Bioactive Phenolics and Antioxidant Capacity of Some Wild Edible Greens as Affected by Different Cooking Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Sample Preparation
2.4. Cooking Conditions
- Boiling. Each sample was immersed in 2 L of boiling tap water in a covered stainless steel pot and cooked on electric heating plate (ARED Heating Magnetic Stirrer, Velp Scientifica, Usmate Velate, MB, Italy). Cooking times: 10 min for B. vulgaris, H. echioides, S. oleraceus; 8 min for A. acutifolius; 7 min for A. lutea, T. officinale, U. picroides; 3 min for U. dioica.
- Steaming. Each sample was placed in a stainless steel steam cooker, suspended in a basket, covered with a lid, and steamed with 500 mL of boiling water at atmospheric pressure using an electric heating plate (ARED Heating Magnetic Stirrer, Velp Scientifica, Usmate Velate, MB, Italy). Cooking times: 4 min for A. acutifolius, 3 min for U. dioica, and 7 min for the other species.
- MW-cooking. Each sample was placed in a glass pot with 200 mL of water. Pots were covered with a micro-perforated plastic film and cooked at 900 W in a commercial microwave oven (mod. MWO 112-WH, 50 Hz, Whirlpool Co., Benton Harbor, MI, USA). Cooking times were as for steaming.
2.5. Dry Matter Determination
2.6. Preparation of Phenolic Extracts
2.7. Total Phenols
2.8. HPLC-DAD Analysis of Phenolic Compounds
2.9. Antioxidant Capacity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Dry Matter Content
3.2. Phenolic Composition of Raw Wild Greens
3.3. The Effect of Cooking on Total Phenolic Content
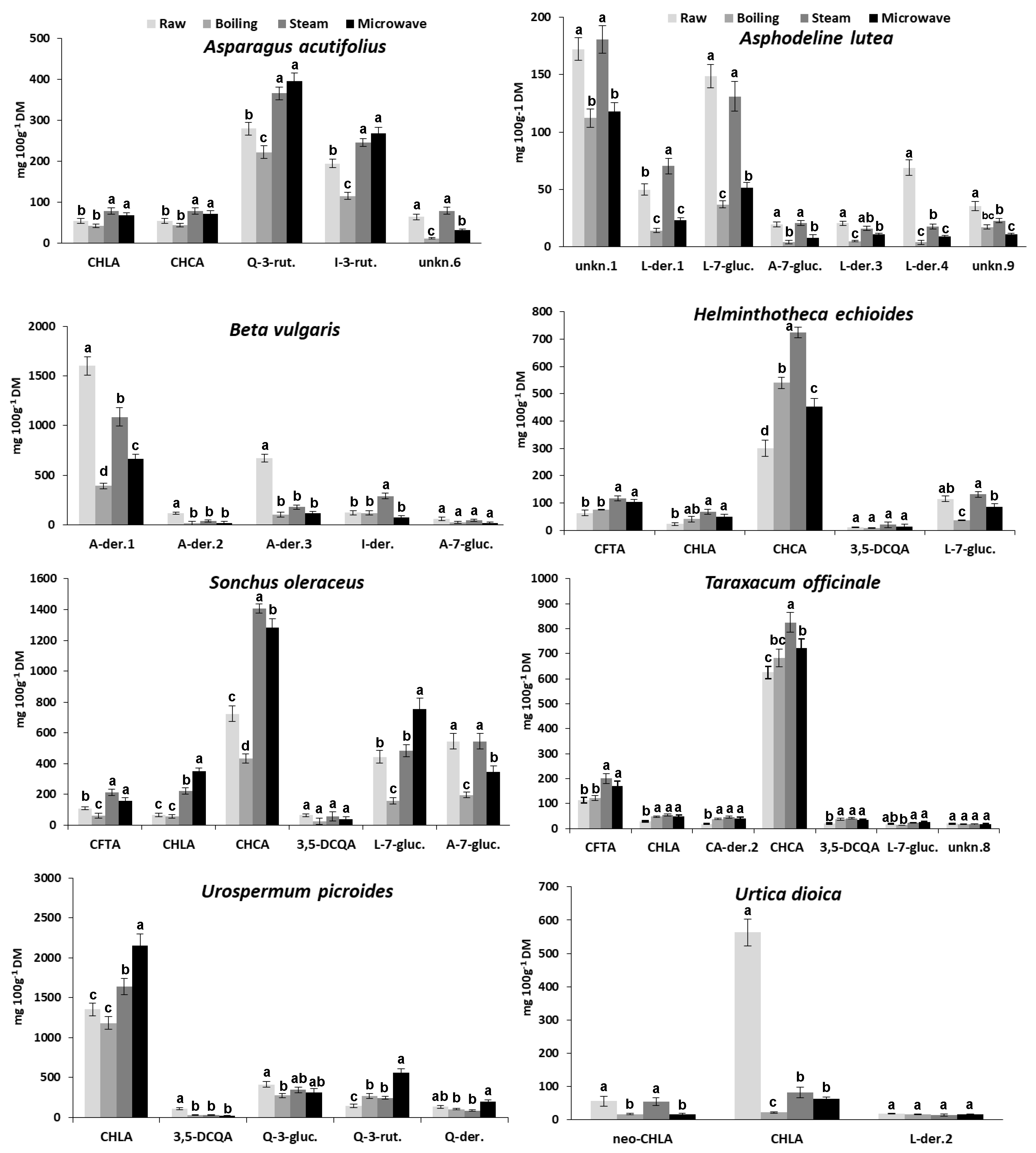
3.4. The Effect of Cooking on Individual Phenolic Compounds
3.5. The Effect of Cooking on Antioxidant Activity
3.6. The Whole Impact of Cooking on Antioxidant Properties of the Wild Edible Greens
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Carvalho, A.M.; Santos-Buelga, C. Use of HPLC–DAD–ESI/MS to profile phenolic compounds in edible wild greens from Portugal. Food Chem. 2011, 127, 169–173. [Google Scholar] [CrossRef]
- Gatto, M.A.; Ippolito, A.; Linsalata, V.; Cascarano, N.A.; Nigro, F.; Vanadia, S.; Di Venere, D. Activity of extracts from wild edible herbs against postharvest fungal diseases of fruit and vegetables. Postharvest Biol. Technol. 2011, 61, 72–82. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C.F.R. Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Res. Int. 2019, 119, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Ferreira, C.F.R.I. Nutritional composition and bioactive properties of commonly consumed wild greens: Potential sources for new trends in modern diets. Food Res. Int. 2011, 44, 2634–2640. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Cabrera Loera, R.D.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Díez Marqués, C.; Pardo-de-Santayana, M.; Tardío, J. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet. Resour. Crop Evol. 2012, 59, 431–443. [Google Scholar] [CrossRef]
