Mitochondria-Mediated Apoptosis and Autophagy Participate in Buprofezin-Induced Toxic Effects in Non-Target A549 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines and Culture
2.3. Cytotoxicity Assay
2.4. Apoptosis Analysis
2.5. Mitochondrial Membrane Potential Analysis
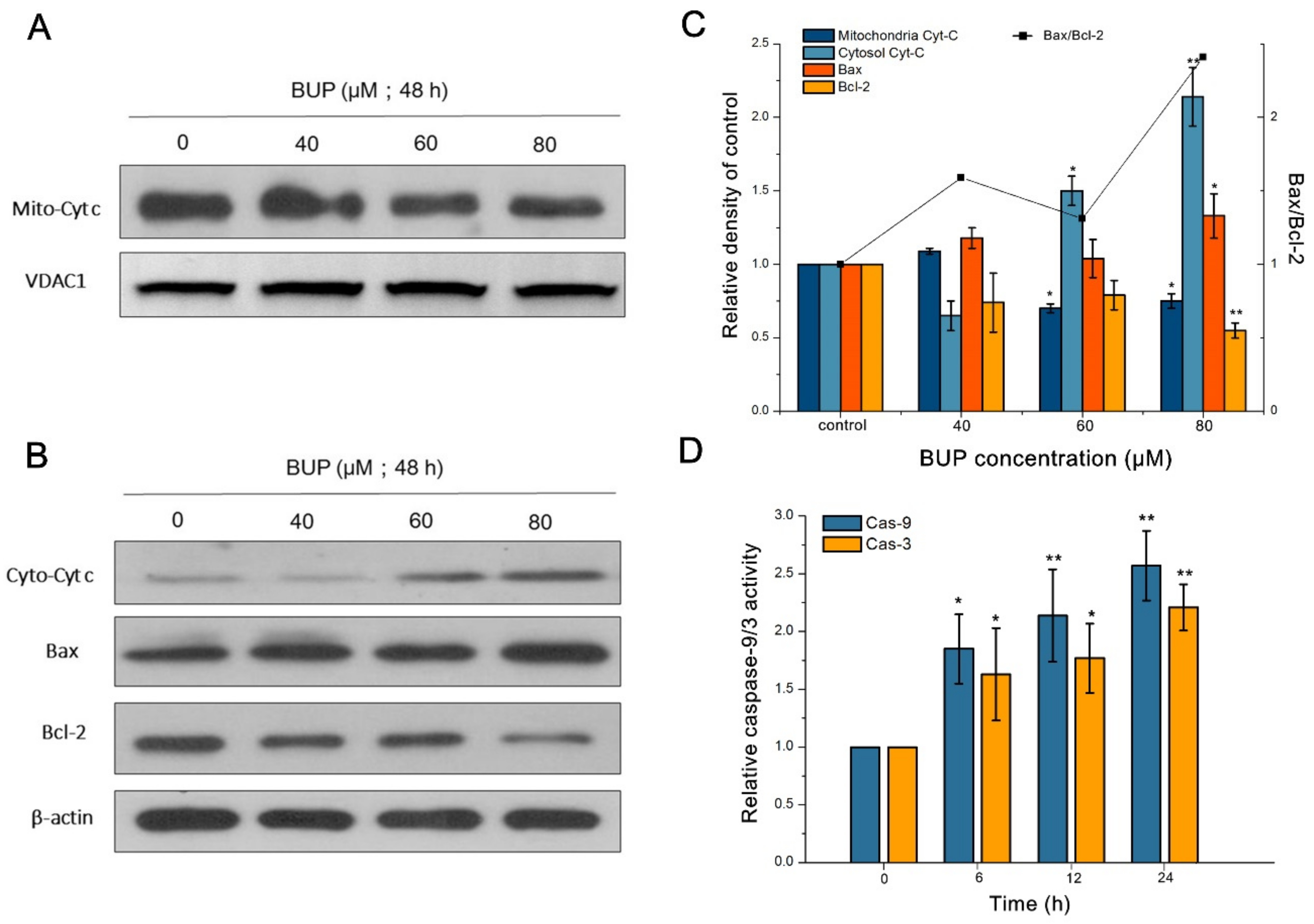
2.6. Western Blot Analysis
2.7. Caspase-9/-3 Activity Analysis
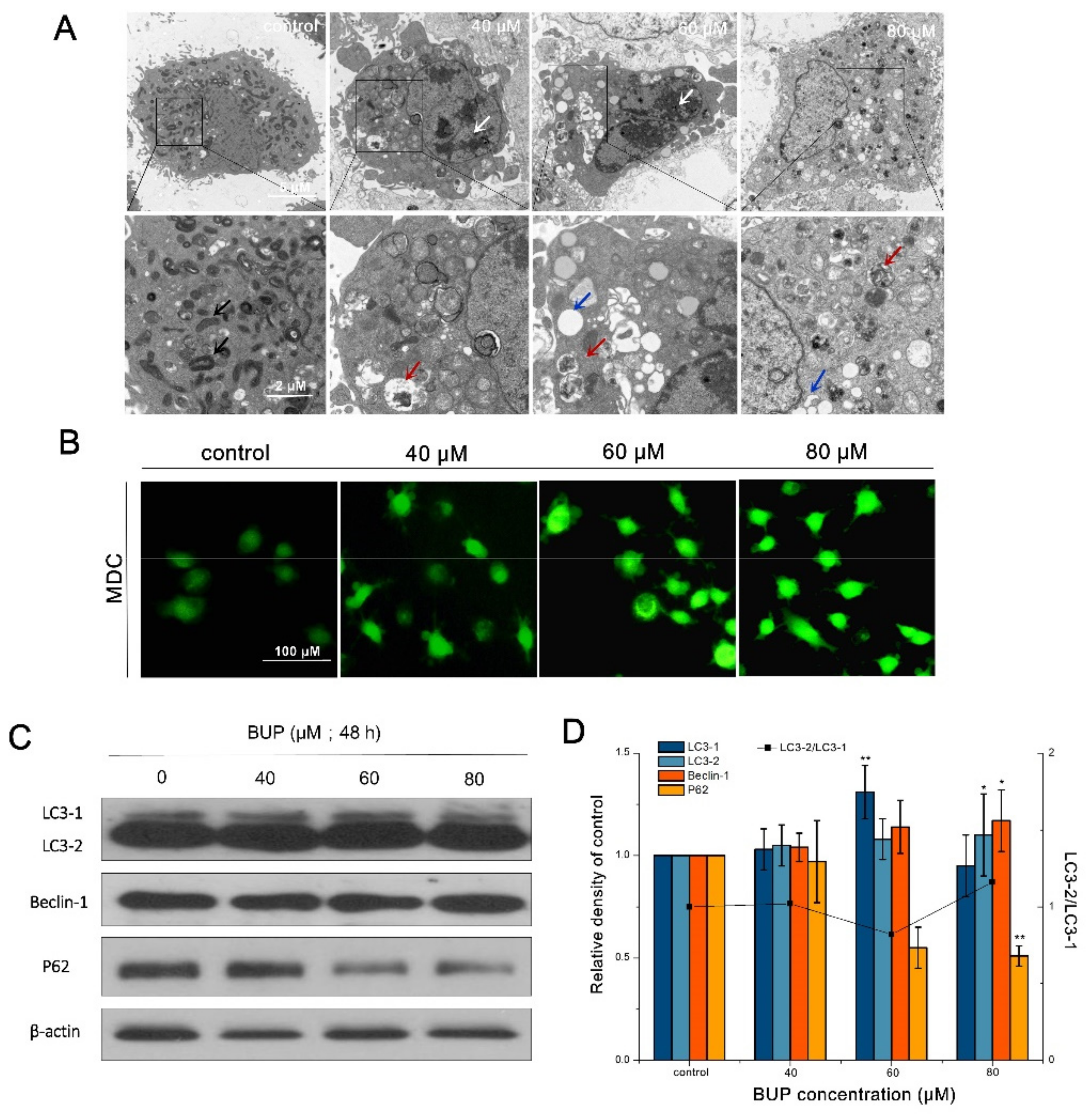
2.8. Autophagy Analysis
2.9. Reactive Oxygen Species Analysis
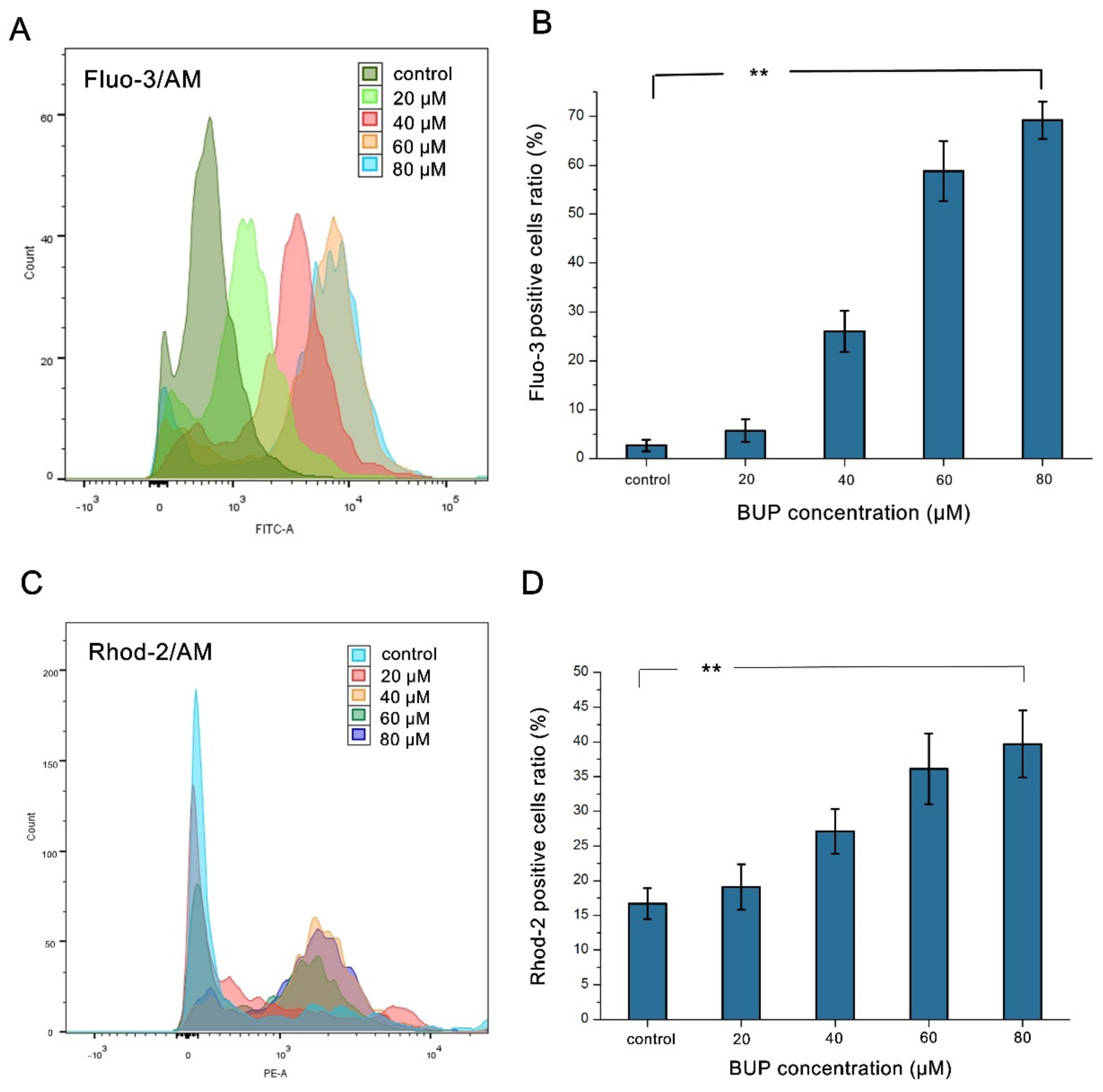
2.10. Cytosolic and Mitochondrial Ca2+ Levels Analysis
2.11. RNA Sequencing Analysis
2.12. Statistics
3. Results
3.1. Cytotoxic Effects of BUP on A549 Cells
3.2. BUP-Induced Apoptosis in A549 Cells
3.3. Production of ROS and Mitochondrial Damage in BUP-Treated Cells
3.4. BUP-Induced Autophagy in A549 Cells
3.5. RNA Sequencing
3.6. Differentially Expressed Genes (DEGs) in BUP-Treated Cells
3.7. Expression of Related Genes after Treatment with BUP
3.8. GO Annotation and Enrichment Analyses of DEGs
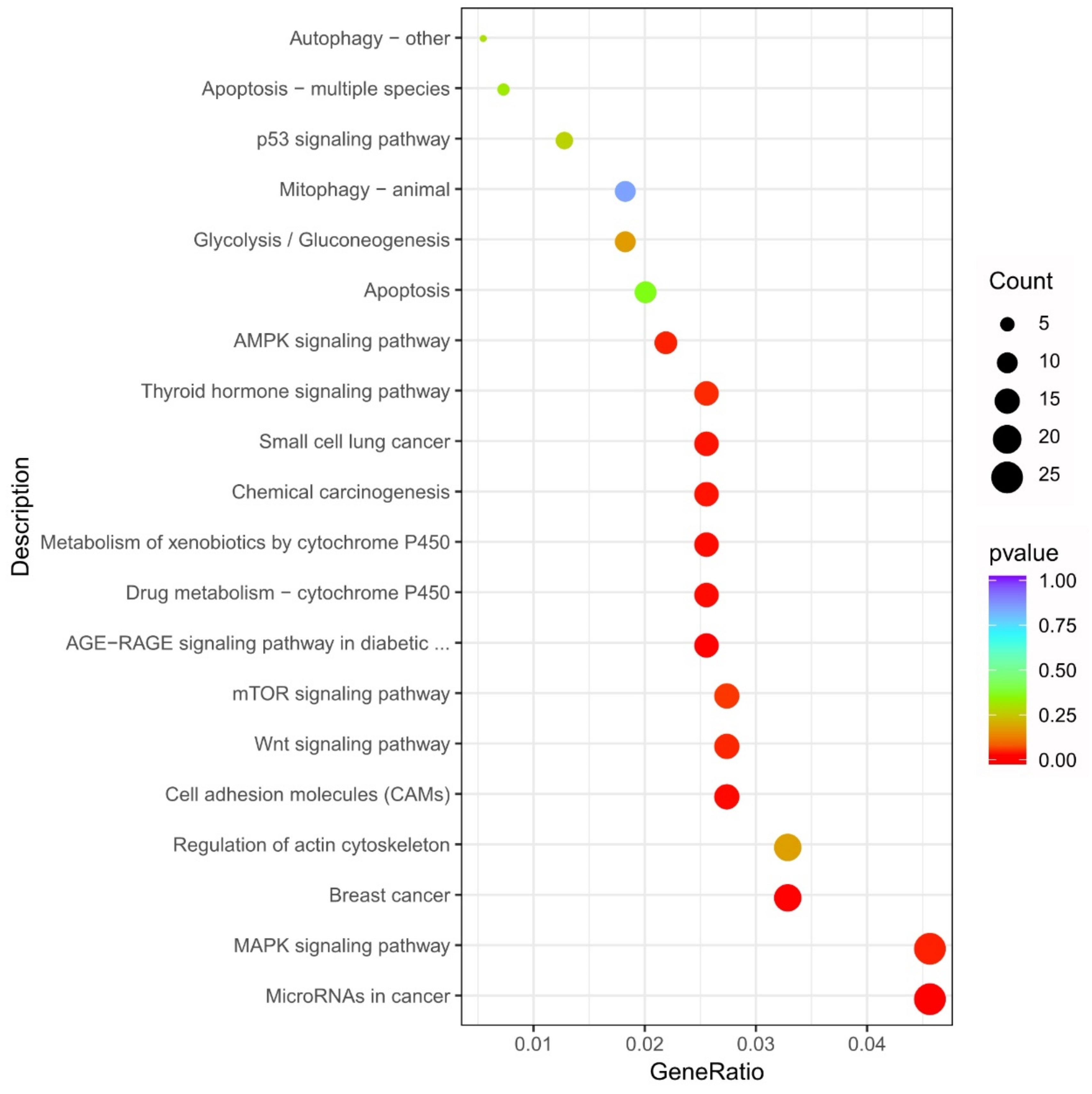
3.9. KEGG Annotation and Enrichment Analyses of DEGs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaluski, R.; Kadri, S.; Alonso, D.; Martins, R.P.; Oliveira, O.R. Fipronil promotes motor and behavioral changes in honey bees (Apis mellifera) and affects the development of colonies exposed to sublethal doses. Environ. Toxicol. Chem. 2015, 34, 1062–1069. [Google Scholar] [CrossRef]
