Digitization of Broccoli Freshness Integrating External Color and Mass Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
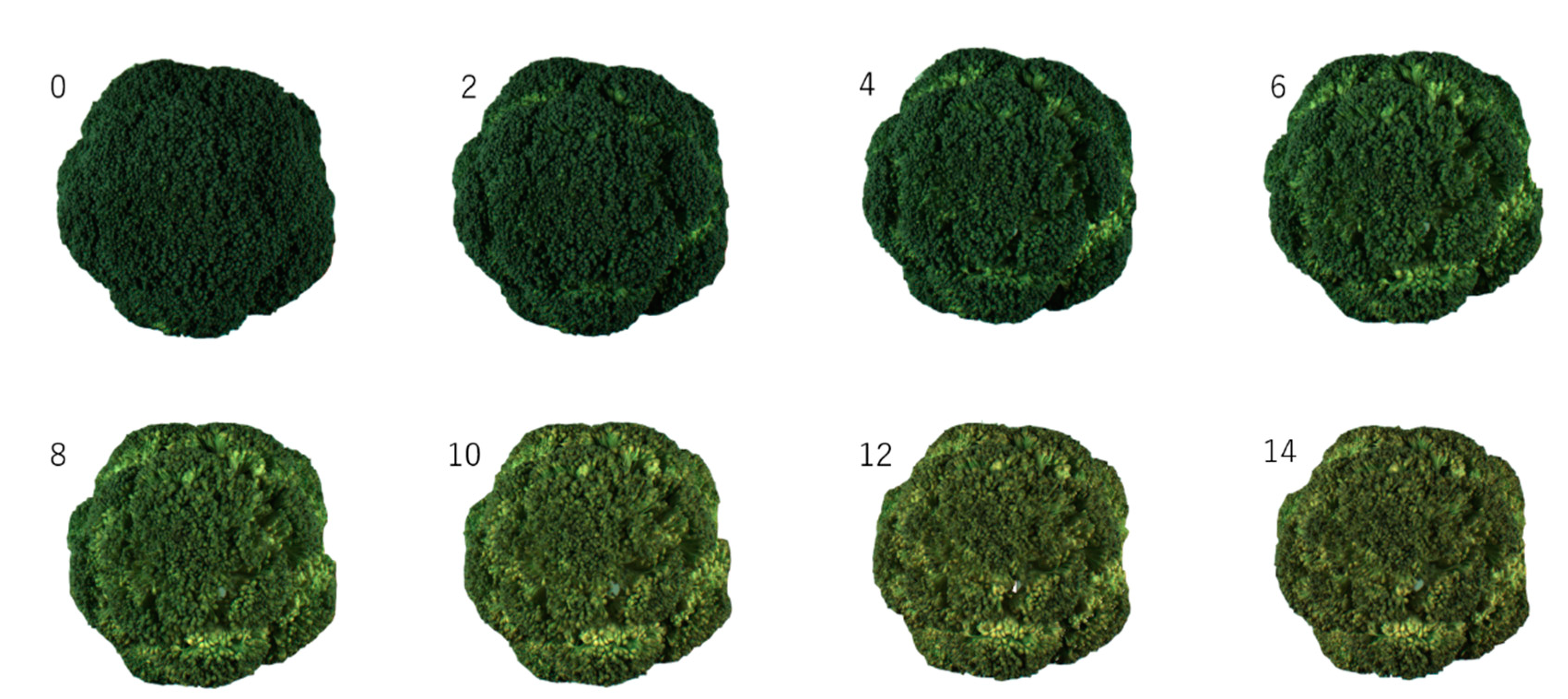
2.2. Storage Method
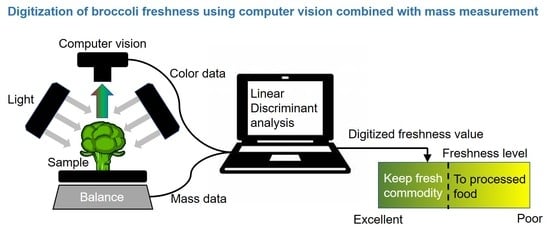
2.3. Digitization of Freshness
3. Results
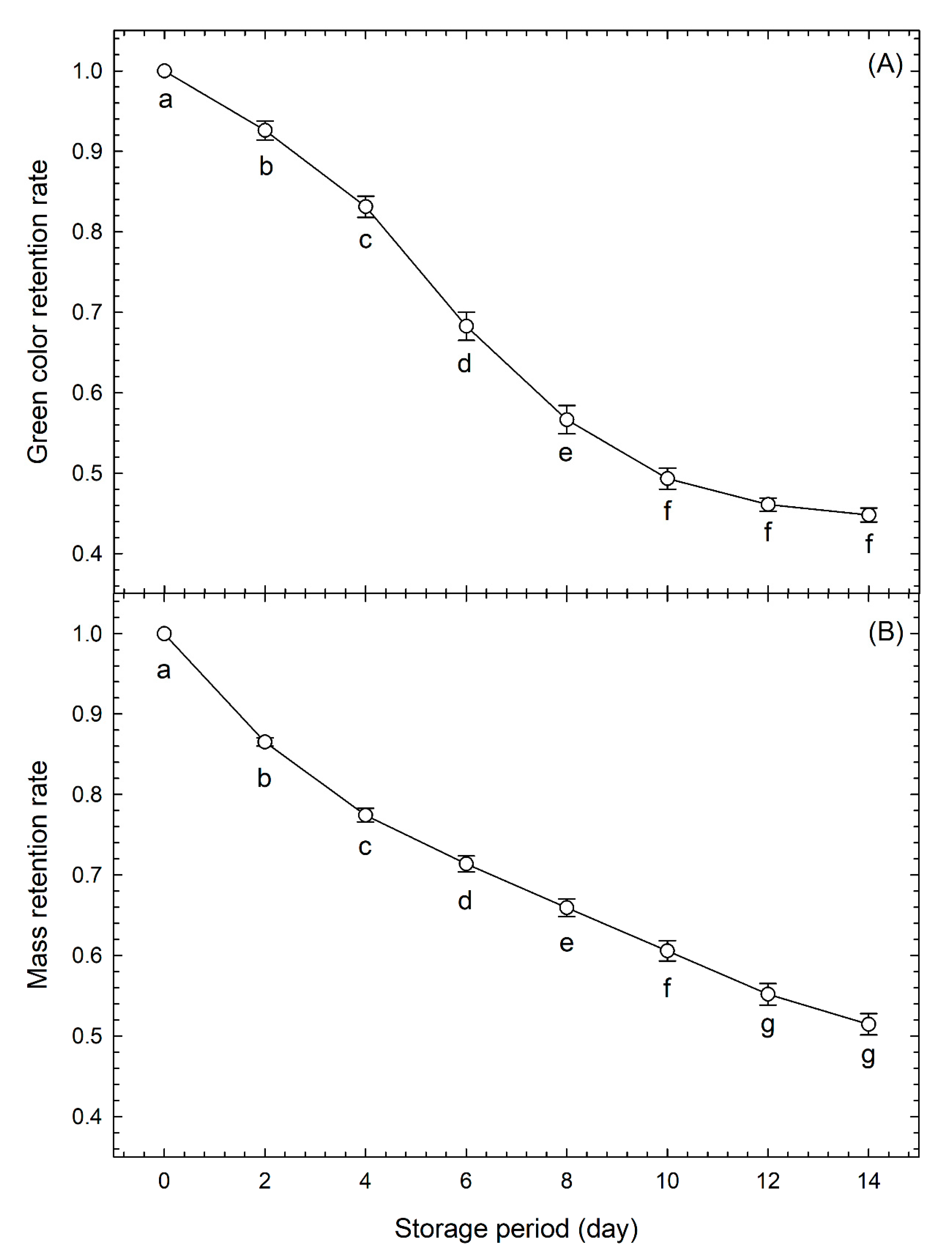
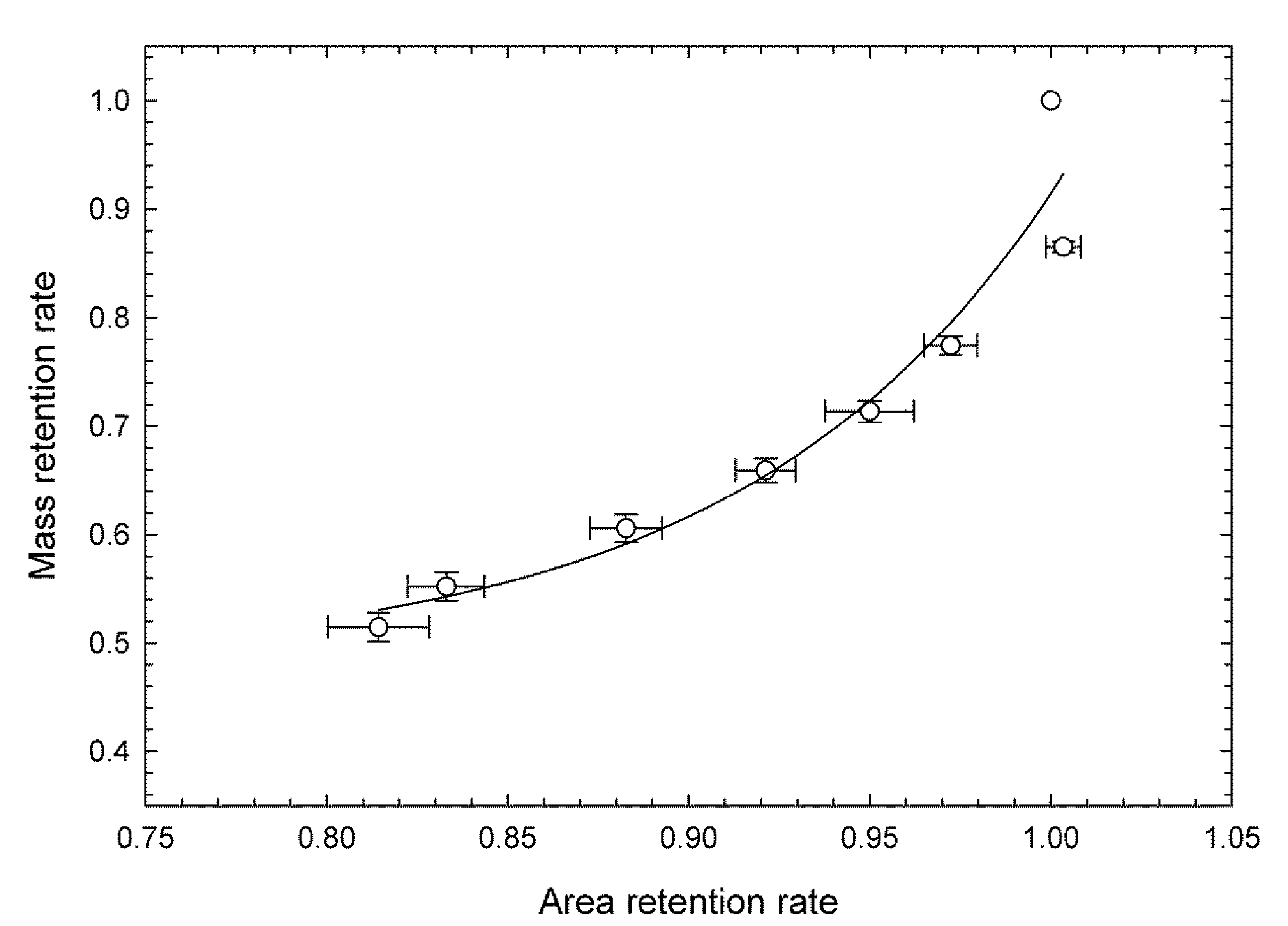
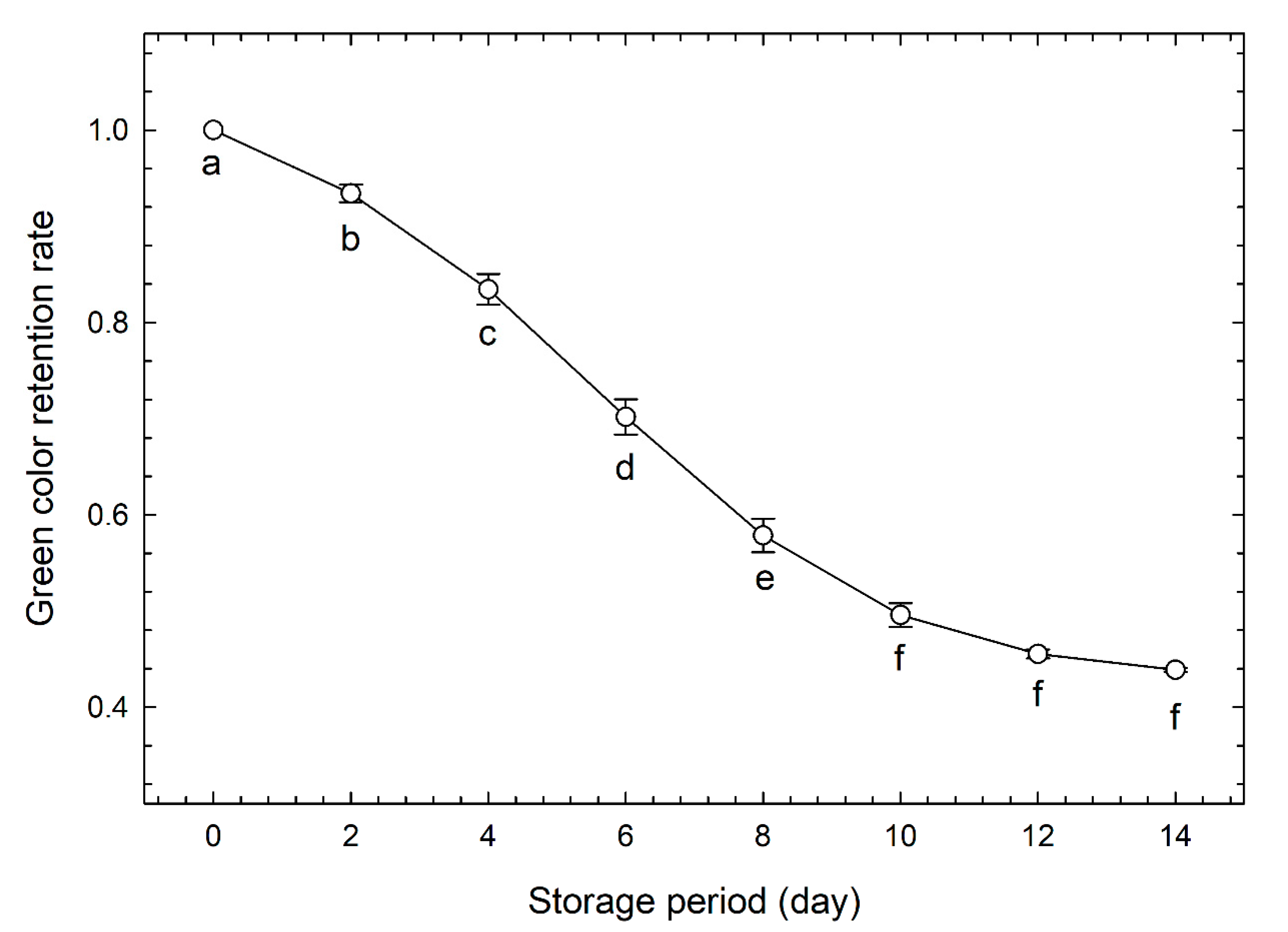
3.1. Evaluation of Freshness by Conventional Methods
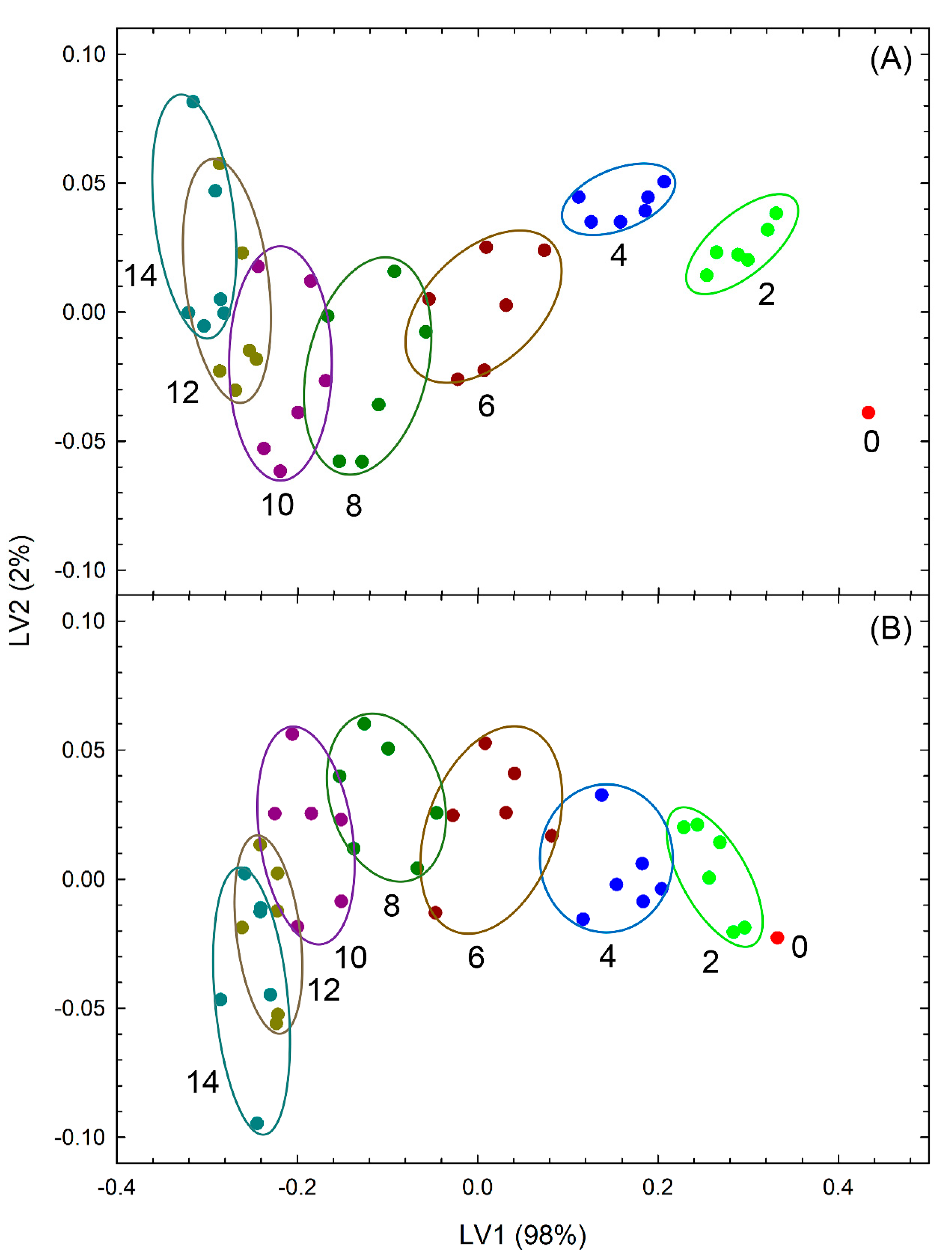
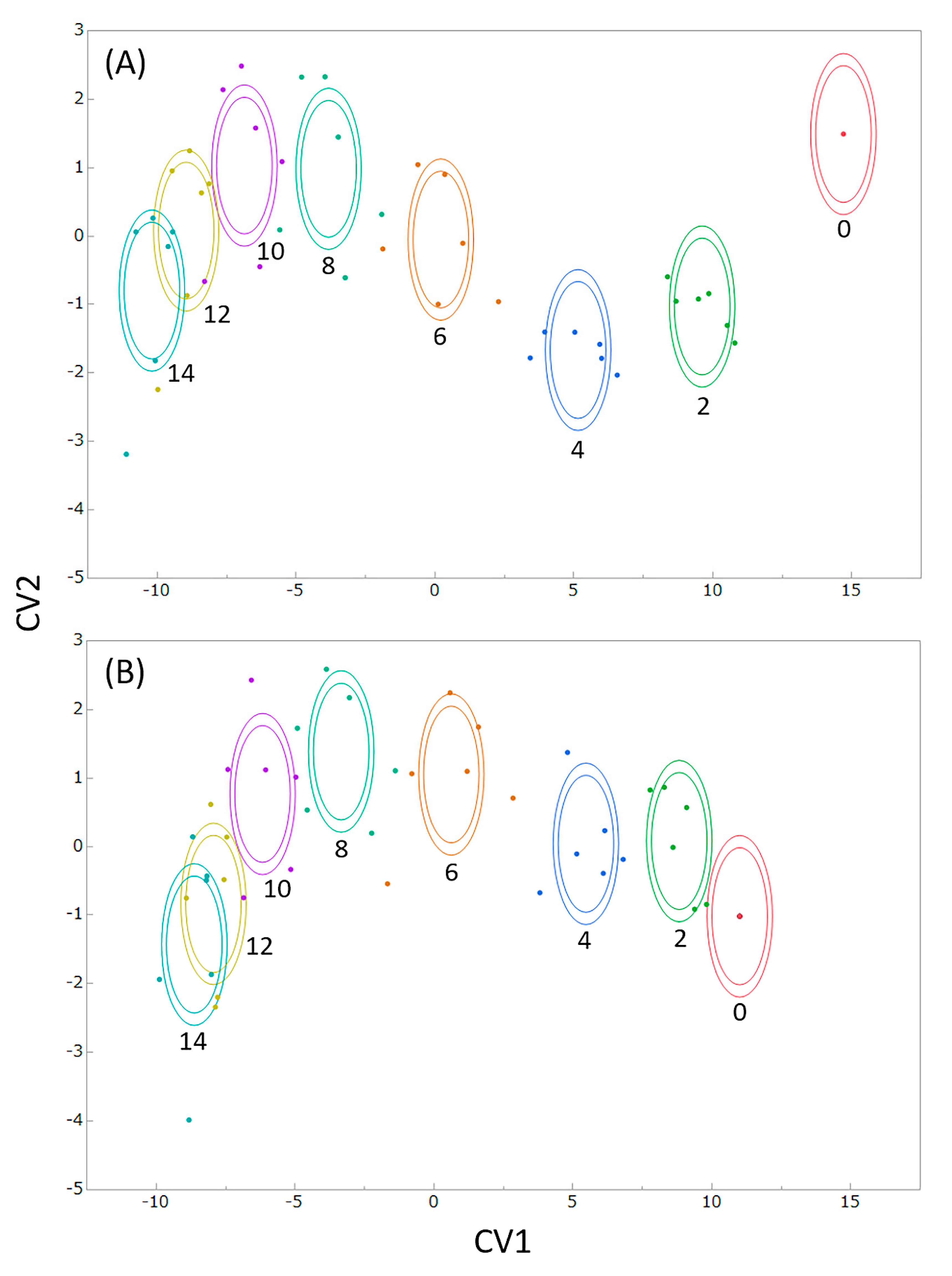
3.2. Evaluation of Freshness by a Novel Evaluation Index Integrating Green Color, Mass, and Area Retention Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baker, G.; Calvin, L.; Gillman, A.; Kitinoja, L.; Osland, T.; Pearson, O.; Prezkop, L.; Roe, B.E.; Spang, E.; Tooley, J.B.; et al. The Economics of Food Loss in the Produce Industry; Minor, T., Thornsbury, S., Mishra, A.K., Eds.; Routledge, Taylor and Francis Group: New York, NY, USA, 2020; pp. 131–204. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Global Food Losses and Food Waste; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; pp. 1–29. [Google Scholar]
- Li, L.; Lichter, A.; Kenigsbuch, D.; Porat, R. Effects of cooling delays at the wholesale market on the quality of fruit and vegetables after retail marketing. J. Food Process. Preserv. 2015, 39, 2533–2547. [Google Scholar] [CrossRef]
