Technological Feature Assessment of Lactic Acid Bacteria Isolated from Cricket Powder’s Spontaneous Fermentation as Potential Starters for Cricket-Wheat Bread Production
Abstract
:1. Introduction
2. Materials and Methods
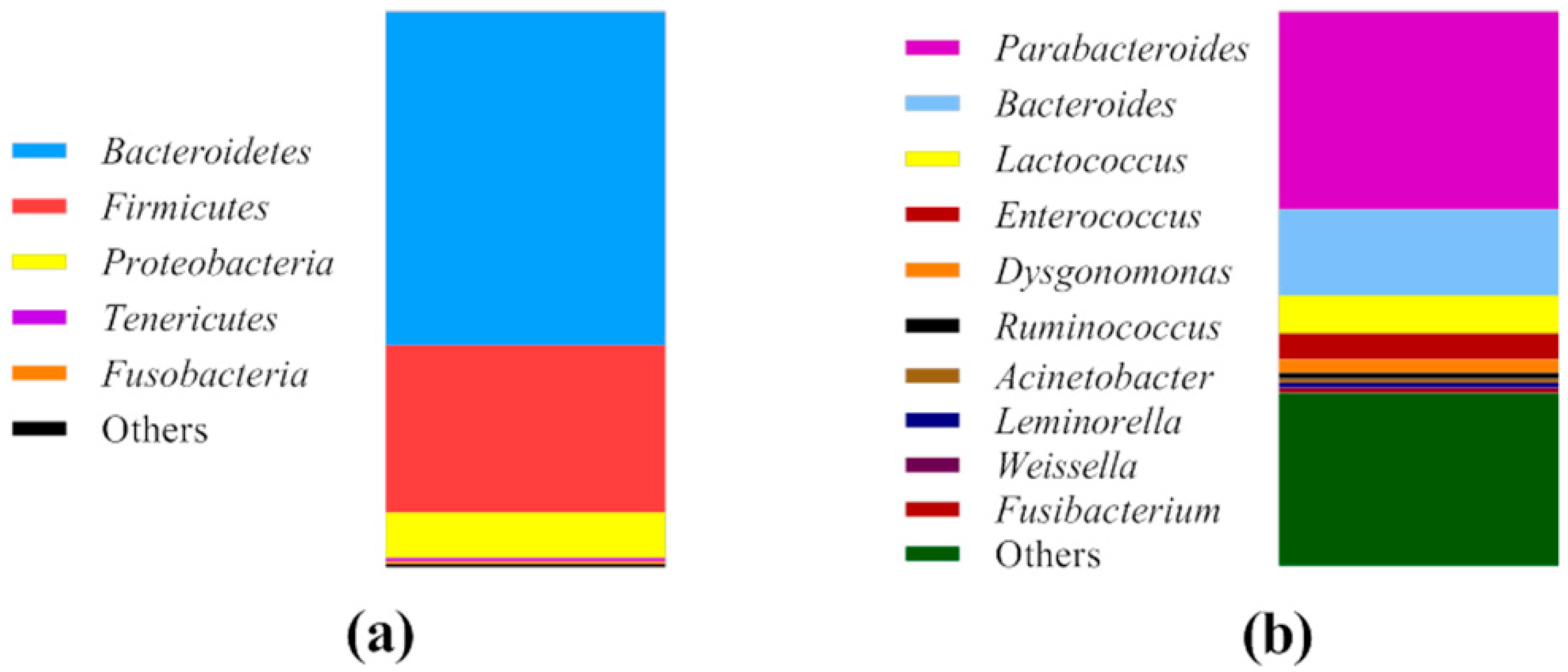
2.1. Bacterial Community Profile of Cricket Powder
2.2. Cricket Powder’s Spontaneous Fermentation
2.3. Microorganism Enumeration
2.4. Monitoring of LAB Populations by RAPD-PCR Analysis
2.5. Genotypic Identification of Lactic Acid Bacteria
2.6. Lactic Acid Bacteria Technological Properties Characterization
2.6.1. Peptidase Activity
2.6.2. Exopolysaccharide Synthesis
2.6.3. Lactic Acid Bacteria Growth and Acidification Kinetics
2.6.4. Cricket-Wheat Sourdough Acidification
2.6.5. Lactic Acid Bacteria Strain Robustness
2.7. Determination of pH, TTA, and Volume
2.8. Determination of Organic Acids
2.9. Bread Preparation
2.10. Approximate Chemical Composition of Breads
2.11. Statistical Analysis
3. Results
3.1. Bacterial Community of Cricket Powder
3.2. Cricket Powder and Spontaneous Fermentation Microbiological Analysis
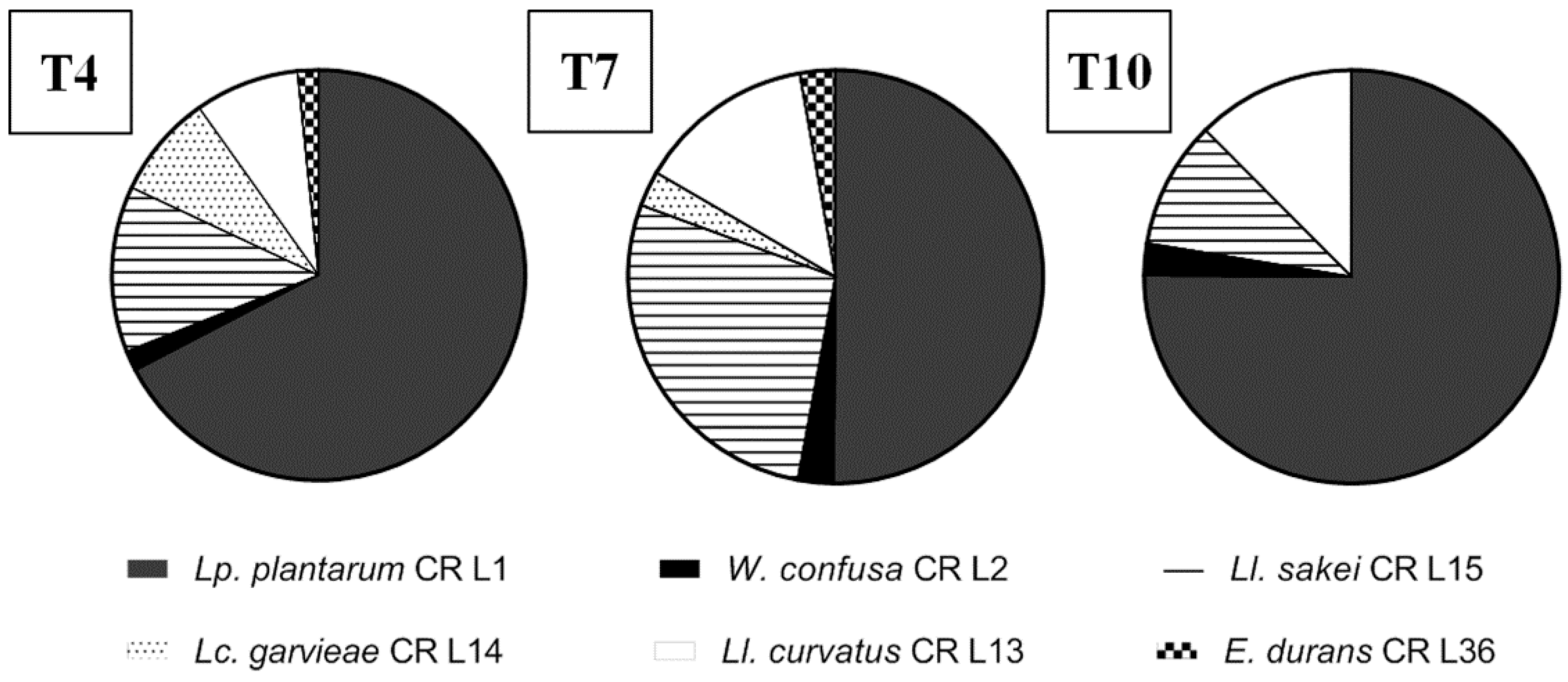
3.3. Lactic Acid Bacteria Identification and Intraspecific Characterization
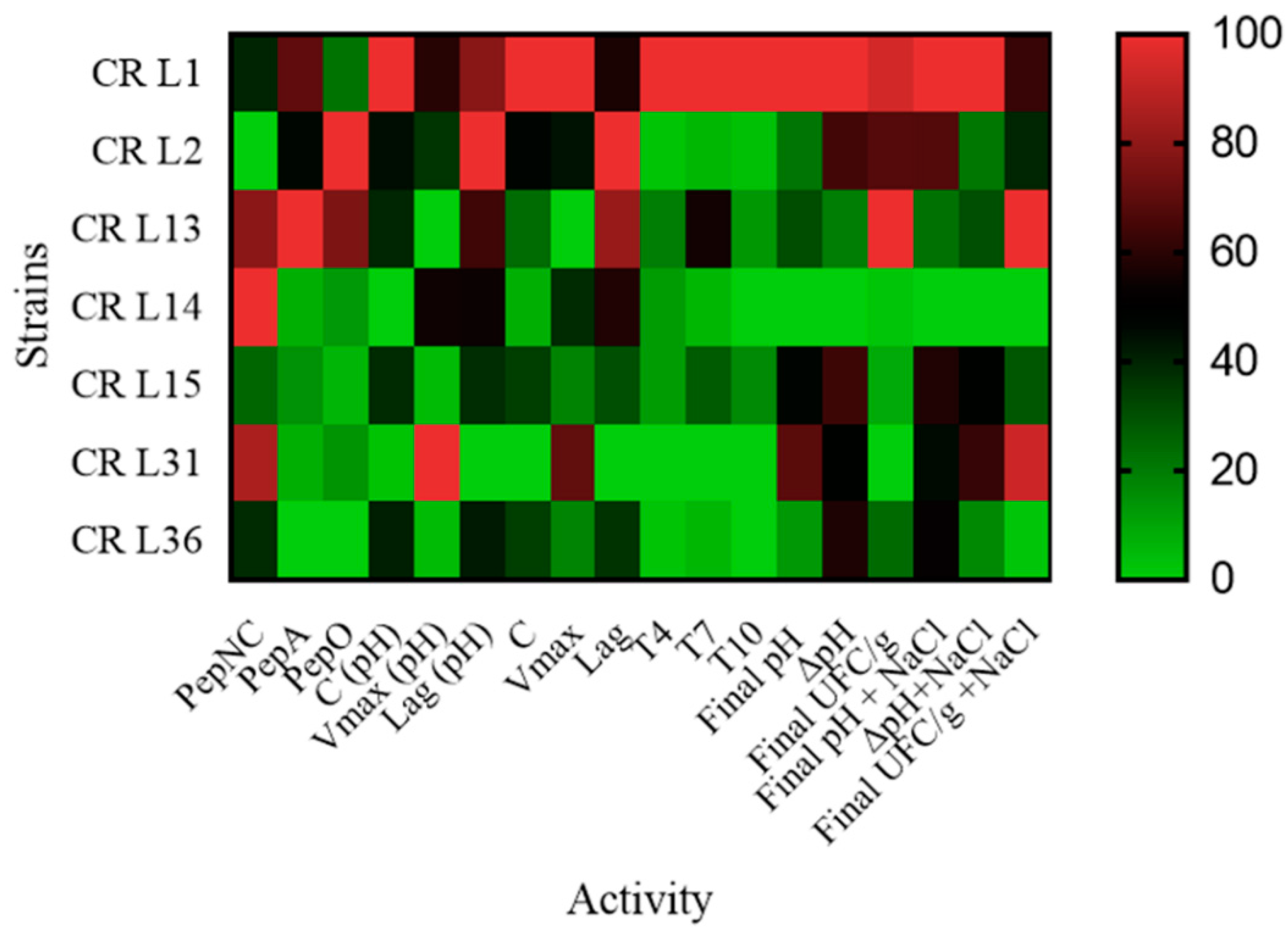
3.4. Lactic Acid Bacteria Technological Properties
3.4.1. Peptidase Activity
3.4.2. Exopolysaccharide Production
3.4.3. LAB Growth and Acidification Kinetics
3.4.4. Effect of Salt on Wheat-Cricket Powder Sourdough Acidification and Cell Concentrations
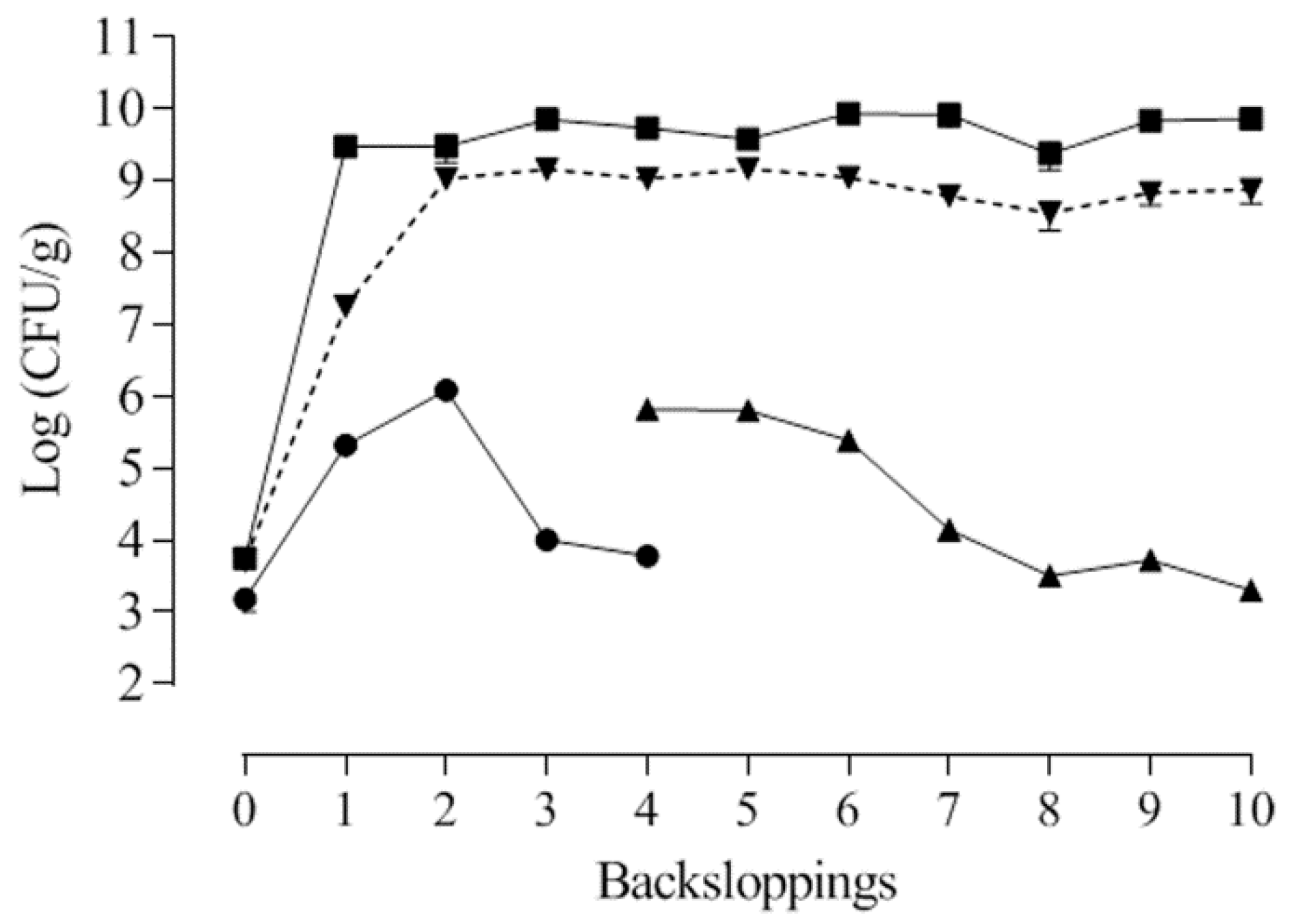
3.4.5. Lactic Acid Bacteria Robustness during Consecutive Backsloppings
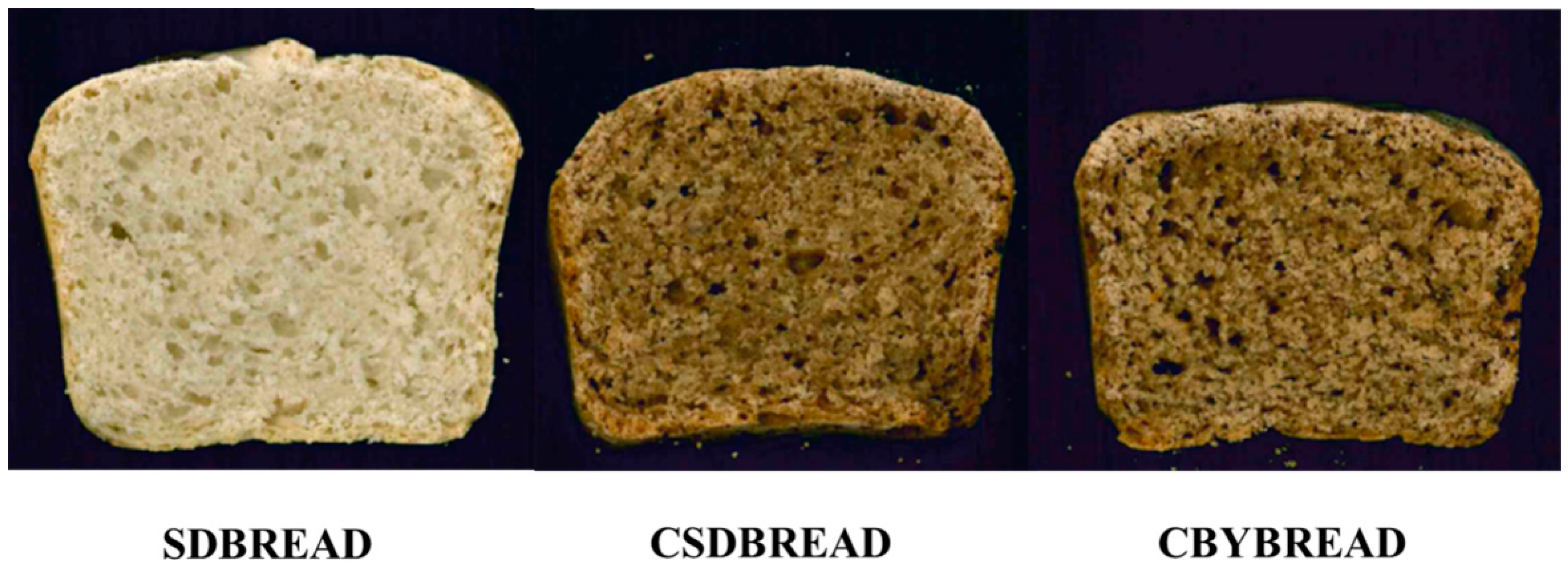
3.5. Dough Fermentation and Bread Making
3.6. Biochemical Characterization of the Breads
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Suleria, H.A.R.; Rauf, A. Edible insects as innovative foods: Nutritional and functional assessments. Trends Food Sci. Technol. 2019, 86, 352–359. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001; European Parliament: Brussels, Belgium, 2015. [Google Scholar]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union. 2005, 50, 1–26. [Google Scholar]
