The Impact of Plant-Based Coatings in “ROCHA” Pear Preservation during Cold Storage: A Metabolomic Approach
Abstract
:1. Introduction
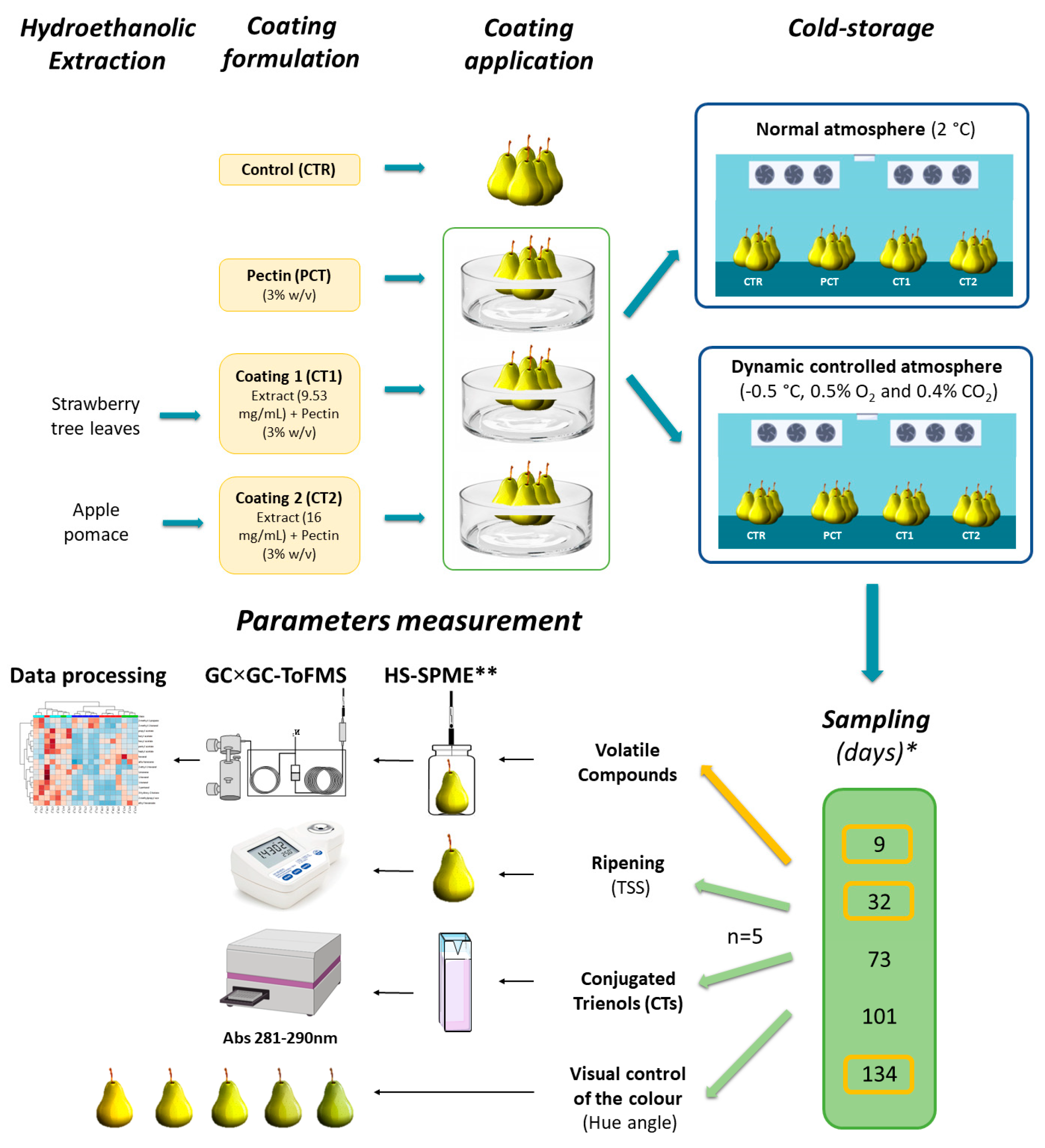
2. Materials and Methods
2.1. Samples, Materials, and Reagents
2.2. Plant-Based Coating
2.2.1. Extracts Preparation
2.2.2. Coating Formulation
2.3. Pilot-Scale Storage Assay
2.4. Conjugated Trienols Analysis
2.5. Assessment of Fruit Maturity
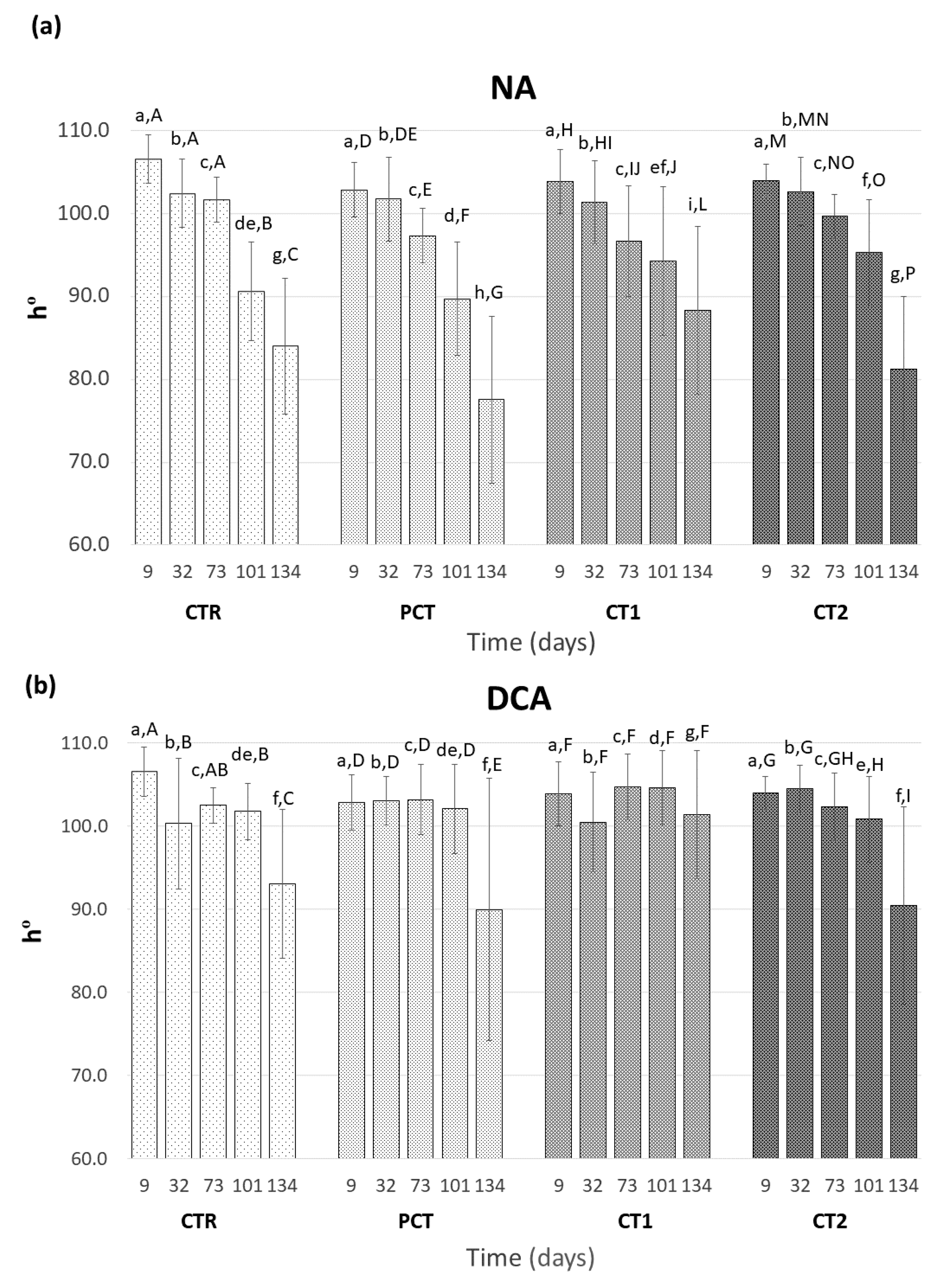
2.6. Surface Color Determination
2.7. Volatile Profile Determination
2.7.1. HS-SPME/GC×GC-ToFMS
2.7.2. Data Processing
3. Results
3.1. Pear Chromatogram Contour Plot Analysis
3.2. Impact of Plant-Based Coatings on Previously Reported Volatile Compounds of “Rocha” Pear
3.3. Impact of Plant-Based Coatings on “Rocha” Pear Physiology during Storage
3.3.1. Fruit Maturity
3.3.2. Accumulation of Conjugated Trienols (CTs)
3.3.3. Volatile Metabolites Analysis
4. Discussion
5. Conclusions
Supplementary Materials
 CTR (control);
CTR (control);  PCT (pectin);
PCT (pectin);  CT1 (coating 1);
CT1 (coating 1);  CT2 (coating 2). Figure S2: Dendrograms of the hierarchical clustering analysis (HCA) for previously reported volatile compounds of ‘Rocha’ pear at 9, 32 and 134 days of the assay in DCA (dynamic controlled atmosphere) storage conditions. Euclidean distances are included on the dendrogram Y-axis. The relative content of each compound, illustrated through a chromatic scale (from low (dark blue) to high chromatographic area (dark red black)), corresponds to its peak area normalized by autoscaling.