- Thompson, A.K. Controlled Atmosphere Storage for Fruit and Vegetables; CAB Int.: Oxon, NY, USA, 1998; pp. 14–55. [Google Scholar]
- Nakhasi, S.; Schlimme, D.; Solomos, T. Storage potential of tomatoes harvested at the breaker stage using modified atmosphere packaging. J. Food Sci. 1991, 56, 55–59. [Google Scholar] [CrossRef]
- Cia, P.; Benato, E.A.; Sigrist, J.M.M.; Sarantopóulos, C.; Oliveira, L.M.; Padula, M. Modified atmosphere packaging for extending the storage life of ‘Fuyu’ persimmon. Postharvest Biol. Technol. 2006, 42, 228–234. [Google Scholar] [CrossRef]
- Boerzhijin, S.; Yoshimura, M.; Sotome, I.; Hirai, M.Y.; Makino, Y. Perforation-mediated modified atmosphere packaging of intact kale. Food Pack. Shelf Life 2020, 23, 100427. [Google Scholar] [CrossRef]
- Makino, Y.; Nishizaka, A.; Yoshimura, M.; Sotome, I.; Kawai, K.; Akihiro, T. Influence of low O2 and high CO2 environment on changes in metabolite concentrations in harvested vegetable soybeans. Food Chem. 2020, 317, 126380. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Snelgar, W.; Lay-Yee, M.; Watkins, C.B.; Bowen, J.H. Expression of heat shock protein genes in apple fruit in the field. Aust. J. Plant Physiol. 1998, 25, 155–163. [Google Scholar] [CrossRef]