- Persistence Market Research. Edible Insects Market: Global Analysis, Size, Share, Value, Demand, Market Growth by 2024. 2018. Available online: https://www.persistencemarketresearch.com/market-research/edible-insectsmarket. (accessed on 18 February 2020).
- TECA. Cricket Farming for Human Consumption. 2013. Available online: http://teca.fao.org/read/7927 (accessed on 11 February 2020).
- EFSA Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef] [Green Version]
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional value, protein and peptide composition of edible cricket powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef]
- Sogari, G.; Menozzi, D.; Mora, C. The food neophobia scale and young adults’ intention to eat insect products. Int. J. Consum. Stud. 2019, 43, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, C.; Shi, J.; Giusto, A.; Siegrist, M. The psychology of eating insects: A cross-cultural comparison between Germany and China. Food Qual. Prefer. 2015, 44, 148–156. [Google Scholar] [CrossRef]
- Schouteten, J.J.; De Steur, H.; De Pelsmaeker, S.; Lagast, S.; Juvinal, J.G.; De Bourdeaudhuij, I.; Verbeke, V.; Gellynck, X. Emotional and sensory profiling of insect-, plant- and meat-based burgers under blind, expected and informed conditions. Food Qual. Prefer. 2016, 52, 27–31. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Bonaccorsi, G.; Lorini, C.; Cini, E. Assessment of the rheological properties and bread characteristics obtained by innovative protein sources (Cicer arietinum, Acheta domesticus, Tenebrio molitor): Novel food or potential improvers for wheat flour? LWT Food Sci. Technol. 2020, 118, 108867. [Google Scholar] [CrossRef]
- González, C.M.; Garzón, R.; Rosell, C.M. Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innov. Food Sci. Emerg. 2019, 51, 205–210. [Google Scholar] [CrossRef]