- Ortelli, D.; Edder, P.; Corvi, C. Multiresidue analysis of 74 pesticides in fruits and vegetables by liquid chromatography-electrospray-tandem mass spectrometry. Anal. Chim. Acta 2004, 520, 33–45. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture, their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Ji, X.; Ku, T.; Zhu, N.; Ning, X.; Wei, W.; Li, G.; Sang, N. Potential hepatic toxicity of buprofezin at sublethal concentrations, ROS-mediated conversion of energy metabolism. J. Hazard. Mater. 2016, 320, 176–186. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, F.; Ou, J. Global pesticide consumption and pollution, with China as a focus. Proc. Int. Acad. Ecol. Environ. Sci. 2011, 1, 125–144. [Google Scholar]
- Tan, Y.; Xiao, L.; Sun, Y.; Zhao, J.; Bai, L. Sublethal effects of the chitin synthesis inhibitor hexaflumuron, in the cotton mirid bug, Apolygus lucorum (Meyer-Dur). Pestic. Biochem. Physiol. 2014, 111, 43–50. [Google Scholar] [CrossRef]
- Toscano, N.; Prabhaker, N.; Castle, S.; Henneberry, T. Inter-regional differences in baseline toxicity of Bemisia argentifolii (Homoptera, aleyrodidae) to the two insect growth regulators, buprofezin andpyriproxyfen. J. Econ. Entomol. 2001, 94, 1538–1546. [Google Scholar] [CrossRef]
- Zhu, Q.; He, Y.; Yao, J.; Liu, Y.; Tao, L.; Huang, Q. Effects of sublethal concentrations of the chitin synthesis inhibitor, hexaflumuron, on the development and hemolymph physiology of the cutworm, Spodoptera litura. J. Insect Sci. 2012, 12, 27. [Google Scholar] [CrossRef]