- Akbudak, B.; Akbudak, N.; Seniz, V.; Eris, A. Sequential treatments of hot water and modified atmosphere packaging in cherry tomatoes. J. Food Qual. 2007, 30, 896–910. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Pu, H.B.; Zhu, Z.W. Development of hyperspectral imaging coupled with chemometric analysis to monitor K value for evaluation of chemical spoilage in fish fillets. Food Chem. 2015, 185, 245–253. [Google Scholar] [CrossRef]
- Kader, A.A. Quality and safety factors: Definition and evaluation for fresh horticultural crops. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California, Agriculture and Natural Resources: Oakland, CA, USA, 2002; pp. 279–285. [Google Scholar]
- Bovi, G.G.; Caleb, O.J.; Linke, M.; Rauf, C.; Mahajan, P. Transpiration and moisture evolution in packaged fresh horticultural produce and the role of integrated mathematical models: A review. Biosys. Eng. 2016, 150, 24–39. [Google Scholar] [CrossRef]
- Tucker, G.A. 1.4 Texture changes. In Biochemistry of Fruit Ripening; Seymour, G.B., Taylor, J.E., Tucker, G.A., Eds.; Chapman & Hall: London, UK, 1993; pp. 17–24. [Google Scholar]
- Yamauchi, N.; Iida, S.; Minamide, T.; Iwata, T. Polar lipids content and their fatty acid composition with reference to yellowing of stored spinach leaves. J. Jpn. Soc. Hort. Sci. 1986, 55, 355–362. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Chen, Y.; Zhang, Z.Q.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Safety 2018, 2, 111–119. [Google Scholar]