- Osimani, A.; Garofalo, C.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Pasquini, M.; Mozzon, M.; Raffaelli, N.; Ruschioni, S.; et al. Insight into the proximate composition and microbial diversity of edible insects marketed in the European Union. Eur. Food Res. Technol. 2017, 243, 1157–1171. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Walkowiak, K.; Masewicz, Ł.; Bartczak, O.; Lewandowicz, J.; Kubiak, P.; Baranowska, H.M. Gluten-Free Bread with Cricket Powder—Mechanical Properties and Molecular Water Dynamics in Dough and Ready Product. Foods 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Rosa Machado, C.; Thys, R.C.S. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Vermeulen, N.; Vogel, R.F. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 2007, 24, 128–138. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2018, 302, 103–113. [Google Scholar] [CrossRef]
- Garofalo, C.; Osimani, A.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Clementi, F. The microbiota of marketed processed edible insects as revealed by high-throughput sequencing. Food Microbiol. 2017, 62, 15–22. [Google Scholar] [CrossRef]
- Messina, C.M.; Gaglio, R.; Morghese, M.; Tolone, M.; Arena, R.; Moschetti, G.; Santulli, A.; Francesca, N.; Settanni, L. Microbiological Profile and Bioactive Properties of Insect Powders Used in Food and Feed Formulations. Foods 2019, 8, 400. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [Green Version]
- Venturi, M.; Guerrini, S.; Granchi, L.; Vincenzini, M. Typing of Lactobacillus sanfranciscensis isolates from traditional sourdoughs by combining conventional and multiplex RAPD–PCR profiles. Int. J. Food Microbiol. 2012, 156, 122–126. [Google Scholar] [CrossRef]
- Seseña, S.; Sánchez, I.; Palop, L. Characterization of Lactobacillus strains and monitoring by RAPD-PCR in controller fermentations of “Almagro” eggplants. Int. J. Food Microbiol. 2005, 104, 325–335. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Corsetti, A.; Tosti, N.; Rossi, J.; Corbo, M.R.; Gobbetti, M. Characterization of non-starter lactic acid bacteria from Italian ewe cheeses based on phenotypic, genotypic, and cell wall protein analyses. Appl. Environ. Microbiol. 