Sub-Chronic Difenoconazole Exposure Induced Gut Microbiota Dysbiosis in Mice
Abstract
:1. Introduction
2. Methods and Materials
2.1. Chemicals and Experimental Animal Husbandry
2.2. Histopathological Analysis
2.3. Colonic RNA Extraction and Real-Time qPCR
2.4. 16S rRNA Analysis
2.5. Statistical Analysis
3. Results
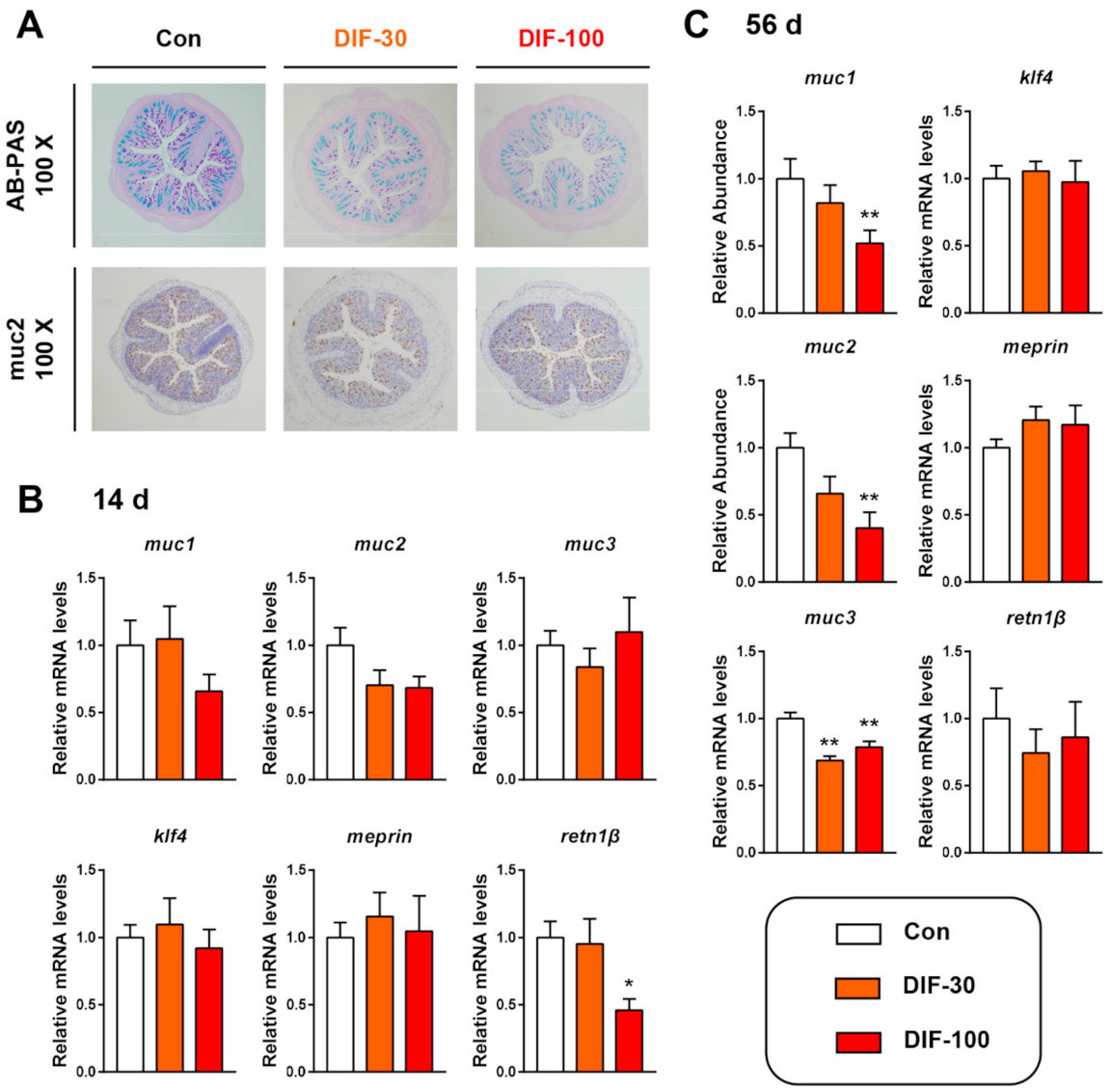
3.1. Exposure to DIF Affected the Colonic Mucus Expression
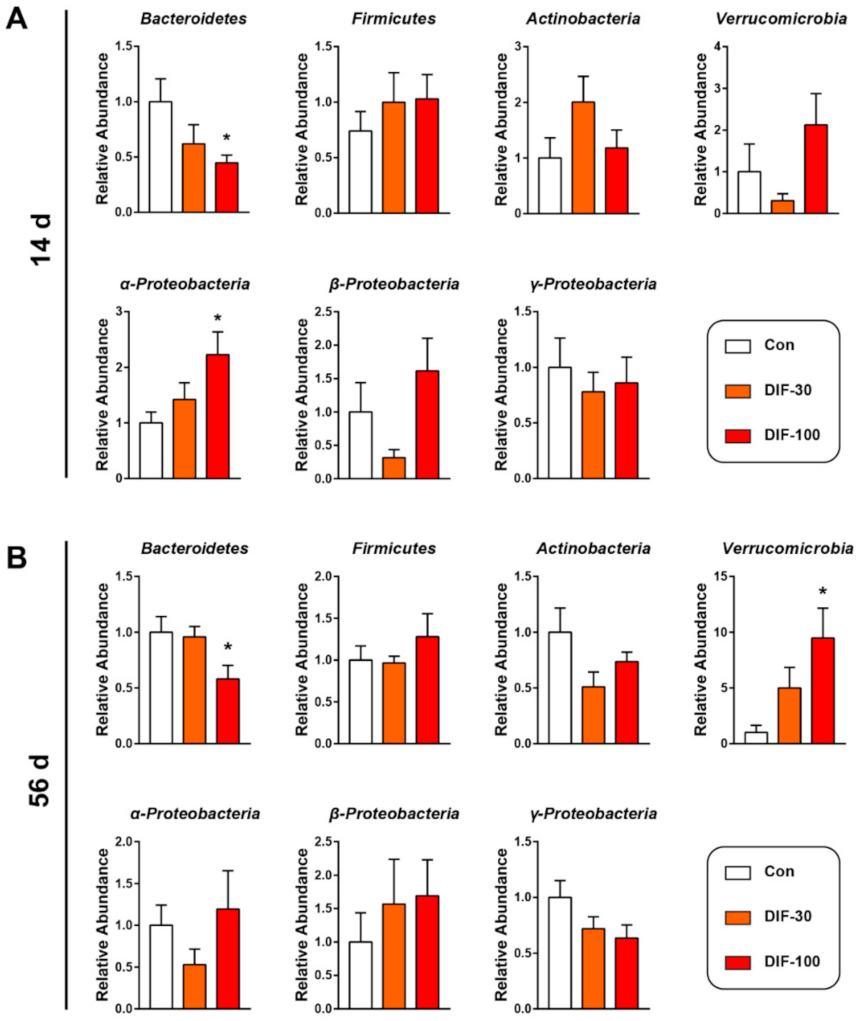
3.2. Exposure to DIF Affected the Gut Microbiota Composition
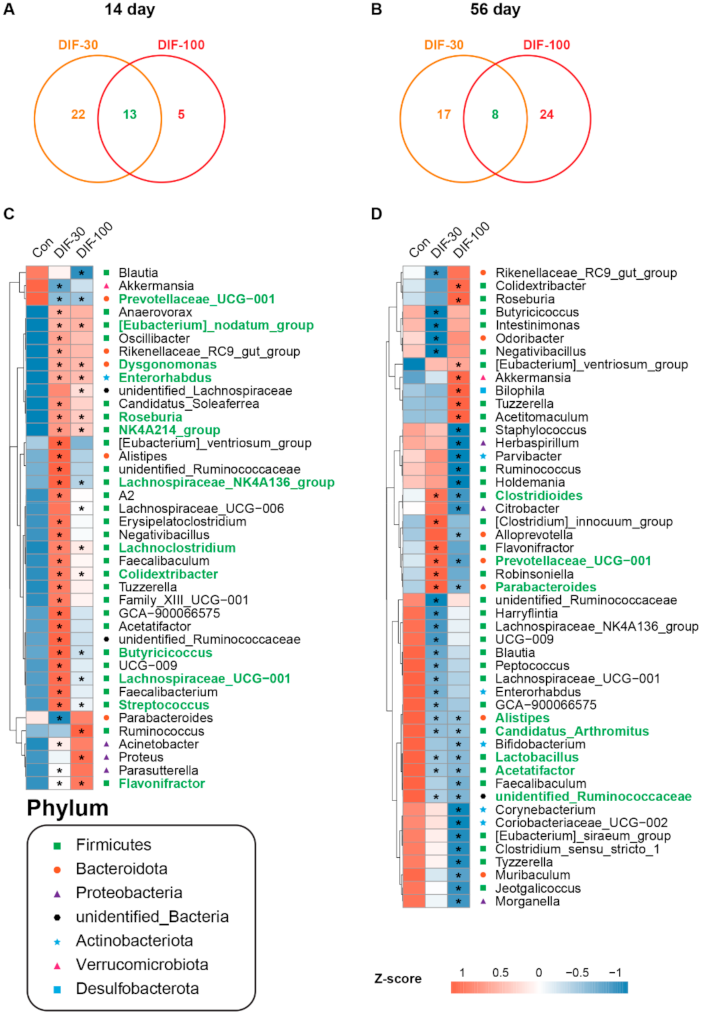
3.3. Data Analysis from 16S rRNA Sequencing
3.4. Differential Genera after DIF Exposure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Q.; Qin, D.; Yang, L.; Liu, B.; Lin, S.; Ma, Q.; Zhang, Z. Dissipation and distribution of difenoconazole in bananas and a risk assessment of dietary intake. Environ. Sci. Pollut. Res. 2020, 27, 15365–15374. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Wu, X.; Zhang, Y.; Cao, J.; Wei, D.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Cumulative risk assessment of dietary exposure to triazole fungicides from 13 daily-consumed foods in China. Environ. Pollut. 2021, 286, 117550. [Google Scholar] [CrossRef] [PubMed]
- Gaouar, Z.L.; Chefirat, B.; Saadi, R.; Djelad, S.; Rezk-Kallah, H. Pesticide residues in tomato crops in Western Algeria. Food Addit. Contam. Part B 2021, 14, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Granger, C.; Dong, H.; Mao, Y.; Duan, S.; Li, J.; Qiang, Z. Occurrences of 29 pesticides in the Huangpu River, China: Highest ecological risk identified in Shanghai metropolitan area. Chemosphere 2020, 251, 126411. [Google Scholar] [CrossRef]
- Tan, H.; Li, Q.; Zhang, H.; Wu, C.; Zhao, S.; Deng, X.; Li, Y. Pesticide residues in agricultural topsoil from the Hainan tropical riverside basin: Determination, distribution, and relationships with planting patterns and surface water. Sci. Total Environ. 2020, 722, 137856. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, H.; Wu, C.; Wang, C.; Li, Q. Pesticides in surface waters of tropical river basins draining areas with rice–vegetable rotations in Hainan, China: Occurrence, relation to environmental factors, and risk assessment. Environ. Pollut. 