CT2 (coating 2). Figure S2: Dendrograms of the hierarchical clustering analysis (HCA) for previously reported volatile compounds of ‘Rocha’ pear at 9, 32 and 134 days of the assay in DCA (dynamic controlled atmosphere) storage conditions. Euclidean distances are included on the dendrogram Y-axis. The relative content of each compound, illustrated through a chromatic scale (from low (dark blue) to high chromatographic area (dark red black)), corresponds to its peak area normalized by autoscaling.  CTR (control);
CTR (control);  PCT (pectin);
PCT (pectin);  CT1 (coating 1);
CT1 (coating 1);  CT2 (coating 2).
CT2 (coating 2).Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Instituto Nacional de Estatística. Estatísticas Agrícolas: 2018; INE: Lisboa, Portugal, 2019; ISBN 978-989-25-0495-7. [Google Scholar]
- Pêra Rocha—A Pera Rocha Distingue-se Pelas suas Características Únicas, que Fazem dela um Sabor de Portugal. Available online: http://perarocha.pt/ (accessed on 5 June 2020).
- Saquet, A.A. Storage of pears. Sci. Hortic. 2019, 246, 1009–1016. [Google Scholar] [CrossRef]
- Saquet, A.A.; Almeida, D.P.F. Ripening physiology and biochemistry of ‘Rocha’ pear as affected by ethylene inhibition. Postharvest Biol. Technol. 2017, 125, 161–167. [Google Scholar] [CrossRef]
- Isidoro, N.; Almeida, D.P.F. α-Farnesene, conjugated trienols, and superficial scald in “Rocha” pear as affected by 1-methylcyclopropene and diphenylamine. Postharvest Biol. Technol. 2006, 42, 49–56. [Google Scholar] [CrossRef]
- Rapparini, F.; Predieri, S. Pear fruit volatiles. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003; Volume 28, pp. 237–324. ISBN 9780471215424. [Google Scholar]
- Zhou, S.; Cheng, Y.; Guan, J. The molecular basis of superficial scald development related to ethylene perception and α-farnesene metabolism in ‘Wujiuxiang’ pear. Sci. Hortic. 2017, 216, 76–82. [Google Scholar] [CrossRef]
- Lurie, S.; Watkins, C.B. Superficial scald, its etiology and control. Postharvest Biol. Technol. 2012, 65, 44–60. [Google Scholar] [CrossRef]
- Dias, C.; Amaro, A.L.; Salvador, Â.C.; Silvestre, A.J.D.; Rocha, S.M.; Isidoro, N.; Pintado, M. Strategies to Preserve Postharvest Quality of Horticultural Crops and Superficial Scald Control: From Diphenylamine Antioxidant Usage to More Recent Approaches. Antioxidants 2020, 9, 356. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Baraiya, N.S.; Rao, T.V.R.; Thakkar, V.R. Improvement of Postharvest quality and storability of jamun fruit (Syzygium cumini L. var. Paras) by zein coating enriched with antioxidants. Food Bioprocess Technol. 2015, 8, 2225–2234. [Google Scholar] [CrossRef]
- Poverenov, E.; Rutenberg, R.; Danino, S.; Horev, B.; Rodov, V. Gelatin-chitosan composite films and edible coatings to enhance the quality of food products: Layer-by-layer vs. Blended formulations. Food Bioprocess Technol. 2014, 7, 3319–3327. [Google Scholar] [CrossRef]
- Gago, C.; Antão, R.; Dores, C.; Guerreiro, A.; Miguel, M.G.; Faleiro, M.L.; Figueiredo, A.C.; Antunes, M.D. The effect of nanocoatings enriched with essential oils on “Rocha” pear long storage. Foods 2020, 9, 240. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Jung, J.; Simonsen, J.; Wang, Y.; Zhao, Y. Cellulose nanocrystal reinforced chitosan coatings for improving the storability of postharvest pears under both ambient and cold storages. J. Food Sci. 2017, 82, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Ankolekar, C.; Greene, D.; Shetty, K. Natural preservatives for superficial scald reduction and enhancement of protective phenolic-linked antioxidant responses in apple during post-harvest storage. J. Food Sci. Technol. 2018, 55, 1767–1780. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T.V.R. Xanthan gum based edible coating enriched with cinnamic acid prevents browning and extends the shelf-life of fresh-cut pears. LWT Food Sci. Technol. 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Dias, C.; Fonseca, A.M.A.; Amaro, A.L.; Vilas-Boas, A.A.; Oliveira, A.; Santos, S.A.O.; Silvestre, A.J.D.; Rocha, S.M.; Isidoro, N.; Pintado, M. Natural-based antioxidant extracts as potential mitigators of fruit browning. Antioxidants 2020, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Yan, S.; Feng, Z.; Xiao, L.; Hu, X.S. Changes in the volatile compounds and chemical and physical properties of Yali pear (Pyrus bertschneideri Reld) during storage. Food Chem. 2006, 97, 248–255. [Google Scholar] [CrossRef]
- Ji, L.C.; Ji, H.W.; Wang, Q.; Deng, H.; Xiao, S.H. Changes in the volatile compounds and chemical and physical properties of Kuerle fragrant pear (Pyrus serotina Reld) during storage. J. Agric. Food Chem. 2006, 54, 8842–8847. [Google Scholar] [CrossRef]
- Zlatić, E.; Zadnik, V.; Fellman, J.; Demšar, L.; Hribar, J.; Čejić, Ž.; Vidrih, R. Comparative analysis of aroma compounds in “Bartlett” pear in relation to harvest date, storage conditions, and shelf-life. Postharvest Biol. Technol. 2016, 117, 71–80. [Google Scholar] [CrossRef]
- Hendges, M.V.; Neuwald, D.A.; Steffens, C.A.; Vidrih, R.; Zlatić, E.; do Amarante, C.V.T. 1-MCP and storage conditions on the ripening and production of aromatic compounds in Conference and Alexander Lucas pears harvested at different maturity stages. Postharvest Biol. Technol. 2018, 146, 18–25. [Google Scholar] [CrossRef]
- Rizzolo, A.; Polesello, A. Use of headspace capillary GC to study the development of volatile compounds in fresh fruit. J. High Resolut. Chromatogr. 1992, 15, 472–477. [Google Scholar] [CrossRef]
- Jayanty, S.; Song, J.; Rubinstein, N.M.; Chong, A.; Beaudry, R.M. Temporal relationship between ester biosynthesis and ripening events in bananas. J. Am. Soc. Hortic. Sci. 2002, 127, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Avelar, M.L.; Rizzolo, A.; Lombardi, P.; Eccher Zerbini, P. Physiological and quality responses of “Rocha” pear to controlled atmospher storage. In Proceedings of the Sixth International Symposium of the European Concerted Action Program COST 94, Oosterbeek, The Netherlands, 19–22 October 1994; Woltering, E.J., Gorris, L.G., Jongen, W.M.F., McKcnna, Β., Höhn, E., Bertolini, P., Woolfe, M.L., de Jager, A., Ahvenainen, R., Calero, F.A., Eds.; European Communities: Oosterbeek, The Netherlands, 1994; pp. 15–22. [Google Scholar]