2001, 67, 2011–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1961, 173, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, A.C.; Vieira, M.; Poças, R.F.; Malcata, X. Peptide hydrolase system of lactic acid bacteria isolated from Serra da Estrela cheese. Int. Dairy J. 2000, 10, 769–774. [Google Scholar] [CrossRef]
- Galli, V.; Mazzoli, L.; Luti, S.; Venturi, M.; Guerrini, S.; Paoli, P.; Vincezini, M.; Granchi, L.; Pazzagli, L. Effect of selected strains of lactobacilli on the antioxidant and anti-inflammatory properties of sourdough. Int. J. Food Microbiol. 2018, 286, 55–65. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Pini, N.; Guerrini, S.; Granchi, L. Exploitation of sourdough lactic acid bacteria to reduce raffinose family oligosaccharides (RFOs) content in breads enriched with chickpea flour. Eur. Food Res. Technol. 2019, 245, 2353–2363. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Micr. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The gut microbiota of insects–diversity in structure and function. FEMS Microbial. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Cini, E.; Lorini, C.; Oliva, N.; Bonaccorsi, G. Insects as food: A review on risks assessments of Tenebrionidae and Gryllidae in relation to a first machines and plants development. Food Control. 2020, 108, 106877. [Google Scholar] [CrossRef]
- Andrighetto, C.; Knijff, E.D.O.; Lombardi, A.; Torriani, S.; Vancanneyt, M.; Kersters, K.; Swings, J.; Dellaglio, F. Phenotypic and genetic diversity of enterococci isolated from Italian cheeses. J. Dairy Res. 2001, 68, 303–316. [Google Scholar] [CrossRef]
- Vandera, E.; Kakouri, A.; Koukkou, A.I.; Samelis, J. Major ecological shifts within the dominant nonstarter lactic acid bacteria in mature Greek Graviera cheese as affected by the starter culture type. Int. J. Food Microbiol. 2019, 290, 15–26. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentations 2017, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Boiocchi, F.; Porcellato, D.; Limonta, L.; Picozzi, C.; Vigentini, I.; Locatelli, D.P.; Foschino, R. Insect frass in stored cereal products as a potential source of Lactobacillus sanfranciscensis for sourdough ecosystem. J. Appl. Microbiol. 2017, 123, 944–955. [Google Scholar] [CrossRef]
