Bioavailability and Bioactivities of Polyphenols Eco Extracts from Coffee Grounds after In Vitro Digestion
Abstract
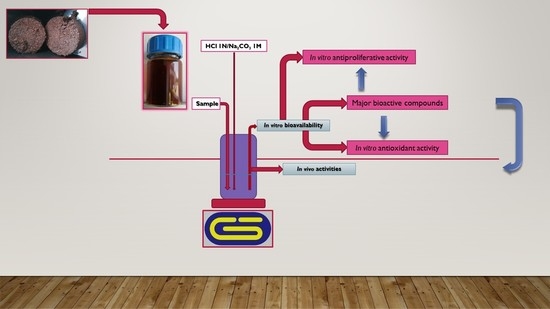
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples and Extraction Process
2.3. Quantification of Polyphenolic Compounds by Capillary Zone Electrophoresis (CZE)
2.4. In Vitro Bioactivity Assays
In Vivo Antioxidant Activity
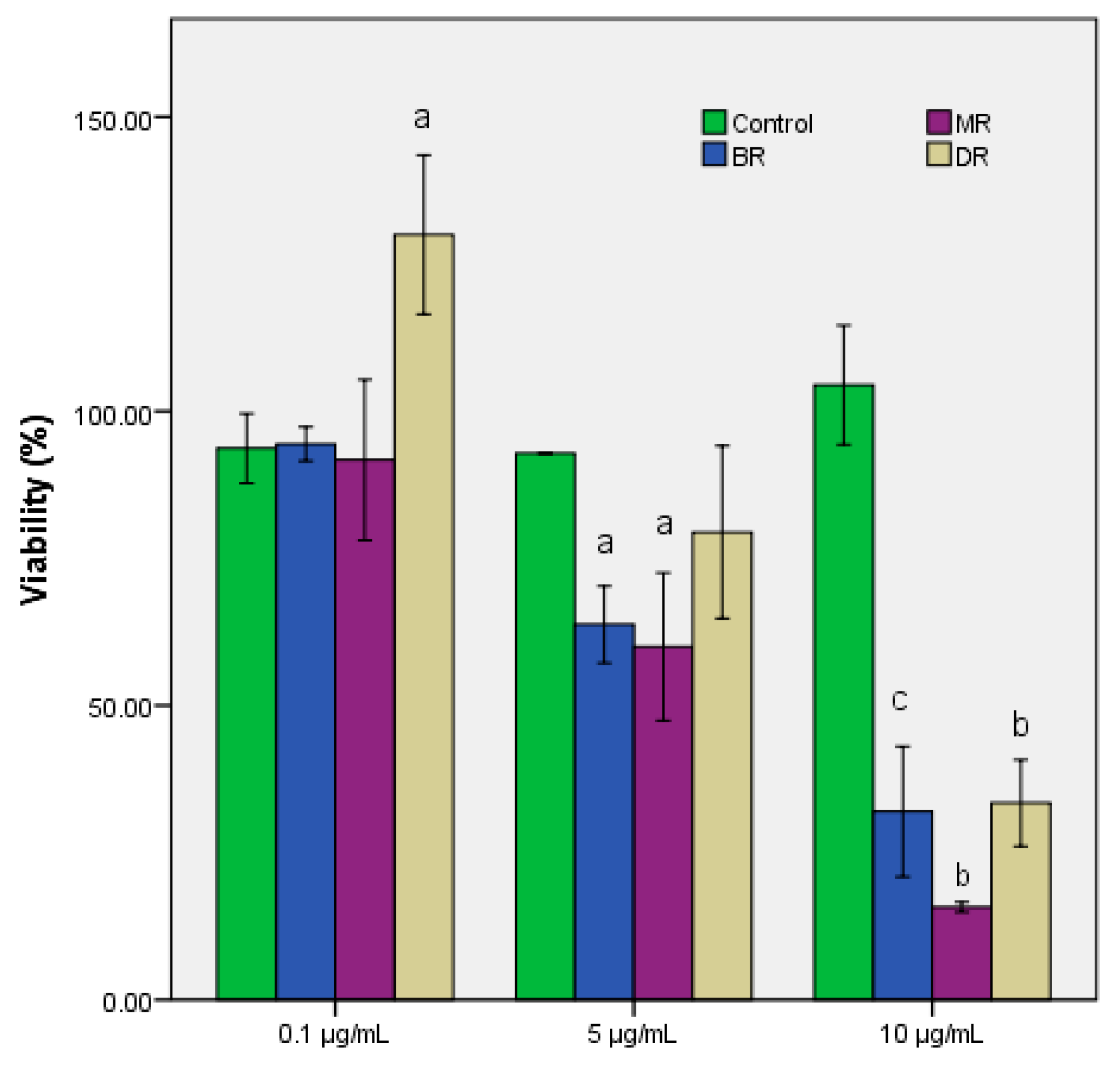
2.5. In Vitro Antiproliferative Assay
2.6. Quantitative Antibacterial Assay by Minimum Inhibitory Concentration (MIC) Determination
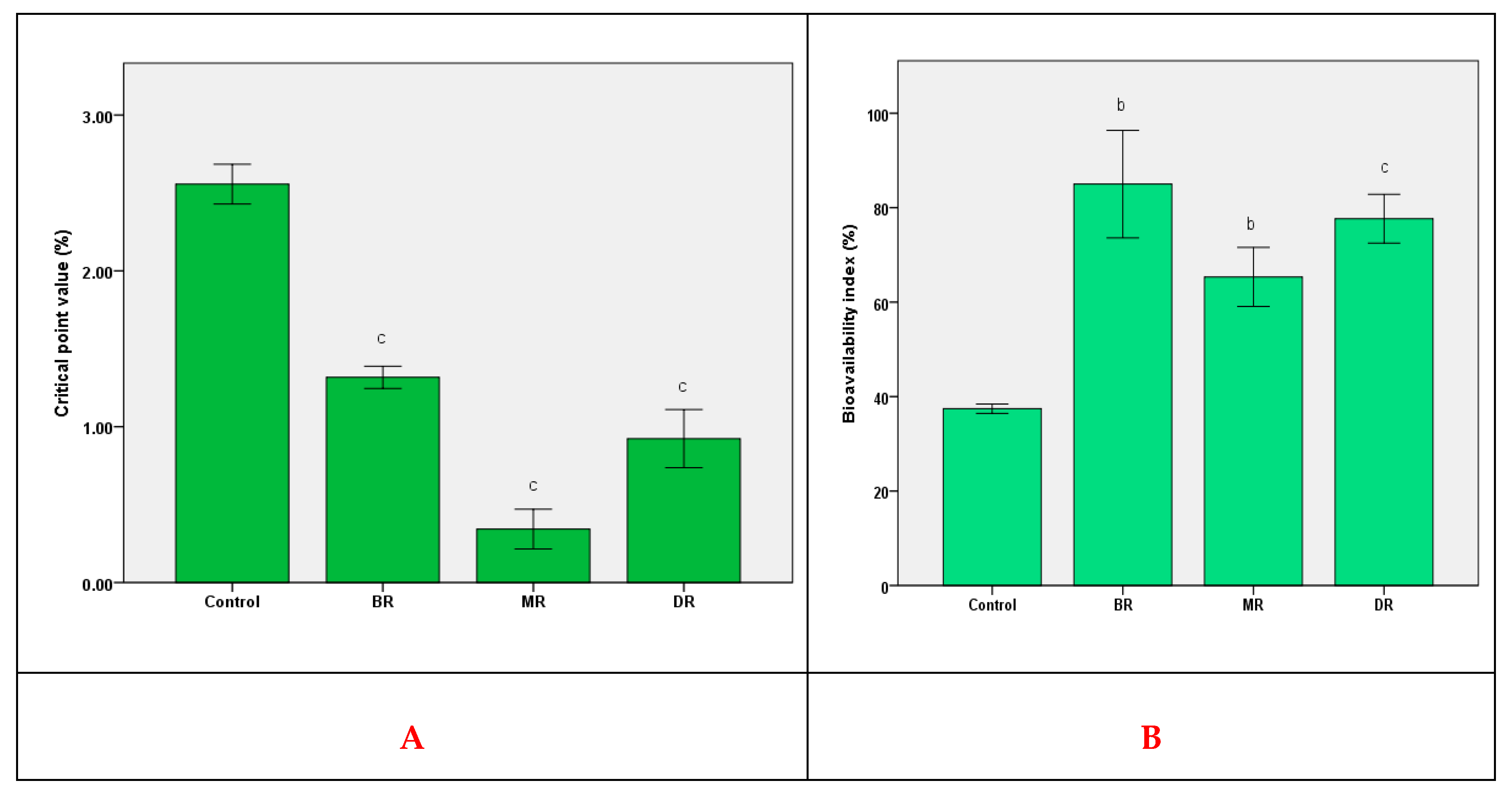
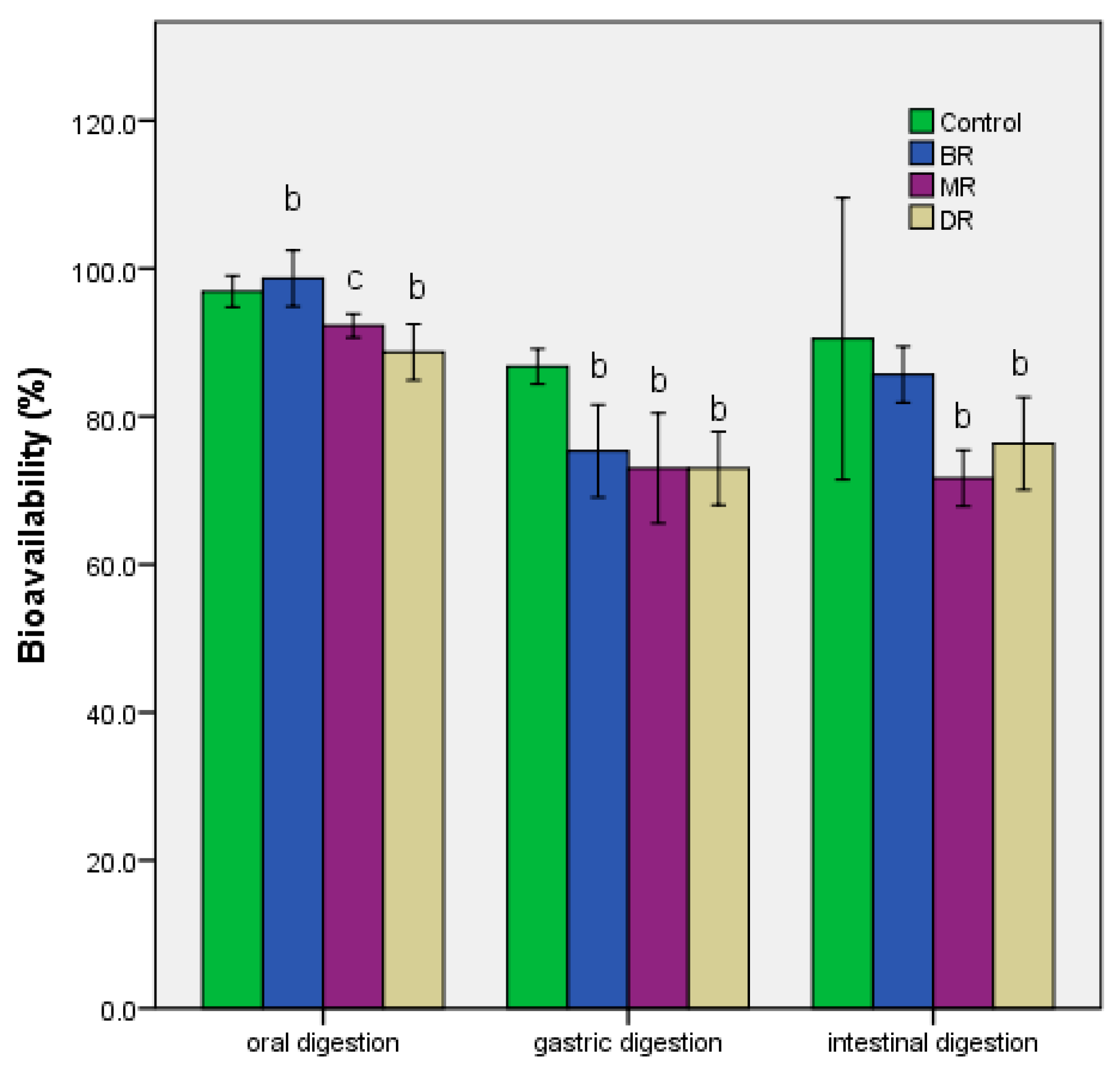
2.7. In Vitro Bioavailability Test
- -
- The tested sample was solubilized in 0.9% NaCl (sterile solution). A volume of 50 mL was made, in which a gelatin capsule was dissolved. 10% of this mixture contained a solution simulating sterilized human saliva, with the formula (g/L): 10 g carboxymethyl cellulose, 0.625 g KCl, 0.059 g MgCl2, 0.166 g CaCl2, 0.804 g K2HPO4, 0.326 g KH2PO4, pH 7—oral phase.