- Gomes, M.H.; Beaudry, R.M.; Almeida, D.P.F. Volatile profile of fresh-cut ‘Rocha’ pear under various temperatures and oxygen levels. Acta Hortic. 2018, 247–250. [Google Scholar] [CrossRef]
- Pott, D.M.; Vallarino, J.G.; Osorio, S. Metabolite changes during postharvest storage: Effects on fruit quality traits. Metabolites 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, W.; Li, H.; Mao, J.; Guo, C.; Ding, R.; Wang, Y.; Fang, L.; Chen, Z.; Yang, G. Analysis of volatile compounds in pears by HS-SPME-GC×GC-TOFMS. Molecules 2019, 24, 1795. [Google Scholar] [CrossRef] [Green Version]
- Górecki, T.; Harynuk, J.; Panić, O. The evolution of comprehensive two-dimensional gas chromatography (GC×GC). J. Sep. Sci. 2004, 27, 359–379. [Google Scholar] [CrossRef]
- Martins, C. Yeasts and Beers Volatile Metabolomic Profiling: Advances In Lager Beer Aroma Comprehension. Ph.D. Thesis, Universidade de Aveiro, Aveiro, Portugal, 2018. [Google Scholar]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Anet, E.F.L.J. Superficial scald, a functional disorder of stored apples. IX. Effect of maturity and ventilation. J. Sci. Food Agric. 1972, 23, 763–769. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Salvador, Â.C.; Rudnitskaya, A.; Silvestre, A.J.D.; Rocha, S.M. Metabolomic-based strategy for fingerprinting of Sambucus nigra L. berry volatile terpenoids and norisoprenoids: Influence of ripening and cultivar. J. Agric. Food Chem. 2016, 64, 5428–5438. [Google Scholar] [CrossRef]
- Martins, C.; Brandão, T.; Almeida, A.; Rocha, S.M. Unveiling the lager beer volatile terpenic compounds. Food Res. Int. 2018, 114, 199–207. [Google Scholar] [CrossRef]
- Dallüge, J.; Beens, J.; Brinkman, U.A.T. Comprehensive two-dimensional gas chromatography: A powerful and versatile analytical tool. J. Chromatogr. A 2003, 1000, 69–108. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. Fatty acids as precursors for aroma volatile biosynthesis in pre-climacteric and climacteric apple fruit. Postharvest Biol. Technol. 2003, 30, 113–121. [Google Scholar] [CrossRef]
- Suwanagul, A. Ripening Pear Flavor Volatiles: Identification, Biosynthesis And Sensory Perception. Ph.D. Thesis, Horticulture, Oregon State University, Corvallis, OR, USA, 1996. [Google Scholar]
- Burdock, G. Fenaroli’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Yan, D.; Shi, J.; Ren, X.; Tao, Y.; Ma, F.; Li, R.; Liu, X.; Liu, C. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’ apple (Malus × domestica). Food Chem. 2020, 327, 127074. [Google Scholar] [CrossRef]
- Tian, H.; Zhan, P.; Deng, Z.; Yan, H.; Zhu, X. Development of a flavour fingerprint by GC-MS and GC-O combined with chemometric methods for the quality control of Korla pear ( Pyrus serotina Reld). Int. J. Food Sci. Technol. 2014, 49, 2546–2552. [Google Scholar] [CrossRef]
- Li, G.; Jia, H.; Wu, R.; Hussain, S.; Teng, Y. Characterization of aromatic volatile constituents in 11 Asian pear cultivars belonging to different species. Afr. J. Agric. Res. 2012, 7, 4761–4770. [Google Scholar] [CrossRef]
- Hui, Y.H.; Chen, F.; Nollet, L.M.L.; Guiné, R.P.F.; Le Quéré, J.L.; Martín-Belloso, O.; Mínguez-Mosquera, M.I.; Paliyath, G.; Pessoa, F.L.P.; Sidhu, J.S.; et al. Handbook of Fruit and Vegetable Flavors; Hui, Y.H., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 9780470622834. [Google Scholar]
- Yi, X.K.; Liu, G.F.; Rana, M.M.; Zhu, L.W.; Jiang, S.L.; Huang, Y.F.; Lu, W.M.; Wei, S. Volatile profiling of two pear genotypes with different potential for white pear aroma improvement. Sci. Hortic. 2016, 209, 221–228. [Google Scholar] [CrossRef]
- Almeida, D.P.; Carvalho, R.; Dupille, E. Efficacy of 1-methylcyclopropene on the mitigation of storage disorders of “Rocha” pear under normal refrigerated and controlled atmospheres. Food Sci. Technol. Int. 2016, 22, 399–409. [Google Scholar] [CrossRef]
- Giné Bordonaba, J.; Matthieu-Hurtiger, V.; Westercamp, P.; Coureau, C.; Dupille, E.; Larrigaudière, C. Dynamic changes in conjugated trienols during storage may be employed to predict superficial scald in “Granny Smith” apples. LWT Food Sci. Technol. 2013, 54, 535–541. [Google Scholar] [CrossRef]
- Shiota, H. Changes in the volatile composition of La France pear during maturation. J. Sci. Food Agric. 1990, 52, 421–429. [Google Scholar] [CrossRef]
- Rapparini, F.; Predieri, S. Volatile constituents of “Harrow” sweet pears by dynamic headspace technique. Acta Hortic. 2002, 811–816. [Google Scholar] [CrossRef]
- Paillard, N.M.M. The flavour of apples, pears and quinces. Dev. Food Sci. 1990, 3, 1–41. [Google Scholar]
- Medeiros, B.G.d.S.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Polysaccharide/Protein nanomultilayer coatings: Construction, characterization and evaluation of their effect on “Rocha” pear (Pyrus communis L.) shelf-life. Food Bioprocess Technol. 2012, 5, 2435–2445. [Google Scholar] [CrossRef] [Green Version]
- Saquet, A.A. Storability of ‘conference’ pear under various controlled atmospheres. Erwerbs Obstbau 2018, 60, 275–280. [Google Scholar] [CrossRef]
- Lima, Á.M.; Cerqueira, M.A.; Souza, B.W.S.; Santos, E.C.M.; Teixeira, J.A.; Moreira, R.A.; Vicente, A.A. New edible coatings composed of galactomannans and collagen blends to improve the postharvest quality of fruits—Influence on fruits gas transfer rate. J. Food Eng. 2010, 97, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Saquet, A.; Almeida, D. Internal disorders of ‘Rocha’ pear affected by oxygen partial pressure and inhibition of ethylene action. Postharvest Biol. Technol. 2017, 128, 54–62. [Google Scholar] [CrossRef]
- Saquet, A.A.; Streif, J.; Almeida, D.P.F. Responses of ‘Rocha’ pear to delayed controlled atmosphere storage depend on oxygen partial pressure. Sci. Hortic. 2017, 222, 17–21. [Google Scholar] [CrossRef]
- Chen, P.M.; Varga, R.J.; Xiao, Y.Q. Inhibition of α-farnesene biosynthesis and its oxidation in the peel tissue of “d’Anjou” pears by low-O2/elevated CO2 atmospheres. Postharvest Biol. Technol. 1993, 3, 215–223. [Google Scholar] [CrossRef]

 CTR (control);
CTR (control);  PCT (pectin);
PCT (pectin);  CT1 (coating 1);
CT1 (coating 1);  CT2 (coating 2).