- Sáez, G.D.; Saavedra, L.; Hebert, E.M.; Zárate, G. Identification and biotechnological characterization of lactic acid bacteria isolated from chickpea sourdough in northwestern Argentina. LWT Food Sci. Technol. 2018, 93, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Corsetti, A.; Settanni, L.; López, C.C.; Felis, G.E.; Mastrangelo, M.; Suzzi, G. A taxonomic survey of lactic acid bacteria isolated from wheat (Triticum durum) kernels and non-conventional flours. Syst. Appl. Microbiol. 2007, 30, 561–571. [Google Scholar] [CrossRef]
- Gobbetti, M.; Minervini, F.; Pontonio, E.; Di Cagno, R.; De Angelis, M. Drivers for the establishment and composition of the sourdough lactic acid bacteria biota. Int. J. Food Microbiol. 2016, 239, 3–18. [Google Scholar] [CrossRef]
- Casaburi, A.; Di Martino, V.; Ferranti, P.; Picariello, L.; Villani, F. Technological properties and bacteriocins production by Lactobacillus curvatus 54M16 and its use as starter culture for fermented sausage manufacture. Food Control. 2016, 59, 31–45. [Google Scholar] [CrossRef]
- Lee, J.S.; Heo, G.Y.; Lee, J.W.; Oh, Y.J.; Park, J.A.; Park, Y.H.; Pyun, Y.R.; Ahn, J.S. Analysis of kimchi microflora using denaturing gradient gel electrophoresis. Int. J. Food Microbiol. 2005, 102, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Kianjam, M.; Pontonio, E.; Verni, M.; Di Cagno, R.; Katina, K.; Rizzello, C.G.; Gobbetti, M. Sourdough-type propagation of faba bean flour: Dynamics of microbial consortia and biochemical implications. Int. J. Food Microbiol. 2017, 248, 10–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granito, M.; Alvarez, G. Lactic acid fermentation of black beans (Phaseolus vulgaris): Microbiological and chemical characterization. J. Sci. Food Agric. 2006, 86, 1164–1171. [Google Scholar] [CrossRef]