- García-Herrera, P.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Díez-Marqués, C.; Molina, M.; Tardío, J. Nutrient composition of six wild edible Mediterranean Asteraceae plants of dietary interest. J. Food Compos. Anal. 2014, 34, 163–170. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Karkanis, A.; Ntatsi, G.; Barros, L.; Ferreira, I.C.F.R. Successive harvesting affects yield, chemical composition and antioxidant activity of Cichorium spinosum L. Food Chem. 2017, 237, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.A.; Fernandes, Â.; Karkanis, A.; Antoniadis, V.; Barros, L.; Ferreira, I.C.F.R. Nutrient solution composition and growing season affect yield and chemical composition of Cichorium spinosum plants. Sci. Hortic. 2018, 231, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Gatto, M.A.; Sanzani, S.M.; Tardia, P.; Linsalata, V.; Pieralice, M.; Sergio, L.; Di Venere, D. Antifungal activity of total and fractionated phenolic extracts from two wild edible herbs. Nat. Sci. 2013, 5, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Pardo de Santayana, M.; Tardío, J. Mediterranean non-cultivated vegetables as dietary sources of compounds with antioxidant and biological activity. LWT Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Di Venere, D.; Gatto, M.A.; Ippolito, A.; Bianco, V.V. Antimicrobial potential of wild edible herbaceous species. In Mediterranean Wild Edible Plants-Ethnobotany and Food Composition Tables; Sánchez-Mata, M.C., Tardío, J., Eds.; Springer Science & Business: New York, NY, USA, 2016; pp. 233–252. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean wild edible plants: Weeds or “new functional crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulos, S.A.; Fernandes, Â.; Vasileios, A.; Ntatsi, G.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and antioxidant activity of Cichorium spinosum L. leaves in relation to developmental stage. Food Chem. 2018, 239, 946–952. [Google Scholar] [CrossRef] [Green Version]
- Finimundy, T.C.; Karkanis, A.; Fernandes, Â.; Petropoulos, S.A.; Calhelha, R.; Petrović, J.; Soković, M.; Rosa, E.; Barros, L.; Ferreira, I.C.F.R. Bioactive properties of Sanguisorba minor L. cultivated in central Greece under different fertilization regimes. Food Chem. 2020, 327, 127043. [Google Scholar] [CrossRef]
- Gatto, M.A.; Sergio, L.; Ippolito, A.; Di Venere, D. Phenolic extracts from wild edible plants to control postharvest diseases of sweet cherry fruit. Postharvest Biol. Technol. 2016, 120, 180–187. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Boari, F.; Cefola, M.; Di Gioia, F.; Pace, B.; Serio, F.; Cantore, V. Effect of cooking methods on antioxidant activity and nitrate content of selected wild Mediterranean plants. Int. J. Food Sci. Nutr. 2013, 64, 870–876. [Google Scholar] [CrossRef]