- Zhan, L.; Hu, J.; Pang, L.; Li, Y.; Shao, J. Light exposure reduced browning enzyme activity and accumulated total phenols in cauliflower heads during cool storage. Postharvest Biol. Technol. 2014, 88, 17–20. [Google Scholar] [CrossRef]
- Aiamla-or, S.; Shigyo, M.; Ito, S.; Yamauchi, N. Involvement of chloroplast peroxidase on chlorophyll degradation in postharvest broccoli florets and its control by UV-B treatment. Food Chem. 2014, 165, 224–231. [Google Scholar] [CrossRef]
- Lipton, W.J.; Harris, C.M. Controlled atmosphere effects on the market quality of stored broccoli (Brassica oleracea L., italica group). J. Am. Soc. Hort. Sci. 1974, 99, 200–205. [Google Scholar]
- Robinson, J.E.; Brown, K.M.; Burton, W.G. Storage characteristics of some vegetables and soft fruits. Ann. Appl. Biol. 1975, 81, 399–408. [Google Scholar] [CrossRef]
- Makino, Y. Oxygen consumption by fruits and vegetables. Food Sci. Technol. Res. 2013, 19, 523–529. [Google Scholar] [CrossRef]
- Brannd, M.; Henderson, R.; Parson, W.W.; Schatz, G.; Smith, A. The respiratory chain and ATP synthase. In Molecular Biology of the Cell, 3rd ed.; Alberts, B., Bray, D., Lewis, J., Ra, M., Roberts, K., Watson, J.D., Eds.; Garland Publishing Inc.: New York, NY, USA, 1994; pp. 672–683. [Google Scholar]
- Makino, Y.; Inoue, J.; Wang, H.W.; Yoshimura, M.; Maejima, K.; Noguchi, S.F.; Yamada, T.; Noguchi, K. Induction of terminal oxidases of electron transport chain in broccoli heads under controlled atmosphere storage. Foods 2020, 9, 380. [Google Scholar] [CrossRef]