2021, 283, 117100. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, C.; Wang, J.; Liang, Y.; Gong, X.; You, L.; Ji, C.; Wang, S.-L.; Wang, C.; Chi, X. Difenoconazole induces cardiovascular toxicity through oxidative stress-mediated apoptosis in early life stages of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 216, 112227. [Google Scholar] [CrossRef]
- Teng, M.; Zhu, W.; Wang, D.; Qi, S.; Wang, Y.; Yan, J.; Dong, K.; Zheng, M.; Wang, C. Metabolomics and transcriptomics reveal the toxicity of difenoconazole to the early life stages of zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 112–120. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, L.; Chen, M.; Yang, Z.; Zuo, Z.; Wang, C. Exposure to difenoconazole inhibits reproductive ability in male marine medaka (Oryzias melastigma). J. Environ. Sci. 2018, 63, 126–132. [Google Scholar] [CrossRef]
- Almasri, H.; Tavares, D.A.; Diogon, M.; Pioz, M.; Alamil, M.; Sené, D.; Tchamitchian, S.; Cousin, M.; Brunet, J.-L.; Belzunces, L.P. Physiological effects of the interaction between Nosema ceranae and sequential and overlapping exposure to glyphosate and difenoconazole in the honey bee Apis mellifera. Ecotoxicol. Environ. Saf. 2021, 217, 112258. [Google Scholar] [CrossRef]
- Wang, X.; Ni, H.; Xu, W.; Wu, B.; Xie, T.; Zhang, C.; Cheng, J.; Li, Z.; Tao, L.; Zhang, Y. Difenoconazole induces oxi-dative DNA damage and mitochondria mediated apoptosis in SH-SY5Y cells. Chemosphere 2021, 283, 131160. [Google Scholar] [CrossRef]
- Wang, T.; Ma, M.; Chen, C.; Yang, X.; Qian, Y. Three widely used pesticides and their mixtures induced cytotoxicity and apoptosis through the ROS-related caspase pathway in HepG2 cells. Food Chem. Toxicol. 2021, 152, 112162. [Google Scholar] [CrossRef]
- Zhang, H.; Sparks, J.B.; Karyala, S.V.; Settlage, R.E.; Luo, X.M. Host adaptive immunity alters gut microbiota. ISME J. 2015, 9, 770–781. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, C.; Wang, D.; Zhou, J.; Yang, G.; Shao, K.; Wang, Q.; Jin, Y. Effects of chlorothalonil, prochloraz and the combination on intestinal barrier function and glucolipid metabolism in the liver of mice. J. Hazard. Mater. 