CT2 (coating 2).
 CTR (control);
CTR (control);  PCT (pectin);
PCT (pectin);  CT1 (coating 1);
CT1 (coating 1);  CT2 (coating 2).
CT2 (coating 2).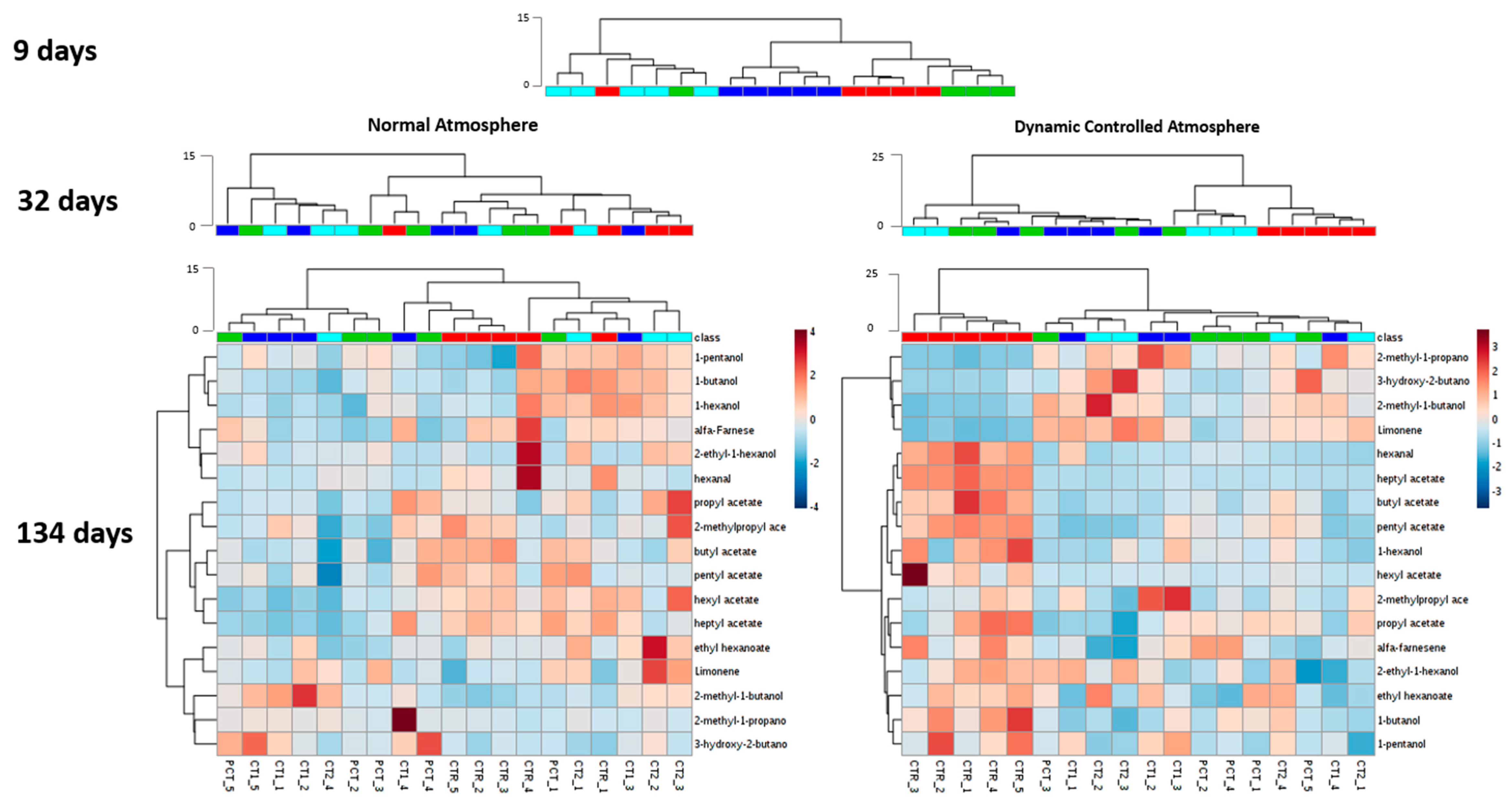
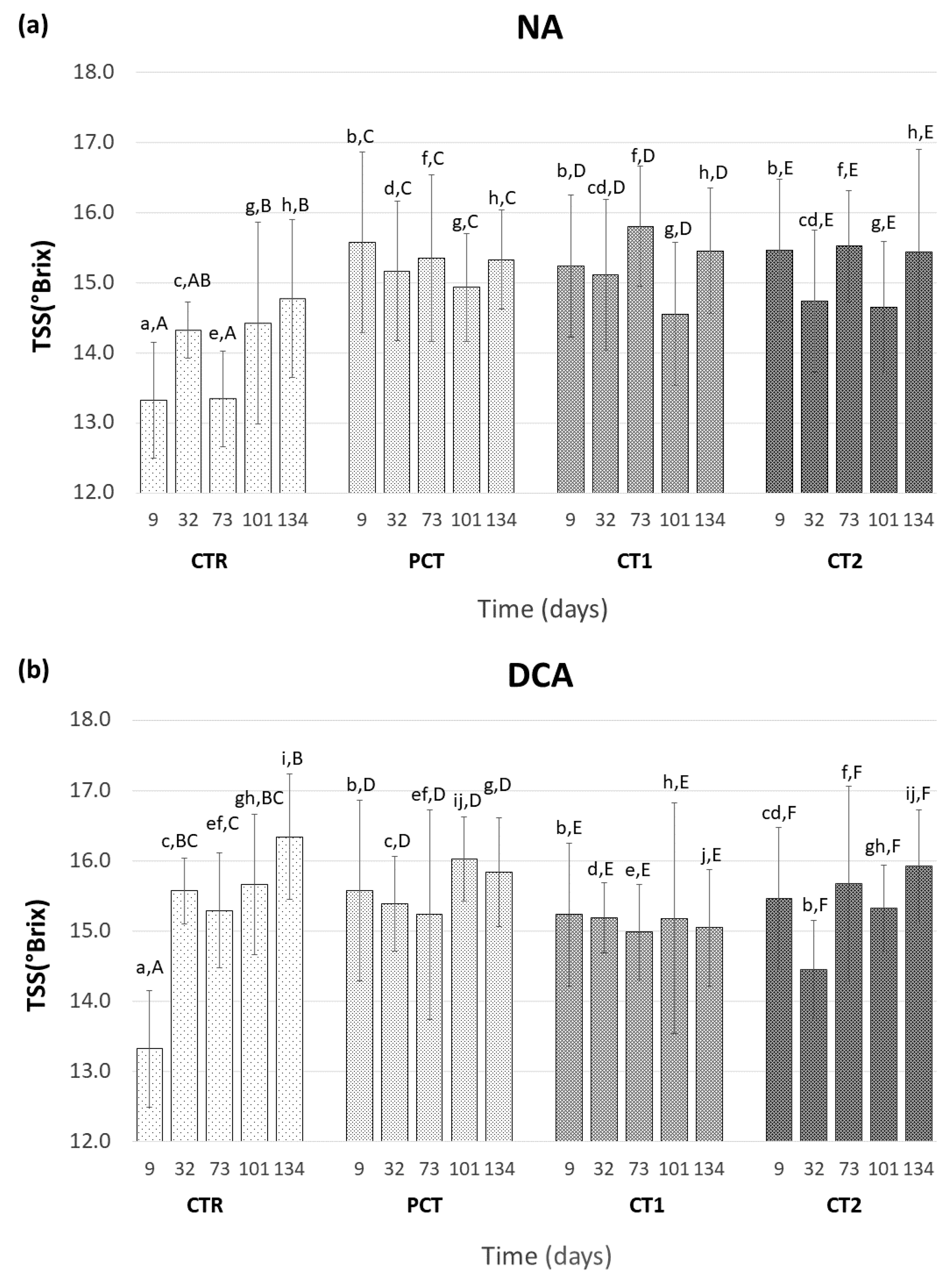
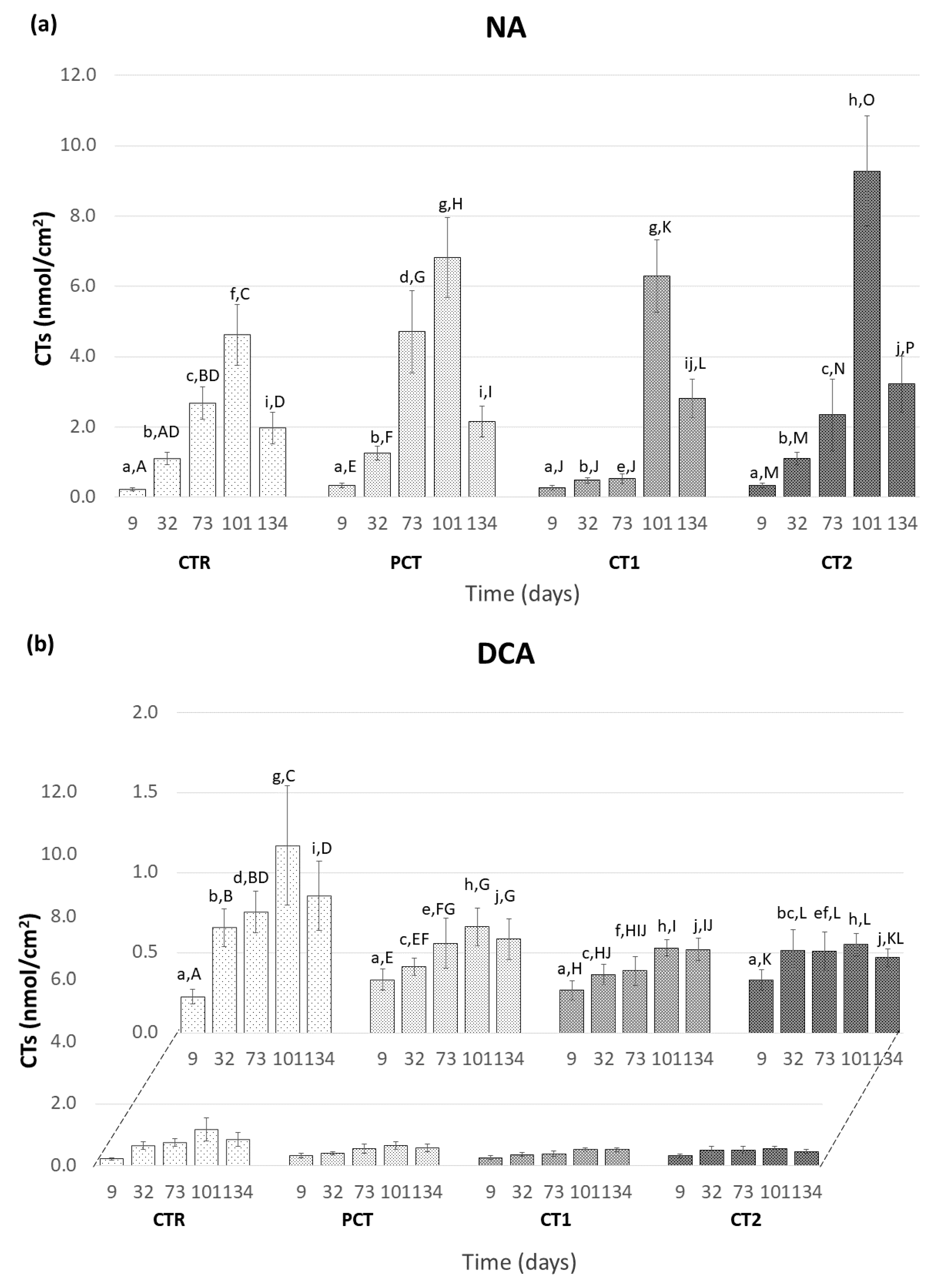
 CTR (control);
CTR (control);  PCT (pectin);
PCT (pectin);  CT1 (coating 1);
CT1 (coating 1);  CT2 (coating 2).
CT2 (coating 2).
 CTR (control);
CTR (control);  PCT (pectin);
PCT (pectin);  CT1 (coating 1);
CT1 (coating 1);  CT2 (coating 2).