- Funayama, S.; Uchida, M.; Kanno, H.; Tsuchiya, K. Degradation of buprofezin in flooded and upland soils under laboratory conditions. J. Pestic. Sci. 1986, 11, 605–610. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Wu, Z.; Cao, L.; Yan, X.; Li, S. Biodegradation of buprofezin by Rhodococcus sp. strain YL-1 isolated from rice field soil. J. Agric. Food Chem. 2012, 60, 2531–2537. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, S.; Zhang, W.; Li, X.; Qiu, L.; Wang, C. Acute and chronic toxicity of buprofezin on daphnia magna and the recovery evaluation. Bull. Environ. Contam. Toxicol. 2012, 89, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Garcia, A.; Gonzalez-Pradas, E.; Aguilera-del, A. Analysis of buprofezin residues in vegetables. Application to the degradation study on eggplant grown in a greenhouse. J. Agric. Food Chem. 1993, 41, 2319–2323. [Google Scholar] [CrossRef]
- Marimuthu, K.; Muthu, N.; Xavier, R.; Arockiaraj, J.; Rahman, M.; Subramaniam, S. Toxicity of buprofezin on the survival of embryo and larvae of African catfish, Clarias gariepinus (Bloch). PLoS ONE 2013, 8, e75545. [Google Scholar] [CrossRef] [PubMed]
- Bibi, R.; Qureshi, I. Short-term exposure of Balb/c mice to buprofezin insecticide induces biochemical, enzymatic, histopathologic and genotoxic damage in liver and kidney tissues. Toxicol. Mech. Method. 2019, 29, 587–603. [Google Scholar] [CrossRef]