- Savo, V.; Salomone, F.; Mattoni, E.; Tofani, D.; Caneva, G. Traditional salads and soups with wild plants as a source of antioxidants: A comparative chemical analysis of five species growing in Central Italy. Evid. Based Complement. Altern. Med. 2019, 2019, 6782472. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized Guidelines for Single Laboratory Validation of Methods of Analysis. Pure Appl. Chem. 2002, 74, 835–855. Available online: http://publications.iupac.org/pac/2002/pdf/7405x0835.pdf (accessed on 16 September 2020). [CrossRef]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts or antioxidant activities applying the 2,2′-azinobis(3-ethylenebenzothiazoline-6-sulfonic) acid radical cation decolorization assay. Methods Enzymol. 1999, 299, 379–389. [Google Scholar] [CrossRef]
- Marston, A.; Hostettmann, K. Separation and quantification of flavonoids. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–36. ISBN 0-8493-2021-6. [Google Scholar]
- Mansour, R.M.A.; Saleh, N.A.M.; Boulos, L. A chemosystematic study of the phenolics of Sonchus. Phytochemistry 1983, 22, 489–492. [Google Scholar] [CrossRef]
- Giner, R.M.; Ubeda, A.; Just, M.J.; Serrano, A.; Máñez, S.; Ríos, J.L. A Chemotaxonomic Survey of Sonchus Subgenus Sonchus. Biochem. Syst. Ecol. 1993, 21, 617–620. [Google Scholar] [CrossRef]
- Giner, R.M.; Recio, M.C.; Cuellar, M.J.; Máñez, S.; Peris, J.B.; Stübing, G.; Mateu, I.; Ríos, J.L. A taxonomical study of the subtribe Leontodontinae based on the distribution of phenolic compounds. Biochem. Syst. Ecol. 1993, 21, 613–616. [Google Scholar] [CrossRef]
- Enke, N.; Gemeinholzer, B.; Zidorn, C. Molecular and phytochemical systematics of the subtribe Hypochaeridinae (Asteraceae, Chicorieae). Org. Divers. Evol. 2012, 12, 1–16. [Google Scholar] [CrossRef]
- Shütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef]
- Ou, Z.-Q.; Schmierera, D.M.; Rades, T.; Larsen, L.; McDowell, A. Application of an online post-column derivatization HPLC-DPPH assay to detect compounds responsible for antioxidant activity in Sonchus oleraceus L. leaf extracts. J. Pharm. Pharmacol. 2012, 65, 271–279. [Google Scholar] [CrossRef]
- Di Maro, A.; Pacifico, S.; Fiorentino, A.; Galasso, S.; Gallicchio, M.; Guida, V.; Severino, V.; Monaco, P.; Parente, A. Raviscanina wild asparagus (Asparagus acutifolius L.): A nutritionally valuable crop with antioxidant and antiproliferative properties. Food Res. Int. 2013, 53, 180–188. [Google Scholar] [CrossRef]
- Salvatore, S.; Pellegrini, N.; Brenna, O.; Del Rio, D.; Frasca, G.; Brighenti, F.; Tumino, R. Antioxidant characterization of some Sicilian edible wild greens. J. Agric. Food Chem. 2005, 53, 9465–9471. [Google Scholar] [CrossRef]