CT2 (coating 2).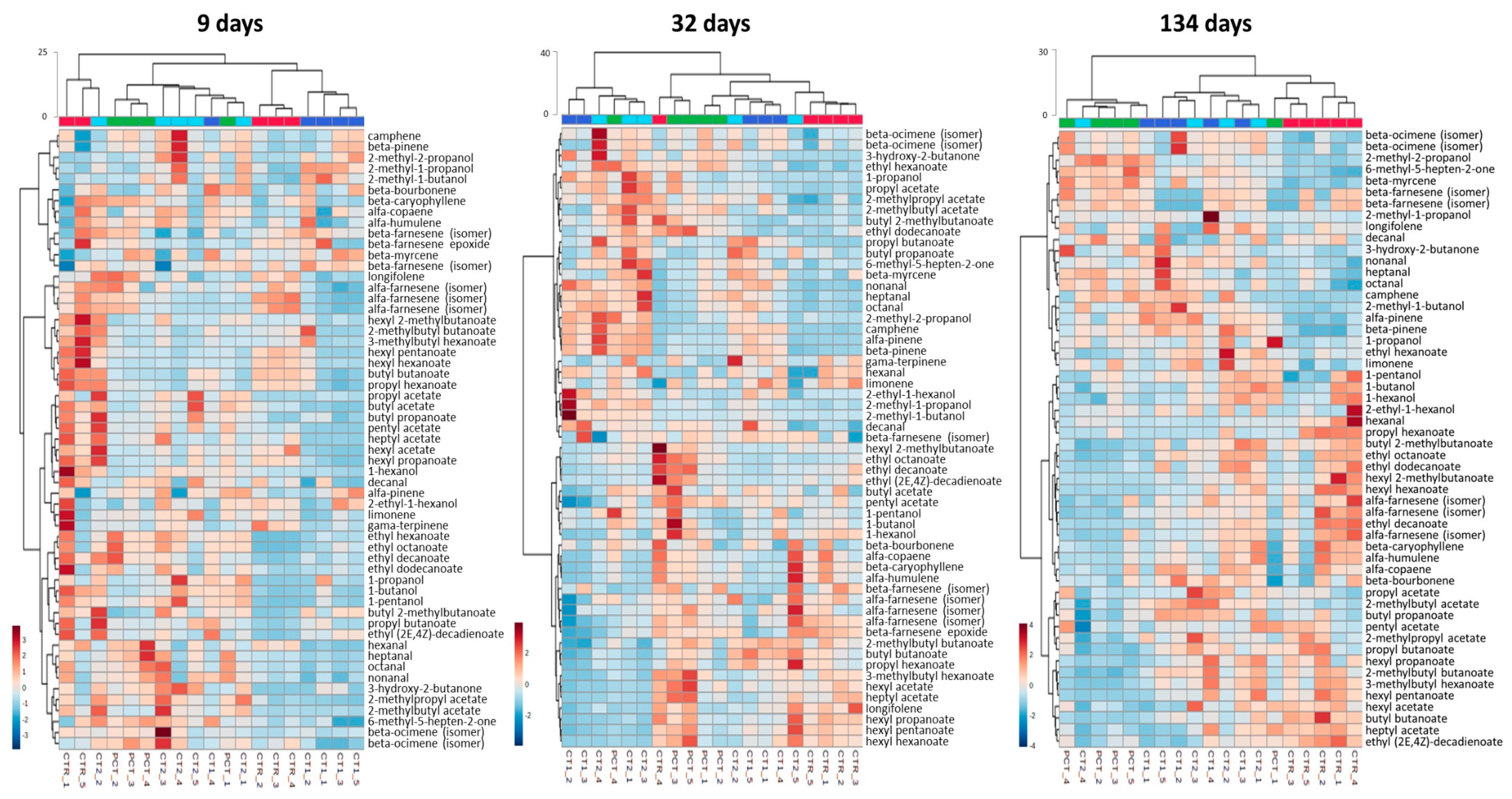
 CTR (control);
CTR (control);  PCT (pectin);
PCT (pectin);  CT1 (coating 1);
CT1 (coating 1);  CT2 (coating 2).
CT2 (coating 2).
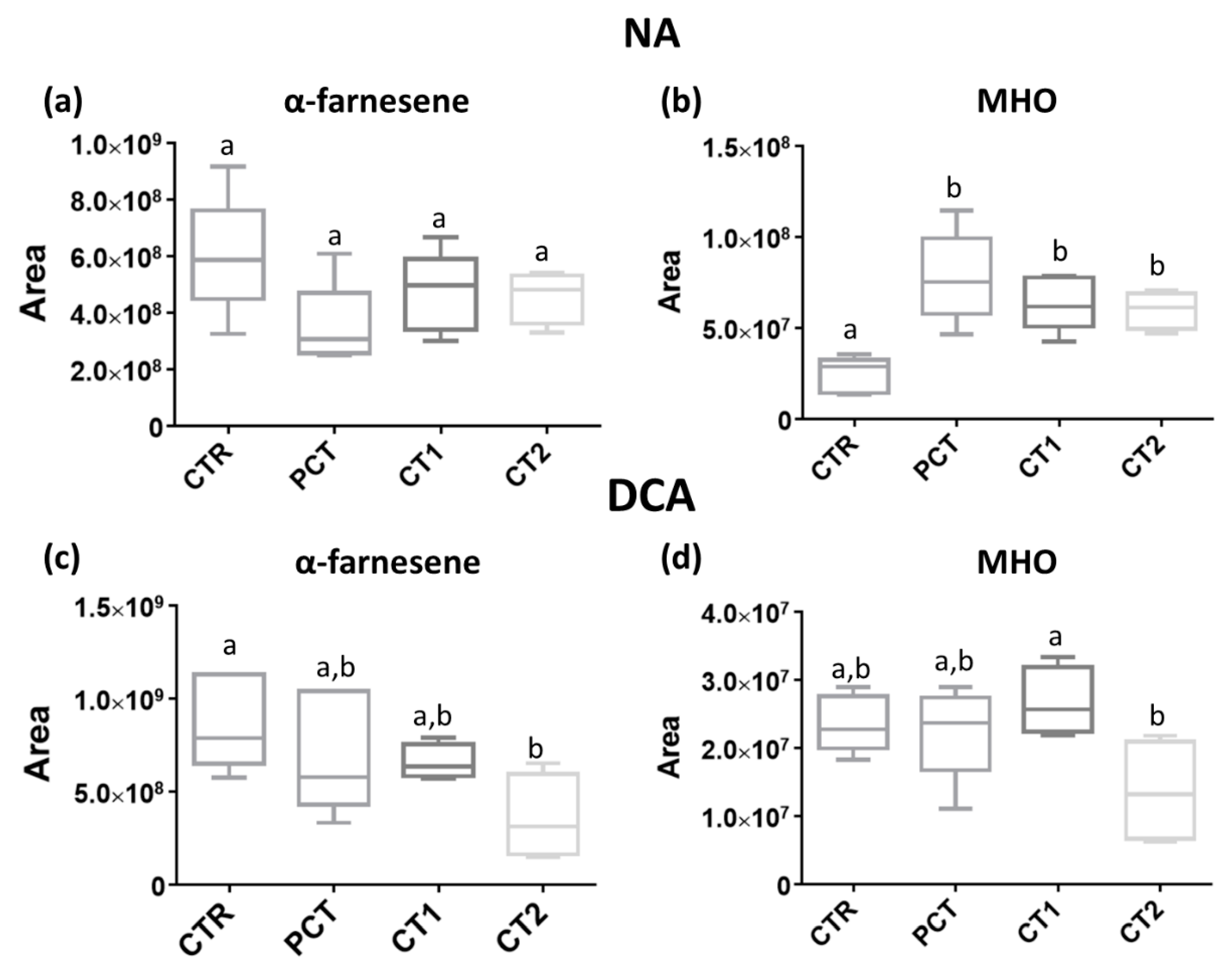
 CTR (control);
CTR (control);  PCT (pectin);
PCT (pectin);  CT1 (coating 1);
CT1 (coating 1);  CT2 (coating 2).