2021, 410, 124639. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; van Goudoever, J.B.; Büller, H.A.; Dekker, J.; VAN Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Jin, C.; Yuan, X.; Wang, C.; Fu, Z.; Jin, Y. Maternal exposure to imazalil disrupts intestinal barrier and bile acids en-terohepatic circulation tightly related IL-22 expression in F0, F1 and F2 generations of mice. J. Hazard. Mater. 2021, 403, 123668. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jin, C.; Wang, Y.; Fu, Z.; Jin, Y. Exposure to the fungicide propamocarb causes gut microbiota dysbiosis and metabolic disorder in mice. Environ. Pollut. 2018, 237, 775–783. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chen, L.; Wu, S.; Lv, L.; Liu, X.; Wang, Q.; Zhao, X. Effects of difenoconazole on hepatotoxicity, lipid metabolism and gut microbiota in zebrafish (Danio rerio). Environ. Pollut. 2020, 265, 114844. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, M.; Wang, J.; Yang, G.; Weng, Y.; Jin, C.; Li, Y.; Jin, Y. Insights into the effects of difenoconazole on the livers in male mice at the biochemical and transcriptomic levels. J. Hazard. Mater. 2021, 422, 126933. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Jin, Y. Microplastic: A potential threat to human and animal health by inter-fering with the intestinal barrier function and changing the intestinal microenvironment. Sci. Total Environ. 2021, 785, 147365. [Google Scholar] [CrossRef]
- Yuan, X.; Pan, Z.; Jin, C.; Ni, Y.; Fu, Z.; Jin, Y. Gut microbiota: An underestimated and unintended recipient for pesticide-induced toxicity. Chemosphere 2019, 227, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Qin, H.; Sun, M.; Tang, M.; Mei, J.; Ma, K.; Fu, X. Chemical conversion of human epidermal stem cells into intestinal goblet cells for modeling mucus-microbe interaction and therapy. Sci. Adv. 2021, 7, eabb2213. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Michaudel, C.; Sokol, H. The Gut Microbiota at the Service of Immunometabolism. Cell Metab. 