- Melucci, D.; Locatelli, M.; Locatelli, C.; Zappi, A.; De Laurentiis, F.; Carradori, S.; Campestre, C.; Leporini, L.; Zengin, G.; Picot, C.M.N.; et al. A comparative assessment of biological effects and chemical profile of Italian Asphodeline lutea extracts. Molecules 2018, 23, 461. [Google Scholar] [CrossRef] [Green Version]
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.R.; Costa, G.; Figueirinha, A.; Liberal, J.; Prior, J.A.V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Urtica spp.: Phenolic composition, safety, antioxidant and anti-inflammatory activities. Food Res. Int. 2017, 99, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Di Ferdinando, M.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer Science & Business: New York, NY, USA, 2012; pp. 159–179. [Google Scholar] [CrossRef]
- Forkmann, G.; Stotz, G. Selection and characterisation of flavanone 3-hydrolase mutants of Dahlia, Streptocarpus, Verbena and Zinnia. Planta 1984, 161, 261–265. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M.; Giannino, D.; Santamaria, P. Quality evaluation of cook-chilled chicory stems (Cichorium intybus L.; Catalogna group) by conventional and sous vide cooking methods. J. Sci. Food Agric. 2014, 94, 656–665. [Google Scholar] [CrossRef]
- Sergio, L.; Cantore, V.; Spremulli, L.; Pinto, L.; Baruzzi, F.; Di Venere, D.; Boari, F. Effect of cooking and packaging conditions on quality of semi-dried green asparagus during cold storage. LWT Food Sci. Technol. 2018, 89, 712–718. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; García-Viguera, C. Phenolic compound contents in edible parts of broccoli inflorescences after domestic cooking. J. Sci. Food Agric. 2003, 83, 1511–1516. [Google Scholar] [CrossRef]
- Pellegrini, N.; Chiavaro, E.; Gardana, C.; Mazzeo, T.; Contino, D.; Gallo, M.; Riso, P.; Fogliano, V.; Porrini, M. Effect of different cooking methods on color, phytochemical concentration, and antioxidant capacity of raw and frozen Brassica vegetables. J. Agric. Food Chem. 2010, 58, 4310–4321. [Google Scholar] [CrossRef]
- Sergio, L.; Gatto, M.A.; Spremulli, L.; Pieralice, M.; Linsalata, V.; Di Venere, D. Packaging and storage conditions to extend the shelf life of semi-dried artichoke hearts. LWT Food Sci. Technol. 2016, 72, 277–284. [Google Scholar] [CrossRef]
- Fanasca, S.; Rouphael, Y.; Venneria, E.; Azzini, E.; Durazzo, A.; Maiani, G. Antioxidant properties of raw and cooked spears of green asparagus cultivars. Int. J. Food Sci. Technol. 2009, 44, 1017–1023. [Google Scholar] [CrossRef]
- Mazzeo, T.; N’Dri, D.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effect of two cooking procedures on phytochemical compounds, total antioxidant capacity and colour of selected frozen vegetables. Food Chem. 2011, 128, 627–633. [Google Scholar] [CrossRef]
- Lutz, M.; Henríquez, C.; Escobar, M. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.), raw and cooked. J. Food Compos. Anal. 2011, 24, 49–54. [Google Scholar] [CrossRef]