- Moroni, A.V.; Arendt, E.K.; Dal Bello, F. Biodiversity of lactic acid bacteria and yeasts in spontaneously-fermented buckwheat and teff sourdoughs. Food Microbiol. 2011, 28, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Calasso, M.; Campanella, D.; De Angelis, M.; Gobbetti, M. Use of sourdough fermentation and mixture of wheat, chickpea, lentil and bean flours for enhancing the nutritional, texture and sensory characteristics of white bread. Int. J. Food Microbiol. 2014, 180, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Rizzello, C.G.; Gobbetti, M. Use of sourdough fermentation and pseudocereals and leguminous flours for the making of a functional bread enriched of γ- aminobutyric acid (GABA). Int. J. Food Microbiol. 2010, 137, 236–245. [Google Scholar] [CrossRef]
- Nionelli, L.; Montemurro, M.; Pontonio, E.; Verni, M.; Gobbetti, M.; Rizzello, C.G. Pro-technological and functional characterization of lactic acid bacteria to be used as starters for hemp (Cannabis sativa L.) sourdough fermentation and wheat bread fortification. Int. J. Food Microbiol. 2018, 279, 14–25. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- Gerez, C.L.; Font de Valdez, G.; Rollán, G.C. Functionality of lactic acid bacteria peptidase activities in the hydrolysis of gliadin-like fragments. Lett. Appl. Microbiol. 2008, 47, 427–432. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations–A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zotta, T.; Ricciardi, A.; Parente, E. Enzymatic activities of lactic acid bacteria isolated from Cornetto di Matera sourdoughs. Int. J. Food Microbiol. 2007, 115, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sorvalia, P.; Laitilab, A.; Ndegwa, H.M.; Coda, R.; Katina, K. Dextran produced in situ as a tool to improve the quality of wheat-faba bean composite bread. Food Hydrocoll. 2018, 84, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Spicher, G. Baked Goods’ in Biotechnology; Verlag Chemie: Weinheim, Germany, 1983; Volume 5, pp. 1–80. [Google Scholar]