2020, 32, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Xu, C.; Qi, C.; Lin, Z.; Li, E.; Wang, C.; Wang, X.; Qin, J.G.; Chen, L. Sodium butyrate can improve intestinal integrity and immunity in juvenile Chinese mitten crab (Eriocheir sinensis) fed glycinin. Fish Shellfish Immunol. 2020, 102, 400–411. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Wan, X.; Li, K.; Jian, F.; Li, W.; Jiang, R.; Han, R.; Li, H.; Kang, X.; et al. Dietary supplementation with Clostridium butyricum improves growth performance of broilers by regulating intestinal microbiota and mucosal epithelial cells. Anim. Nutr. 2021, 7, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Lluansí, A.; Llirós, M.; Oliver, L.; Bahí, A.; Elias-Masiques, N.; Gonzalez, M.; Benejam, P.; Cueva, E.; Termes, M.; Ramió-Pujol, S.; et al. In vitro Prebiotic Effect of Bread-Making Process in Inflammatory Bowel Disease Microbiome. Front. Microbiol. 2021, 12, 2808. [Google Scholar] [CrossRef]
- Alatawi, H.; Mosli, M.; Saadah, O.I.; Annese, V.; Al-Hindi, R.; Alatawy, M.; Al-Amrah, H.; Alshehri, D.; Bahieldin, A.; Edris, S. Attributes of intestinal microbiota composition and their correlation with clinical primary nonresponse to anti-TNF-α agents in inflammatory bowel disease patients. Bosn. J. Basic Med Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Vasquez, R.; Kim, S.H.; Hwang, I.-C.; Song, J.H.; Park, J.H.; Kim, I.H.; Kang, D.-K. Multispecies probiotics alter fecal short-chain fatty acids and lactate levels in weaned pigs by modulating gut microbiota. J. Anim. Sci. Technol. 2021, 63, 1142–1158. [Google Scholar] [CrossRef]
- Chen, L. Gut Microbiota Manipulation to Mitigate the Detrimental Effects of Environmental Pollutants. Toxics 2021, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, M.; Tang, L.; Hu, C.; Huang, Z.; Zhou, X.; Chen, L. Probiotic supplementation mitigates the developmental toxicity of perfluorobutanesulfonate in zebrafish larvae. Sci. Total Environ. 2021, 799, 149458. [Google Scholar] [CrossRef]