- -
- A total of 20 mL of simulated gastric juice was added (pepsin 3 g/L, 3 g/L mucin were dissolved in 0.9% NaCl).
- -
- The pH was adjusted to 2 with 1N HCl with a Behrotest peristaltic pump, Type PLP 33, flow rate 0.4–2.0 L/h and kept for 1.5–2 h—Gastric phase.
- -
- It was supplemented with 25 mL 0.9% NaCl in which 7 mg/mL pancreatin and 5 mg/mL bile salts were dissolved.
- -
- The pH was adjusted to 7.5 with 1M Na2CO3, with a Behrotest peristaltic pump, Type PLP 33, flow 0.4–2.0 L/h and kept for 2–4 h—Intestinal phase;
- -
- At the end of each phase, a volume of 10 mL was collected and analyzed.
In Vivo Bioavailability Test
2.8. Statistical Analysis
3. Results
3.1. Phenolic Compounds from Coffee Grounds Eco Extracts
3.2. Biologic Activities of Coffee Grounds Eco Extracts
3.3. In Vivo Antioxidant Activity and Bioavailability
3.4. Effect of In Vitro Gastrointestinal Digestion
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gebeyehu, B.T.; Bikila, S.L. Determination of Caffeine Content and Antioxidant Activity of Coffee. Am. J. Appl. Chem. 2015, 3, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Kim, M.H.; Park, J.H.; Jeong, Y.; Ko, K.S. Cellular Antioxidant and Anti-Inflammatory Effects of Coffee Extracts with Different Roasting Levels. J. Med. Food 2017, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Freedman, N.D.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 2012, 366, 1891–1904. [Google Scholar] [CrossRef]
- DePaula, J.; Farah, A. Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Mena, P.; Tassotti, M.; Martini, D.; Rosi, A.; Brighenti, F.; Del Rio, D. The Pocket-4-Life project, bioavailability and beneficial properties of the bioactive compounds of espresso coffee and cocoa-based confectionery containing coffee: Study protocol for a randomized cross-over trial. Trials 2017, 18, 527. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Affonso, R.C.; Voytena, A.P.; Fanan, S.; Heloísa, P.; Coelho, D.S.; Horstmann, A.L.; Pereira, A.; Uarrota, V.G.; Hillmann, M.C.; Calbusch Varela, L.A.; et al. Phytochemical Composition, Antioxidant Activity, and the Effect of the Aqueous Extract of Coffee (Coffea arabica L.) Bean Residual Press Cake on the Skin Wound Healing. Oxidative Med. Cell. Longev. 2016, 2016, 1923754. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farah, A.; Monteiro, M.; Donangelo, C.; Lafay, S. Chlorogenic Acids from Green Coffee Extract are Highly Bioavailable in Humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, B.; Koh, E. Spent coffee as a rich source of antioxidative compounds. Food Sci. Biotechnol. 2017, 26, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Chaita, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. LWT—Food Sci. Technol. 2020, 117, 108623. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Münchow, M.; Alstrup, J.; Steen, I.; Giacalone, D. Roasting Conditions and Coffee Flavor: A Multi-Study Empirical Investigation. Beverages 2020, 6, 29. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zhang, B.; Chen, G.; Fu, X. Analysis of solvent effects on polyphenols profile, antiproliferative and antioxidant activities of mulberry (Morus alba L.) extracts. Int. J. Food Sci. Technol. 2017, 52, 1690–1698. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F.; Sârbu, I.; Pelinescu, D. An In Vitro Study of the Influence of Curcuma longa Extracts on the Microbiota Modulation Process, In Patients with Hypertension. Pharmaceutics 2019, 11, 191. [Google Scholar] [CrossRef] [Green Version]
- Matei, A.O.; Gatea, F.; Teodor, E.D.; Radu, G.L. Polyphenols analysis from different medicinal plants extracts using capillary zone electrophoresis (CZE). Rev. Chim. 2016, 67, 1051–1055. Available online: https://www.revistadechimie.ro/Articles.asp?ID=5027 (accessed on 9 September 2020).