- Herrer, L.; Ostrosky-Wegman, P.; Schiffmann, D.; Chen, Q.; Ziegler-Skylakakis, K.; Andrae, U. The insecticide buprofezin induces morphological transformation and kinetochore-positive micronuclei in cultured Syrian hamster embryo cells in the absence of detectable DNA damage. Mutat. Res. 1993, 303, 121–125. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Y.; Cheng, J.; Xu, W.; Xu, Z.; Gao, J.; Tao, L. Adjuvant contributes Roundup’s unexpected effects on A549 cells. Environ. Res. 2020, 184, 109306. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Q.; Lu, L.; Jin, H.; Tao, K.; Hou, T. Toxicity and physiological actions of biflavones on potassium current in insect neuronal cells. Pestic. Biochem. Phys. 2021, 171, 104735. [Google Scholar] [CrossRef]
- Ren, Y.; Mu, Y.; Yue, Y.; Jin, H.; Tao, K.; Hou, T. Neochamaejasmin A extracted from Stellera chamaejasme L. induces apoptosis involved mitochondrial dysfunction and oxidative stress in Sf9 cells. Pestic. Biochem. Phys. 2019, 43, 169–177. [Google Scholar] [CrossRef]
- Ren, Y.; He, L.; Jin, H.; Tao, K.; Hou, T. Cytotoxicity evaluation and apoptosis-inducing effects of furanone analogs in insect cell line SL2. Food Agric. Immunol. 2018, 29, 964–975. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, N.; Yue, Y.; Jin, H.; Tao, K.; Hou, T. Investigation of novel pyrazole carboxamides as new apoptosis inducers on neuronal cells in Helicoverpa zea. Bioorg. Med. Chem. 2018, 26, 2280–2286. [Google Scholar] [CrossRef]