- Wang, H.W.; Makino, Y.; Inoue, J.; Maejima, K.; Funayama-Noguchi, S.; Yamada, T.; Noguchi, K. Influence of modified atmosphere on induction and activity of respiratory enzymes in broccoli florets during the early stage of postharvest storage. J. Agric. Food Chem. 2017, 65, 8538–8543. [Google Scholar] [CrossRef]
- McLaren, K. The development of the CIE 1976 (L*a*b*), Uniform colour space and colour-difference formula. J. Soc. Dyers Colour. 1976, 92, 338–341. [Google Scholar] [CrossRef]
- Yokota, Y.; Akihiro, T.; Boerzhijin, S.; Yamada, T.; Makino, Y. Effect of the storage atmosphere on metabolomics of harvested tomatoes (Solanum lycopersicum L.). Food Sci. Nutr. 2019, 7, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Goto, K.; Oshita, S.; Sato, A.; Tsukada, M. A grading method for mangoes on the basis of peel color measurement using a computer vision system. Agric. Sci. 2016, 7, 327–334. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Makino, Y.; Duan, Z.N.; Yoshimura, M.; Sotome, I. Nondestructive detection of decay in vegetable soybeans stored at different temperatures using chlorophyll fluorescence imaging. Environ. Control Biol. 2020, 58. in press. [Google Scholar] [CrossRef]