- Gatea, F.; Teodor, E.D.; Matei, A.O.; Badea, I.G.; Radu, G.L. Capillary Electrophoresis Method for 20 Polyphenols Separation in Propolis and Plant Extracts. Food Anal. Methods 2015, 8, 1197–1206. Available online: https://link.springer.com/article/10.1007/s12161-014-0006-5 (accessed on 9 September 2020). [CrossRef]
- Nguyen, T.T.K.; Laosinwattana, C.; Teerarak, M.; Pilasombut, K. Potential antioxidant and lipid peroxidation inhibition of Phyllanthus acidus leaf extract in minced pork. Asian-Australas. J. Anim. Sci. 2017, 30, 1323–1331. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. In Vitro Anti-Inflammatory Properties of Selected Green Leafy Vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Vamanu, E. Antioxidant properties of mushroom mycelia obtained by batch cultivation and tocopherol content affected by extraction procedures. BioMed Res. Int. 2014, 2014, 974804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabulici, C.M.; Sârbu, I.; Vamanu, E. The Bioactive Potential of Functional Products and Bioavailability of Phenolic Compounds. Foods 2020, 9, 953. [Google Scholar] [CrossRef]
- Prochor, P.; Mierzejewska, Z.A. Influence of the Surface Roughness of PEEK GRF30 and Ti6Al4V SLM on the Viability of Primary Human Osteoblasts Determined by the MTT Test. Materials 2019, 12, 4189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihai, M.M.; Holban, A.M.; Giurcăneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazǎr, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408. [Google Scholar]
- Sarbu, I.; Vassu, T.; Chifiriuc, M.C.; Bucur, M.; Stoica, I.; Stefana, P.; Rusu, E.; Horatiu, M.; Pelinescu, D. Assessment the Activity of Some Enzymes and Antibiotic Substances Sensitivity on Pathogenic Bacteria Species. Rev. Chim. 2017, 68, 3015–3021. Available online: https://revistadechimie.ro/Articles.asp?ID=6029 (accessed on 8 June 2020). [CrossRef]
- Marhuenda, J.; Alemán, M.D.; Gironés-Vilaplana, A.; Pérez, A.; Caravaca, G.; Figueroa, F.; Mulero, J.; Zafrilla, P. Phenolic Composition, Antioxidant Activity, and In Vitro Availability of Four Different Berries. J. Chem. 2016, 2016, 5194901. [Google Scholar] [CrossRef] [Green Version]
- Wessel, M.D.; Jurs, P.C.; Tolan, J.W.; Muskal, S.M. Prediction of Human Intestinal Absorption of Drug Compounds from Molecular Structure. J. Chem. Inf. Comput. Sci. 1998, 38, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Pali-Schöll, I.; Untersmayr, E.; Klems, M.; Jensen-Jarolim, E. The Effect of Digestion and Digestibility on Allergenicity of Food. Nutrients 2018, 10, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, B.J.; Bergström, C.A.S.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef] [Green Version]
- Bauer, D.; Abreu, J.; Jordão, N.; Rosa, J.; Freitas-Silva, O.; Teodoro, A. Effect of Roasting Levels and Drying Process of Coffea canephora on the Quality of Bioactive Compounds and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3407. [Google Scholar] [CrossRef] [Green Version]
- Gayoso, L.; Claerbout, A.S.; Calvo, M.I.; Cavero, Y.R.; Astiasarán, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, Z.; Dai, M.; Cao, X.; Xiong, X.; He, S.; Yuan, Y.; Zhang, M.; Dong, L.; Zhang, R.; et al. Effects of simulated digestion on the phenolic composition and antioxidant activity of different cultivars of lychee pericarp. BMC Chem. 2019, 13, 27. [Google Scholar] [CrossRef]
- Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients 2017, 9, 597. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Vaddady, P.K.; Lee, R.E.; Meibohm, B. In vitro pharmacokinetic/pharmacodynamic models in anti-infective drug development: Focus on TB. Future Med. Chem. 2010, 2, 1355–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morano, K.A.; Grant, C.M.; Moye-Rowley, W.S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 2012, 190, 1157–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [Green Version]
- Vamanu, E.; Gatea, F.; Sârbu, I. In Vitro Ecological Response of the Human Gut Microbiome to Bioactive Extracts from Edible Wild Mushrooms. Molecules 2018, 23, 2128. [Google Scholar] [CrossRef] [Green Version]
| Compound (µg/mL) | BR | MR | DR |
|---|---|---|---|
| Caffeic Acid | 4.18 ± 0.51 b | 2.04 ± 0.18 c | nd |
| Cinnamic Acid | 150.95 ± 2.08 a | 72.59 ± 0.23 b | 82.08 ± 2.84 a |
| Chlorogenic Acid | 135.07 ± 2.04 a | 47.75 ± 0.38 b | 56.67 ± 1.32 b |
| Ferulic Acid | 13.5 ± 0.38 c | nd | nd |
| Coumaric Acid | 13.98 ± 0.22 c | 4.56 ± 0.72 a | 4.67 ± 0.86 b |
| Quercetol | 11.82 ± 1.00 a | 4.26 ± 0.41 b | 4.47 ± 0.36 c |
| Kaempferol | 2.12 ± 0.22 c | 2.24 ± 0.17 c | 6.84 ± 0.56 b |
| Isoquercitrin | 8.28 ± 0.54 b | 6.86 ± 0.49 b | 22.69 ± 1.38 a |
| Methods for Antioxidant Activity | BR | MR | DR | AA | TBHQ |
| Inhibition of lipid peroxidation | 1.07 ± 0.08 a/* | 1.35 ± 0.03 a/** | 1.78 ± 0.09 b/*** | 1.00 ± 0.07 | 0.75 ± 0.06 |
| Reducing power | 0.40 ± 0.003 a/** | 0.49 ± 0.002 b/** | 0.47 ± 0.01 ** | 0.64 ± 0.02 | 0.60 ± 0.01 |
| DPPH scavenging activity | 0.76 ± 0.11 c/** | 1.20 ± 0.10 c/*** | 1.54 ± 0.05 c/*** | 0.93 ± 0.07 | 0.45 ± 0.11 |
| Methods for Anti-Inflammatory Activity | BR | MR | DR | AAS | DKP |
| Inhibition of protein denaturation | 1.35 ± 0.09 b/* | 1.40 ± 0.02 c/* | 1.47 ± 0.01 b/* | 0.90 ± 0.10 | 1.20 ± 0.53 |
| Inhibition of hemolysis | 1.90 ± 0.03 b/** | 1.25 ± 0.16 b/* | 1.31 ± 0.06 c/* | 1.17 ± 0.08 | 1.37 ± 0.07 |
| Microbial Strains | BR | MR | DR | Control |
|---|---|---|---|---|
| S. aureus ATCC BAA 1026 | 12.5 | 6.25 | 12.5 | 12.5 |
| S. aureus 1004 | 25 | 6.25 | 12.5 | 25 |
| E. coli ACTCC 25922 | 12.5 | 6.25 | 25 | 12.5 |
| E. coli B11 | 12.5 | 6.25 | 25 | 12.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamanu, E.; Gatea, F.; Pelinescu, D.R. Bioavailability and Bioactivities of Polyphenols Eco Extracts from Coffee Grounds after In Vitro Digestion. Foods 2020, 9, 1281. https://doi.org/10.3390/foods9091281
Vamanu E, Gatea F, Pelinescu DR. Bioavailability and Bioactivities of Polyphenols Eco Extracts from Coffee Grounds after In Vitro Digestion. Foods. 2020; 9(9):1281. https://doi.org/10.3390/foods9091281
Chicago/Turabian StyleVamanu, Emanuel, Florentina Gatea, and Diana Roxana Pelinescu. 2020. "Bioavailability and Bioactivities of Polyphenols Eco Extracts from Coffee Grounds after In Vitro Digestion" Foods 9, no. 9: 1281. https://doi.org/10.3390/foods9091281
APA StyleVamanu, E., Gatea, F., & Pelinescu, D. R. (2020). Bioavailability and Bioactivities of Polyphenols Eco Extracts from Coffee Grounds after In Vitro Digestion. Foods, 9(9), 1281. https://doi.org/10.3390/foods9091281