- Ren, Y.; Jin, H.; Tao, K.; Hou, T. Apoptotic effects of 1,5-bis-(5-nitro-2-furanyl)-1,4-pentadien-3-one on Drosophila SL2 cells. Mol. Cell. Toxicol. 2015, 11, 187–192. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Q.; Lu, L.; Jin, H.; Tao, K.; Hou, T. Isochamaejasmin induces toxic effects on Helicoverpa zea via DNA damage and mitochondria-associated apoptosis. Pest Manag. Sci. 2021, 1, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; He, X.; Yan, X.; Yang, Y.; Li, Q.; Yao, T.; Lu, L.; Peng, L.; Zou, L. Unravelling the polytoxicology of chlorfenapyr on non-target HepG2 cells: The involvement of mitochondria-mediated programmed cell death and DNA damage. Molecules 2022, 27, 5722. [Google Scholar] [CrossRef]
- Luo, W.; Wang, J.; Wang, Y.; Tang, J.; Ren, Y.; Geng, F. Bacteriostatic effects of high-intensity ultrasonic treatment on Bacillus subtilis vegetative cells. Ultrason. Sonochem. 2021, 81, 105862. [Google Scholar] [CrossRef]
- Sedlic, F.; Sepac, A.; Pravdic, D.; Camara, A.; Bienengraeber, M.; Brzezinska, A.; Wakatsuki, T.; Bosnjak, Z. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane, roles of ROS and Ca2+. Am. J. Physiol. Cell Physiol. 2010, 2, C506–C515. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.; Hoek, J.; Sheu, S. Mitochondrial Ca2+ and regulation of the permeability transition pore. J. Bioenerg. Biomembr. 2017, 1, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Azouz1, R.A.; AbuBakr, H.O.; Khattab, M.S.; Abou-Zeid, M. Buprofezin toxication implicates health hazards in Nile tilapia (Oreochromis niloticus). Aquac. Res. 2020, 52, 1–12. [Google Scholar] [CrossRef]