- Makhlouf, J.; Castaigne, F.; Arul, J.; Willemot, C.; Gosselin, A. Long-term storage of broccoli under controlled-atmosphere. Hortscience 1989, 24, 637–639. [Google Scholar]
- Makino, Y.; Wakatsuki, A.; Amino, G.; Oshita, S.; Sato, A.; Tsukada, M. Objective evaluation of external quality of broccoli heads using a computer vision system. Jpn. J. Food Eng. 2016, 17, 107–113. [Google Scholar] [CrossRef]
- Makino, Y.; Kousaka, Y. Prediction of degreening velocity of broccoli buds using hyperspectral camera combined with artificial neural networks. Foods 2020, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, F.; Dejmek, P.; Aguilera, J.M. Calibrated Color Measurements of Agricultural Foods Using Image Analysis. Postharvest Biol. Technol. 2006, 41, 285–295. [Google Scholar] [CrossRef]
- Nagano, T.; Shimaji, H. Internal plant-water-status and its control (1) Measurement of internal plant water status. J. Agric. Met. 1976, 32, 67–71. [Google Scholar] [CrossRef][Green Version]
- Lee, L.C.; Liong, C.Y.; Jemain, A.A. Partial least squares-discriminant analysis (PLS-DA) for classification of high-dimensional (HD) data: A review of contemporary practice strategies and knowledge gaps. Analyst 2018, 143, 3526. [Google Scholar] [CrossRef]
- Coomans, D.; Massart, D.L.; Kaufman, L. Optimization by statistical linear discriminant-analysis in analytical-chemistry. Anal. Chim. Acta-Comput. Tech. Optim. 1979, 3, 97–122. [Google Scholar] [CrossRef]