| Scientific Name | Family Name | Common Name | Used Part | Traditional Uses |
|---|---|---|---|---|
| Asparagus acutifolius L. | Liliaceae | Wild Asparagus | Stems | Boiled, fried, omelets, soup, pickles, in oil |
| Asphodeline lutea (L.) Rchb. | Liliaceae | Yellow Asfodel | Stems, roots | Salad, boiled, fried, grilled, omelets |
| Beta vulgaris subsp. maritima (L.) Arcang. | Chenopodiaceae | Common Beet | Leaves, stems | Salad, boiled, vegetable pie |
| Helminthotheca echioides L. | Asteraceae | Bristy Ox-Tongue | Leaves, stems, roots | Salad, boiled |
| Sonchus oleraceus L. | Asteraceae | Sow Thistle | Leaves, stems, flowers | Salad, boiled, soup, vegetable pie |
| Taraxacum officinale Weber | Asteraceae | Common Dandelion | Leaves, roots, flowers, germ | Salad, boiled, fried, soup, vegetable pie, pickles, in oil |
| Urospermum picroides (L.) Schmidt | Asteraceae | Prickly Golden Fleece | Leaves, stems | Boiled |
| Urtica dioica L. | Urticaceae | Stinging Nettle | Leaves, stems, flowers | Boiled, fried, omelets, soup, vegetable pie |
| Peak Number | Retention Time (a) (min) | λmax (b) (nm) | Phenolic Compound Identity (c) | Phenolic Compound Content (mg 100g −1 DM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||||
| Asparagus acutifolius | Asphodeline lutea | Beta vulgaris | Helminthotheca echioides | Sonchus oleraceus | Taraxacum officinale | Urospermum picroides | Urtica dioica | ||||
| 1 | 5.15 | 326 | neo-CHLA | 56 ± 7 | |||||||
| 2 | 5.81 | 275 | unknown 1 | 172 ±13 | |||||||
| 3 | 5.87 | 327 | CFTA | 63 ± 5 | 109 ± 11 | 114 ± 10 | |||||
| 4 | 8.18 | 326 | CHLA | 55 ± 5 | 23 ± 3 | 66 ± 8 | 29 ± 3 | 1355 ± 148 | 563 ± 67 | ||
| 5 | 8.29 | 303 | unknown 2 | 18 ± 3 | |||||||
| 6 | 9.80 | 326 | CA-der. 1 | 12 ± 3 | 14 ± 4 | ||||||
| 7 | 10.80 | 325 | CA (I.S.) | tr.(d) | tr.(d) | tr.(d) | tr.(d) | ||||
| 8 | 11.77 | 315 | unknown 3 | 15 ± 4 | |||||||
| 9 | 12.71 | 327 | CA-der. 2 | tr.(d) | tr.(d) | 28 ± 4 | tr.(d) | ||||
| 10 | 13.93 | 325 | CA-der. 3 | 18 ± 5 | |||||||
| 11 | 16.45 | 331 | CHCA | 65 ± 7 | 301 ± 42 | 725 ± 85 | 625 ± 72 | ||||
| 12 | 16.93 | 255; 349 | L-der. 1 | 50 ± 4 | |||||||
| 13 | 18.00 | 267; 344 | unknown 4 | 17 ± 3 | |||||||
| 14 | 20.01 | 269; 338 | A-der. 1 | 1600 ± 192 | |||||||
| 15 | 21.31 | 269; 338 | A-der. 2 | 115 ± 9 | |||||||
| 16 | 21.20 | 326 | 3,5-DCQA | 11 ± 4 | 25 ± 2 | 64 ± 5 | 40 ± 5 | 110 ± 13 | |||
| 17 | 22.25 | 326 | 1,5-DCQA | 14 ± 5 | |||||||
| 18 | 22.53 | 256; 351 | L-7-glucoside | 149 ± 18 | 115 ± 12 | 444 ± 51 | 11 ± 3 | ||||
| 19 | 22.92 | 257; 356 | Q-3-glucoside | 412 ± 48 | |||||||
| 20 | 23.65 | 257; 356 | Q-3-rutinoside | 299 ± 27 | 147 ± 16 | ||||||
| 21 | 24.96 | 269; 337 | A-der. 3 | 672 ± 55 | |||||||
| 22 | 25.56 | 257; 356 | Q-der. | 133 ± 18 | tr.(d) | ||||||
| 23 | 25.90 | 256; 352 | I-der. | 122 ± 15 | |||||||
| 24 | 27.50 | 317 | unknown 5 | 28 ± 5 | |||||||