- Ku, T.; Yan, W.; Jia, W.; Yun, Y.; Zhu, N.; Li, G.; Sang, N. Characterization of synergistic embryotoxicity of nickel and buprofezin in zebrafish. Environ. Sci. Technol. 2015, 7, 4600–4608. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis, a review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Johnson, V.; Ko, S.; Holmstrom, T.; Eriksson, J.; Chow, S. Effector caspases are dispensable for the early nuclear morphological changes during chemical-induced apoptosis. J. Cell Sci. 2000, 113, 2941–2953. [Google Scholar] [CrossRef]
- Yip, K.; Reed, J. Bcl-2 family proteins and cancer. Oncogene 2008, 27, 6398–6406. [Google Scholar] [CrossRef] [Green Version]
- Granville, D.; Cassidy, B.; Ruehlmann, D.; Choy, J.; Brenner, C.; Kroemer, G.; Breemen, C.; Margaron, P.; Hunt, D.; Mcmanus, B. Mitochondrial release of apoptosis-inducing factor and cytochrome c during smooth muscle cell apoptosis. Am. J. Pathol. 2001, 159, 305–311. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress, cross-talk and redox signalling. Biochem. J. 2012, 2, 523–540. [Google Scholar] [CrossRef]
- Fujioka, Y.; Noda, N.; Nakatogawa, H.; Ohsumi, Y.; Inagaki, F. Dimeric coiled-coil structure of saeeharomyces cerevisiae Atg16 and its functional significance in autophagy. J. Biol. Chem. 2010, 2, 1508–1515. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell B. 2004, 36, 2503–2518. [Google Scholar] [CrossRef]
- Masuda, G.; Yashiro, M.; Kitayama, K.; Miki, Y.; Hirakawa, K. Clinicopathological correlations of autophagy-related proteins LC3, Beclin 1 and p62 in gastric cancer. Anticancer Res. 2016, 36, 129. [Google Scholar]
