25 | 28.71 |
| 6 | 6.00 | 5.26 | 14.04 | ||
| 10 | 6.00 | 5.34 | 6.67 | ||
| 12 | 6.00 | 5.42 | 4.34 | ||
| Middle part | 3 | 6.00 | 5.39 | 19.49 | |
| 6 | 6.00 | 5.47 | 7.38 | ||
| 10 | 6.00 | 5.59 | 2.94 | ||
| 12 | 6.00 | 5.63 | 2.08 | ||
| Basal | 3 | 6.00 | 6.52 | −4.36 | |
| 6 | 6.00 | 6.57 | −2.28 | ||
| 10 | 6.00 | 6.61 | −1.42 | ||
| 12 | 6.00 | 6.71 | −1.26 | ||
| Ce | Root tip | 3 | 6.00 | 5.01 | 54.27 |
| 6 | 6.00 | 5.09 | 22.22 | ||
| 10 | 6.00 | 5.23 | 9.10 | ||
| 12 | 6.00 | 5.26 | 7.04 | ||
| Middle part | 3 | 6.00 | 5.25 | 28.78 | |
| 6 | 6.00 | 5.29 | 12.77 | ||
| 10 | 6.00 | 5.38 | 5.90 | ||
| 12 | 6.00 | 5.43 | 4.26 | ||
| Basal | 3 | 6.00 | 6.48 | −4.17 | |
| 6 | 6.00 | 6.54 | −2.22 | ||
| 10 | 6.00 | 6.58 | −1.38 | ||
| 12 | 6.00 | 6.63 | −1.20 |
| Treatment | Time (h) | Initial pH | pH Value | Proton Secretion (nmol/h) |
|---|---|---|---|---|
| Control | 2 | 6.00 | 6.83 | −7.98 |
| 4 | 6.00 | 6.89 | −4.09 | |
| 6 | 6.00 | 6.92 | −2.75 | |
| 8 | 6.00 | 6.94 | −2.08 | |
| 10 | 6.00 | 6.93 | −1.66 | |
| 12 | 6.00 | 7.06 | −1.43 | |
| Ce | 2 | 6.00 | 6.95 | −8.33 |
| 4 | 6.00 | 7.11 | −4.33 | |
| 6 | 6.00 | 7.14 | −2.90 | |
| 8 | 6.00 | 7.18 | −2.19 | |
| 10 | 6.00 | 7.32 | −1.79 | |
| 12 | 6.00 | 7.35 | −1.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, G.; Wei, Y.; Zhao, N.; Gu, M.; He, B.; Wang, X. Effects of Claroideoglomus etunicatum Fungi Inoculation on Arsenic Uptake by Maize and Pteris vittata L. Toxics 2022, 10, 574. https://doi.org/10.3390/toxics10100574
Pan G, Wei Y, Zhao N, Gu M, He B, Wang X. Effects of Claroideoglomus etunicatum Fungi Inoculation on Arsenic Uptake by Maize and Pteris vittata L. Toxics. 2022; 10(10):574. https://doi.org/10.3390/toxics10100574
Chicago/Turabian StylePan, Guofei, Yanyan Wei, Ningning Zhao, Minghua Gu, Bing He, and Xueli Wang. 2022. "Effects of Claroideoglomus etunicatum Fungi Inoculation on Arsenic Uptake by Maize and Pteris vittata L." Toxics 10, no. 10: 574. https://doi.org/10.3390/toxics10100574
APA StylePan, G., Wei, Y., Zhao, N., Gu, M., He, B., & Wang, X. (2022). Effects of Claroideoglomus etunicatum Fungi Inoculation on Arsenic Uptake by Maize and Pteris vittata L. Toxics, 10(10), 574. https://doi.org/10.3390/toxics10100574