CT2 (coating 2).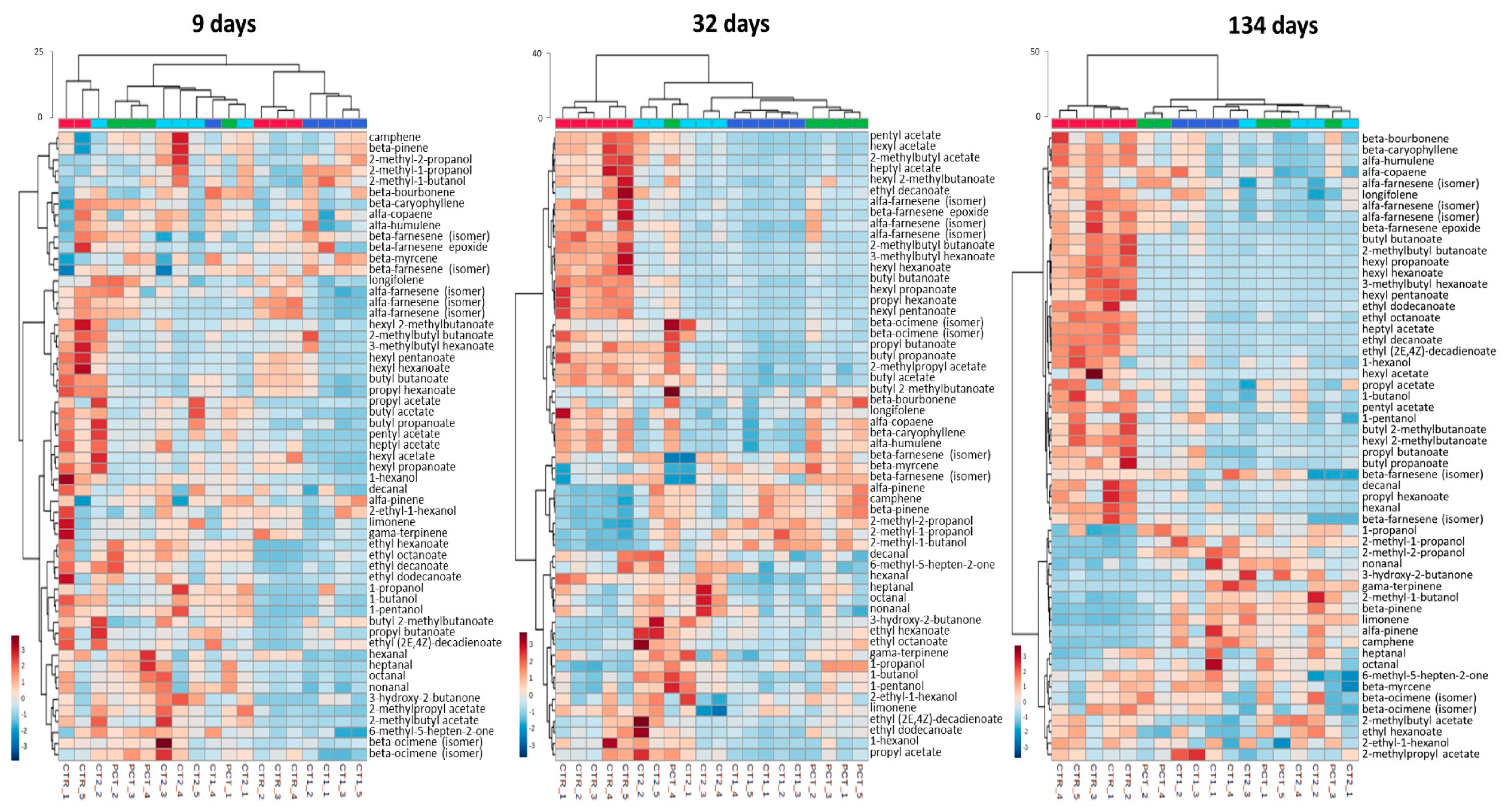
| 1tR (s) a | 2tR (s) a | Compound | Formula | CAS Number | RICalc. b | RILit c | Odor Descriptor | Ref. d |
|---|---|---|---|---|---|---|---|---|
| Alcohols | ||||||||
| 75 | 0.85 | 1-propanol | C3H8O | 71-23-8 | 590 | 591 | oxidized pear, aldehyde | [39] |
| 85 | 1.04 | 2-methyl-1-propanol | C4H10O | 78-83-1 | 614 | 615 | wine | [40] |
| 95 | 1.38 | 1-butanol | C4H10O | 71-36-3 | 638 | 637 | medicinal, metallic | [6,39] |
| 125 | 1.80 | 2-methyl-1-butanol | C5H12O | 1565-80-6 | 710 | 728 | acidic, sharp, spicy | [41] |
| 145 | 2.26 | 1-pentanol | C5H12O | 71-41-0 | 758 | 754 | roasted | [6] |
| 230 | 1.34 | 2-methyl-2-propanol | C4H10O | 75-65-0 | 855 | - | - | - |
| 250 | 3.43 | 1-hexanol | C6H14O | 111-27-3 | 873 | 877 | oxidized, soapy, fresh rose, fresh, grass, engine | [6,39] |
| 560 | 3.56 | 2-ethyl-1-hexanol | C8H18O | 104-76-7 | 1036 | 1036 | oily, sweet, floral | [40] |
| Aldehydes | ||||||||
| 170 | 0.91 | Hexanal | C6H12O | 66-25-1 | 804 | 801 | fruity, green | [6,40] |
| 295 | 1.18 | heptanal | C7H14O | 111-71-7 | 905 | 906 | fatty | [40] |
| 490 | 1.40 | octanal | C8H16O | 124-13-0 | 1006 | 1005 | fruity, orange | [42] |
| 730 | 1.52 | nonanal | C9H18O | 124-19-6 | 1104 | 1106 | orange, fresh rose, fatty | [40,42] |
| 995 | 1.54 | decanal | C10H20O | 112-31-2 | 1206 | 1207 | sweet, waxy, floral, citrus, fatty | [40] |
| Ketones | ||||||||
| 115 | 2.84 | 3-hydroxy-2-butanone | C4H8O2 | 513-86-0 | 689 | 697 | woody, yogurt | [40] |
| 460 | 1.89 | 6-methyl-5-hepten-2-one | C8H14O | 110-93-0 | 993 | 990 | fatty, green, citrus | [40] |
| Esters | ||||||||
| 115 | 0.63 | propyl acetate | C5H12O2 | 109-60-4 | 683 | 684 | floral, estery | [39] |
| 150 | 0.71 | 2-methylpropyl acetate | C6H12O2 | 110-19-0 | 766 | 769 | pear, apple, fruity, sweet, floral | [39] |
| 185 | 0.87 | butyl acetate | C6H12O2 | 123-86-4 | 817 | 819 | fruity, pear, floral, sweet | [20,39,43] |
| 265 | 0.93 | 2-methylbutyl acetate | C7H14O2 | 624-41-9 | 884 | 879 | apple peel, banana | [40] |
| 295 | 0.90 | propyl butanoate | C7H14O2 | 105-66-8 | 905 | 900 | pineapple, apricot, rancid, sweaty | [40] |
| 310 | 0.95 | butyl propanoate | C7H14O2 | 590-01-2 | 913 | 912 | earthy, sweet | [40] |
| 320 | 1.08 | pentyl acetate | C7H14O2 | 628-63-7 | 918 | 919 | pear, fruity, estery, sweet, candy, floral | [39] |
| 475 | 1.06 | butyl butanoate | C8H16O2 | 109-21-7 | 1000 | 996 | pear, estery | [39] |
| 490 | 1.10 | ethyl hexanoate | C8H16O2 | 123-66-0 | 1006 | 1001 | pear, floral, fruity, estery, sweet, cooked pear, oxidized, medicine | [39] |
| 520 | 1.33 | hexyl acetate | C8H16O2 | 142-92-7 | 1018 | 1024 | pear, floral, sweet, fruity, estery | [39] |
| 585 | 0.95 | butyl 2-methylbutanoate | C9H18O2 | 15706-73-7 | 1045 | 1048 | fruity, cocoa | [40] |
| 625 | 1.03 | 2-methylbutyl butanoate | C9H18O2 | 51115-64-1 | 1061 | - | - | - |
| 715 | 1.12 | propyl hexanoate | C9H18O2 | 626-77-7 | 1098 | 1098 | ether, pineapple, blackberry | [40] |
| 745 | 1.17 | hexyl propanoate | C9H18O2 | 2445-76-3 | 1110 | 1114 | earthy | [40] |
| 765 | 1.32 | heptyl acetate | C9H18O2 | 112-06-1 | 1117 | 1118 | fermented | [6] |
| 980 | 1.22 | ethyl octanoate | C10H20O2 | 106-32-1 | 1200 | 1193 | floral, sweet, cooked apple, fruity | [39] |
| 1080 | 1.03 | hexyl 2-methylbutanoate | C11H22O2 | 10032-15-2 | 1239 | 1236 | green, fruity | [40] |