- Kasim, R.; Kasim, M.U.; Erkal, S. The effect of packaging after 1-MCP treatment on color changes and chlorophyll degradation of broccoli (Brassica oleracea var. italica cv. Monopoly). J. Food Agric. Environ. 2007, 5, 48–51. [Google Scholar]
- Ren, K.; Tu, K.; Pan, L.Q.; Chen, Y.Y. Kinetic modelings of broccoli color changes during chilled storage. J. Food Process. Preserv. 2006, 30, 180–193. [Google Scholar] [CrossRef]
| Variable | Effect | Degree of Freedom | Sum of Square | F | p |
|---|---|---|---|---|---|
| Green color retention rate | Storage period | 7 | 2.01 | 309 | <0.001 *** |
| Error | 40 | 0.0371 | |||
| Total | 47 | 2.05 | |||
| Mass retention rate | Storage period | 7 | 1.13 | 261 | <0.001 *** |
| Error | 40 | 0.0248 | |||
| Total | 47 | 1.16 | |||
| Area retention rate | Storage period | 7 | 0.223 | 60.6 | <0.001 *** |
| Error | 40 | 0.0210 | |||
| Total | 47 | 0.244 |
| (A) Covariates: Green Color and Mass Retention Rate | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ac(days) | Pr(days) | |||||||
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | |
| 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| 6 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| 12 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| (B) Covariates: Green Color and Area Retention Rate | ||||||||
| Ac(days) | Pr(days) | |||||||
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | |
| 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| 12 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| 14 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| (A) Covariates: Green Color and Mass Retention Rate | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ac(days) | Pr(days) | |||||||
| 0 | 2 | 5 | 6 | 8 | 10 | 12 | 14 | |
| 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| (B) Covariates: Green Color and Area Retention Rate | ||||||||
| Ac(days) | Pr(days) | |||||||
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | |
| 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| 12 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makino, Y.; Amino, G. Digitization of Broccoli Freshness Integrating External Color and Mass Loss. Foods 2020, 9, 1305. https://doi.org/10.3390/foods9091305
Makino Y, Amino G. Digitization of Broccoli Freshness Integrating External Color and Mass Loss. Foods. 2020; 9(9):1305. https://doi.org/10.3390/foods9091305
Chicago/Turabian StyleMakino, Yoshio, and Genki Amino. 2020. "Digitization of Broccoli Freshness Integrating External Color and Mass Loss" Foods 9, no. 9: 1305. https://doi.org/10.3390/foods9091305
APA StyleMakino, Y., & Amino, G. (2020). Digitization of Broccoli Freshness Integrating External Color and Mass Loss. Foods, 9(9), 1305. https://doi.org/10.3390/foods9091305