| 25 | 27.67 | 268; 339 | A-7-glucoside | 19 ± 3 | 59 ± 8 | 544 ± 67 | |||||
| 26 | 28.33 | 255; 349 | L-der. 2 | 18 ± 4 | |||||||
| 27 | 28.83 | 266; 349 | K-3-rutinoside | 18 ± 4 | |||||||
| 28 | 29.85 | 256; 351 | L-der. 3 | 21 ± 5 | |||||||
| 29 | 29.97 | 255; 356 | I-3-rutinoside | 215 ± 18 | |||||||
| 30 | 39.66 | 255; 351 | L-der. 4 | 69 ± 8 | |||||||
| 31 | 43.88 | 317 | unknown 6 | 64 ± 10 | |||||||
| 32 | 44.02 | 319 | unknown 7 | 35 ± 4 | |||||||
| 33 | 45.32 | 303 | unknown 8 | 29 ± 2 | |||||||
| 34 | 50.38 | 275 | unknown 9 | 36 ± 4 | |||||||
| Species | Total Phenolic Content (TPC) (mg CAE 100g−1 DM) | |||
|---|---|---|---|---|
| Raw | Boiling | Steam | Microwave | |
| A. acutifolius | 1200 ± 57 b,A | 873 ± 33 d,B | 1196 ± 60 d,A | 1136 ± 52 c,A |
| A. lutea | 1055 ± 52 c,A | 756 ± 42 d,B | 955 ± 60 e,A | 777 ± 53 d,B |
| B. vulgaris | 2477 ± 255 a,A | 1443 ± 81 b,B | 2375 ± 108 b,A | 1962 ± 264 b,AB |
| H. echioides | 810 ± 115 d,C | 1410 ± 117 bc,AB | 1184 ± 124 d,B | 1495 ± 268 c,A |
| S. oleraceus | 1394 ± 231 b,B | 644 ± 44 de,C | 2005 ± 212 c,A | 2371 ± 472 b,A |
| T. officinale | 1044 ± 265 bcd,A | 1108 ± 212 c,A | 1315 ± 210 d,A | 1151 ± 192 c,A |
| U. picroides | 2574 ± 247 a,BC | 2109 ± 245 a,C | 2811 ± 152 a,B | 4632 ± 104 a,A |
| U. dioica | 735 ± 87 d,A | 184 ± 65 f,C | 443 ± 73 f,B | 326 ± 75 e,BC |
| Cooking Method | TPC | Chlorogenic Acid | Chicoric Acid | Caftaric Acid | Apigenin-7-Glucoside | Luteolin-7-Glucoside | Sum of Caffeic Acid Derivatives | Sum of Flavonoids |
|---|---|---|---|---|---|---|---|---|
| Raw | ** | ns | ns | ns | ns | *** | * | ns |
| Boiling | *** | ** | ns | * | ns | ns | * | * |
| Steam | *** | ns | ns | ns | * | *** | ** | ns |
| Microwave | **** | ** | ns | ns | * | *** | ** | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergio, L.; Boari, F.; Pieralice, M.; Linsalata, V.; Cantore, V.; Di Venere, D. Bioactive Phenolics and Antioxidant Capacity of Some Wild Edible Greens as Affected by Different Cooking Treatments. Foods 2020, 9, 1320. https://doi.org/10.3390/foods9091320
Sergio L, Boari F, Pieralice M, Linsalata V, Cantore V, Di Venere D. Bioactive Phenolics and Antioxidant Capacity of Some Wild Edible Greens as Affected by Different Cooking Treatments. Foods. 2020; 9(9):1320. https://doi.org/10.3390/foods9091320
Chicago/Turabian StyleSergio, Lucrezia, Francesca Boari, Maria Pieralice, Vito Linsalata, Vito Cantore, and Donato Di Venere. 2020. "Bioactive Phenolics and Antioxidant Capacity of Some Wild Edible Greens as Affected by Different Cooking Treatments" Foods 9, no. 9: 1320. https://doi.org/10.3390/foods9091320
APA StyleSergio, L., Boari, F., Pieralice, M., Linsalata, V., Cantore, V., & Di Venere, D. (2020). Bioactive Phenolics and Antioxidant Capacity of Some Wild Edible Greens as Affected by Different Cooking Treatments. Foods, 9(9), 1320. https://doi.org/10.3390/foods9091320