- Bobillo, M.; Marshall, V.M. Effect of salt and culture aeration on lactate and acetate production by Lactobacillus plantarum. Food Microbiol. 1991, 8, 153–160. [Google Scholar] [CrossRef]
- Simonson, L.; Salovaara, H.; Korhola, M. Response of wheat sourdough parameters to temperature, NaCl and sucrose variations. Food Microbiol. 2003, 20, 193–199. [Google Scholar] [CrossRef]
- Minervini, F.; De Angelis, M.; Di Cagno, R.; Gobbetti, M. Ecological parameters influencing microbial diversity and stability of traditional sourdough. Int. J. Food Microbiol. 2014, 171, 136–146. [Google Scholar] [CrossRef]
- Ercolini, D.; Pontonio, E.; De Filippis, F.; Minervini, F.; La Storia, A.; Gobbetti, M.; Di Cagno, R. Microbial ecology dynamics during rye and wheat sourdough preparation. Appl. Environ. Microbiol. 2013, 79, 7827–7836. [Google Scholar] [CrossRef] [Green Version]
- Galli, V.; Venturi, M.; Pini, N.; Guerrini, S.; Granchi, L.; Vincenzini, M. Liquid and firm sourdough fermentation: Microbial robustness and interactions during consecutive backsloppings. LWT Food Sci. Technol. 2019, 105, 9–15. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. 2018, 48, 150–163. [Google Scholar] [CrossRef]

| Ingredients | SDBread | CSDBread | CBYBread | |
|---|---|---|---|---|
| Sourdough 18 h at 30 °C | Wheat flour (g) | 162.5 | 162.5 | -- |
| Water (mL) | 87.5 | 87.5 | -- | |
| L. plantarum CR L1 L. curvatus CR L 13 7 log (CFU/g) | 7 | 7 | -- | |
| Breads 2 h at 30 °C | Sourdough (g) | 250 | 250 | |
| Wheat flour (g) | 474.2 | 347.5 | 510.0 | |
| Cricket powder (g) | -- | 126.7 | 126.7 | |
| Water (mL) | 275.8 | 275.8 | 363.3 | |
| Salt (g) | 9 | 9 | 9 | |
| Baker’s yeast(g) | 9 | 9 | 9 | |
| Dough yield | 157 | 157 | 157 |
| Strains | Growth Kinetic Parameters | pH Kinetic Parameters | |||
|---|---|---|---|---|---|
| µ Max | Lag (h) | C | µ Max (-dpHh−1) | Lag (h) | |
| L. plantarum CR L1 | 0.29 ± 0.02 d | 6.92 ± 0.2 bc | 2.06 ± 0.05 c | 0.28 ± 0.02 bc | 5.77 ± 0.3 ab |
| W. confusa CR L2 | 0.18 ± 0.01 bc | 5.79 ± 0.2 a | 1.63 ± 0.04 b | 0.23 ± 0.02 ab | 5.16 ± 0.3 a |