- Hou, T.; Sun, X.; Zhu, J.; Hon, K.L.; Jiang, P.; Chu, I.M.; Tsang, M.S.; Lam, C.W.; Zeng, H.; Wong, C.K. IL-37 Amelio-rating Allergic Inflammation in Atopic Dermatitis Through Regulating Microbiota and AMPK-mTOR Signaling Path-way-Modulated Autophagy Mechanism. Front. Immunol. 2020, 11, 752. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, S.; Zhong, R.; Su, D.; Xia, B.; Liu, L.; Chen, L.; Zhang, H. Time-course alterations of gut microbiota and short-chain fatty acids after short-term lincomycin exposure in young swine. Appl. Microbiol. Biotechnol. 2021, 105, 8441–8456. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Shamsaddini, A.; Acharya, C.; Fagan, A.; Sikaroodi, M.; Gavis, E.; McGeorge, S.; Khoruts, A.; Fuchs, M.; Sterling, R.K.; et al. Multiple bacterial virulence factors focused on adherence and biofilm formation associate with outcomes in cirrhosis. Gut Microbes 2021, 13, 1993584. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, X.; Wei, W.; Li, R.; Hu, K.; Liu, X.; Jiang, W.; Liu, S.; Wang, W.; Sun, H.; et al. The Association of Fried Meat Consumption with the Gut Microbiota and Fecal Metabolites and Its Impact on Glucose Homoeostasis, Intestinal Endotoxin Levels, and Systemic Inflammation: A Randomized Controlled-Feeding Trial. Diabetes Care 2021, 44, 1970–1979. [Google Scholar] [CrossRef]
- Xu, H.-M.; Huang, H.-L.; Liu, Y.-D.; Zhu, J.-Q.; Zhou, Y.-L.; Chen, H.-T.; Xu, J.; Zhao, H.-L.; Guo, X.; Shi, W.; et al. Selection strategy of dextran sulfate sodium-induced acute or chronic colitis mouse models based on gut microbial profile. BMC Microbiol. 2021, 21, 279. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.H.; Ko, G.; Jo, H.; Oh, T.; Ahn, B.; Unno, T. Anti-Inflammatory Properties and Gut Microbiota Modulation of Porphyra tenera Extracts in Dextran Sodium Sulfate-Induced Colitis in Mice. Antioxidants 2020, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huang, X.; Luo, Z.; Wang, Z.; He, G.; Tan, Y.; Zhang, B.; Zhou, H.; Li, P.; Shen, T.; et al. Electro-magnetic field exposure-induced depression features could be alleviated by heat acclimation based on remodeling the gut microbiota. Ecotoxicol. Environ. Saf. 2021, 228, 112980. [Google Scholar] [CrossRef]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal Integrity and Akkermansia muciniphila, a Mucin-Degrading Member of the Intestinal Microbiota Present in Infants, Adults, and the Elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Zhang, Y.; Wang, X.; Yang, R.; Zhu, X.; Zhang, Y.; Chen, C.; Yuan, H.; Yang, Z.; Sun, L. Gut bacteria Ak-kermansia is associated with reduced risk of obesity: Evidence from the American Gut Project. Nutr. Metab. 