| 1120 | 1.08 | 3-methylbutyl hexanoate | C11H22O2 | 2198-61-0 | 1255 | 1259 | apple, pineapple, fruity, green, sweet | [40] |
| 1215 | 1.14 | hexyl pentanoate | C11H22O2 | 1117-59-5 | 1292 | 1293 | - | - |
| 1455 | 1.15 | hexyl hexanoate | C12H24O2 | 6378-65-0 | 1388 | 1392 | floral, candy | [39] |
| 1485 | 1.23 | ethyl decanoate | C12H24O2 | 110-38-3 | 1400 | 1391 | fermented food | [39] |
| 1660 | 1.89 | ethyl (2E,4Z)-decadienoate | C12H20O2 | 3025-30-7 | 1475 | - | pear | [39,43] |
| 1945 | 1.20 | ethyl dodecanoate | C14H28O | 106-33-2 | 1600 | 1601 | floral, fruity | [40] |
| Monoterpenes | ||||||||
| 340 | 0.59 | α-pinene | C10H16 | 80-56-8 | 929 | 932 | pine, turpentine | [40] |
| 365 | 0.66 | camphene | C10H16 | 79-92-5 | 942 | 959 | oily, camphor | [44] |
| 420 | 0.72 | β-pinene | C10H16 | 127-91-3 | 971 | 982 | green, turpentine | [40] |
| 460 | 0.85 | β-myrcene | C10H16 | 123-35-3 | 992 | 994 | sweet, balsamic, plastic | [40] |
| 535 | 0.89 | Limonene | C10H16 | 138-86-3 | 1024 | 1027 | lemon, camphor, turpentine | [40] |
| 570 | 0.97 | β-ocimene (isomer) | C10H16 | 13877-91-3 | 1039 | 1042 | herbaceous | [40] |
| 595 | 1.00 | β-ocimene (isomer) | C10H16 | 13877-91-3 | 1049 | 1048 | herbaceous | [40] |
| 610 | 0.96 | γ-terpinene | C10H16 | 99-85-4 | 1055 | 1058 | woody, terpene, tropical, lemon | [40] |
| Sesquiterpenes | ||||||||
| 1400 | 0.90 | α-copaene | C15H24 | 3856-25-5 | 1366 | 1370 | - | - |
| 1420 | 0.93 | β-bourbonene | C15H24 | 5208-59-3 | 1374 | 1379 | - | - |
| 1460 | 1.04 | longifolene | C15H24 | 475-20-7 | 1390 | 1395 | - | - |
| 1500 | 1.06 | β-caryophyllene | C15H24 | 87-44-5 | 1406 | 1416 | terpene, clove, turpentine | [40] |
| 1560 | 1.07 | β-farnesene (isomer) | C15H24 | 18794-84-8 | 1432 | - | citrus, herbaceous | [40] |
| 1585 | 1.17 | α-humulene | C15H24 | 6753-98-6 | 1443 | 1450 | - | - |
| 1620 | 1.10 | β-farnesene (isomer) | C15H24 | 18794-84-8 | 1457 | 1455 | citrus, herbaceous | [40] |
| 1720 | 1.18 | α-farnesene (isomer) | C15H24 | 502-61-4 | 1500 | - | flowery, balsam | [45] |
| 1740 | 1.23 | α-farnesene (isomer) | C15H24 | 502-61-4 | 1509 | 1505 | flowery, balsam | [45] |
| 1785 | 1.28 | α-farnesene (isomer) | C15H24 | 502-61-4 | 1529 | - | flowery, balsam | [45] |
| 1995 | 2.00 | β-farnesene epoxide | C15H24O | 83637-40-5 | 1624 | - | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, A.M.A.; Dias, C.; Amaro, A.L.; Isidoro, N.; Pintado, M.; Silvestre, A.J.D.; Rocha, S.M. The Impact of Plant-Based Coatings in “ROCHA” Pear Preservation during Cold Storage: A Metabolomic Approach. Foods 2020, 9, 1299. https://doi.org/10.3390/foods9091299
Fonseca AMA, Dias C, Amaro AL, Isidoro N, Pintado M, Silvestre AJD, Rocha SM. The Impact of Plant-Based Coatings in “ROCHA” Pear Preservation during Cold Storage: A Metabolomic Approach. Foods. 2020; 9(9):1299. https://doi.org/10.3390/foods9091299
Chicago/Turabian StyleFonseca, Alexandre M. A., Cindy Dias, Ana L. Amaro, Nélson Isidoro, Manuela Pintado, Armando J. D. Silvestre, and Sílvia M. Rocha. 2020. "The Impact of Plant-Based Coatings in “ROCHA” Pear Preservation during Cold Storage: A Metabolomic Approach" Foods 9, no. 9: 1299. https://doi.org/10.3390/foods9091299
APA StyleFonseca, A. M. A., Dias, C., Amaro, A. L., Isidoro, N., Pintado, M., Silvestre, A. J. D., & Rocha, S. M. (2020). The Impact of Plant-Based Coatings in “ROCHA” Pear Preservation during Cold Storage: A Metabolomic Approach. Foods, 9(9), 1299. https://doi.org/10.3390/foods9091299