| L. curvatus CR L13 | 0.08 ± 0.01 a | 6.11 ± 0.4 ab | 1.59 ± 0.07 b | 0.15 ± 0.01 a | 6.19 ± 0.6 ab |
| L. garvieae CR L14 | 0.17 ± 0.02 bc | 6.94 ± 0.2 bc | 1.28 ± 0.04 a | 0.27 ± 0.04 b | 6.46 ± 0.3 abc |
| L. sakei CR L15 | 0.12 ± 0.01 ab | 7.69 ± 0.2 cd | 1.58 ± 0.07 b | 0.16 ± 0.02 a | 6.92 ± 0.2 bc |
| L. garvieae CR L31 | 0.24 ± 0.04 cd | 8.52 ± 0.1 d | 1.30 ± 0.03 a | 0.37 ± 0.04 c | 7.99 ± 0.2 c |
| E. durans CR L36 | 0.11 ± 0.01 ab | 7.21 ± 0.4 bc | 1.60 ± 0.08 b | 0.16 ± 0.02 a | 6.80 ± 0.6 abc |
| Protein | Carbohydrates | Lipids | Dry Matter | |
|---|---|---|---|---|
| SDBread | 12.6 ± 0.8 a | 75.3 ± 2.1 b | 0.7 ± 0.1 a | 69.6 ± 0.42 a |
| CSDBread | 22.9 ± 1.1 b | 61.6 ± 3.4 a | 5.8 ± 0.9 b | 70.1 ± 0.52 a |
| CBYBread | 24.5 ± 1.6 b | 60.9 ± 2.8 a | 6.0 ± 0.8 b | 69.4 ± 0.43 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galli, V.; Venturi, M.; Pini, N.; Granchi, L. Technological Feature Assessment of Lactic Acid Bacteria Isolated from Cricket Powder’s Spontaneous Fermentation as Potential Starters for Cricket-Wheat Bread Production. Foods 2020, 9, 1322. https://doi.org/10.3390/foods9091322
Galli V, Venturi M, Pini N, Granchi L. Technological Feature Assessment of Lactic Acid Bacteria Isolated from Cricket Powder’s Spontaneous Fermentation as Potential Starters for Cricket-Wheat Bread Production. Foods. 2020; 9(9):1322. https://doi.org/10.3390/foods9091322
Chicago/Turabian StyleGalli, Viola, Manuel Venturi, Niccolò Pini, and Lisa Granchi. 2020. "Technological Feature Assessment of Lactic Acid Bacteria Isolated from Cricket Powder’s Spontaneous Fermentation as Potential Starters for Cricket-Wheat Bread Production" Foods 9, no. 9: 1322. https://doi.org/10.3390/foods9091322
APA StyleGalli, V., Venturi, M., Pini, N., & Granchi, L. (2020). Technological Feature Assessment of Lactic Acid Bacteria Isolated from Cricket Powder’s Spontaneous Fermentation as Potential Starters for Cricket-Wheat Bread Production. Foods, 9(9), 1322. https://doi.org/10.3390/foods9091322