2020, 17, 90. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.; Xue, X.; Zhang, L.; Yang, X.; Zhao, L.; Zhang, C. Strain-Specific Anti-inflammatory Properties of Two Ak-kermansia muciniphila Strains on Chronic Colitis in Mice. Front. Cell. Infect. Microbiol. 2019, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.; Florin, T.H.J. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Hu, Y.; Zhao, L.; Zhang, C. Initial gut microbiota structure affects sensitivity to DSS-induced colitis in a mouse model. Sci. China Life Sci. 2017, 61, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, Å.; Tormobadia, N.; Baridi, A.; Xu, J.; Molin, G.; Hagslätt, M.-L.; Karlsson, C.; Jeppsson, B.; Cilio, C.M.; Ahrné, S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin. Exp. Med. 2015, 15, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yang, G.; Bao, Z.; Jin, Y.; Wang, J.; Chen, J.; Qian, M. Stereoselective effects of fungicide difenoconazole and its four stereoisomers on gut barrier, microbiota, and glucolipid metabolism in male mice. Sci. Total Environ. 2021, 805, 150454. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Z.; Wang, W.; Wang, X.; Qian, M.; Jin, Y. Sub-Chronic Difenoconazole Exposure Induced Gut Microbiota Dysbiosis in Mice. Toxics 2022, 10, 34. https://doi.org/10.3390/toxics10010034
Bao Z, Wang W, Wang X, Qian M, Jin Y. Sub-Chronic Difenoconazole Exposure Induced Gut Microbiota Dysbiosis in Mice. Toxics. 2022; 10(1):34. https://doi.org/10.3390/toxics10010034
Chicago/Turabian StyleBao, Zhiwei, Weitao Wang, Xiaofang Wang, Mingrong Qian, and Yuanxiang Jin. 2022. "Sub-Chronic Difenoconazole Exposure Induced Gut Microbiota Dysbiosis in Mice" Toxics 10, no. 1: 34. https://doi.org/10.3390/toxics10010034
APA StyleBao, Z., Wang, W., Wang, X., Qian, M., & Jin, Y. (2022). Sub-Chronic Difenoconazole Exposure Induced Gut Microbiota Dysbiosis in Mice. Toxics, 10(1), 34. https://doi.org/10.3390/toxics10010034