- Sheehan, J.; Swerdlow, R.; Miller, S.; Davis, E.; Parks, J.; Parker, W.; Tuttle, J. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic alzheimer’s disease. J. Neurosci. 1997, 12, 4612–4622. [Google Scholar] [CrossRef]
- Zhang, Y.; Gou, W.; Chen, H.; Gao, J.; Xu, Z.; Tao, L.; Li, Z.; Xu, W. Spinetoram confers its cytotoxic effects by inducing AMPK/mTOR-mediated autophagy and oxidative DNA damage. Ecotox. Environ. Safe 2019, 183, 109480. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; He, X.; Yang, Y.; Cao, Y.; Li, Q.; Lu, L.; Peng, L.; Zou, L. Mitochondria-Mediated Apoptosis and Autophagy Participate in Buprofezin-Induced Toxic Effects in Non-Target A549 Cells. Toxics 2022, 10, 551. https://doi.org/10.3390/toxics10100551
Ren Y, He X, Yang Y, Cao Y, Li Q, Lu L, Peng L, Zou L. Mitochondria-Mediated Apoptosis and Autophagy Participate in Buprofezin-Induced Toxic Effects in Non-Target A549 Cells. Toxics. 2022; 10(10):551. https://doi.org/10.3390/toxics10100551
Chicago/Turabian StyleRen, Yuanhang, Xuan He, Yanting Yang, Yanan Cao, Qiang Li, Lidan Lu, Lianxin Peng, and Liang Zou. 2022. "Mitochondria-Mediated Apoptosis and Autophagy Participate in Buprofezin-Induced Toxic Effects in Non-Target A549 Cells" Toxics 10, no. 10: 551. https://doi.org/10.3390/toxics10100551
APA StyleRen, Y., He, X., Yang, Y., Cao, Y., Li, Q., Lu, L., Peng, L., & Zou, L. (2022). Mitochondria-Mediated Apoptosis and Autophagy Participate in Buprofezin-Induced Toxic Effects in Non-Target A549 Cells. Toxics, 10(10), 551. https://doi.org/10.3390/toxics10100551







