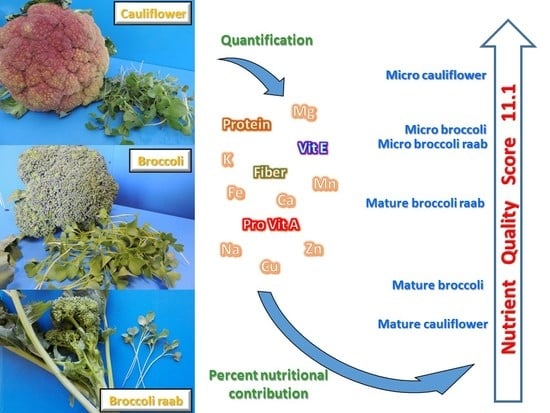
The Use of a Nutrient Quality Score is Effective to Assess the Overall Nutritional Value of Three Brassica Microgreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Microgreens and Sample Preparation
2.2. Determination of Protein and Dietary Fiber
2.3. Determination of α-Tocopherol and β-Carotene
2.4. Elemental Analysis
2.5. Development and Calculation of Nutrient Quality Score
2.6. Statistical Analysis
3. Results
3.1. Nutrient Content in Microgreens
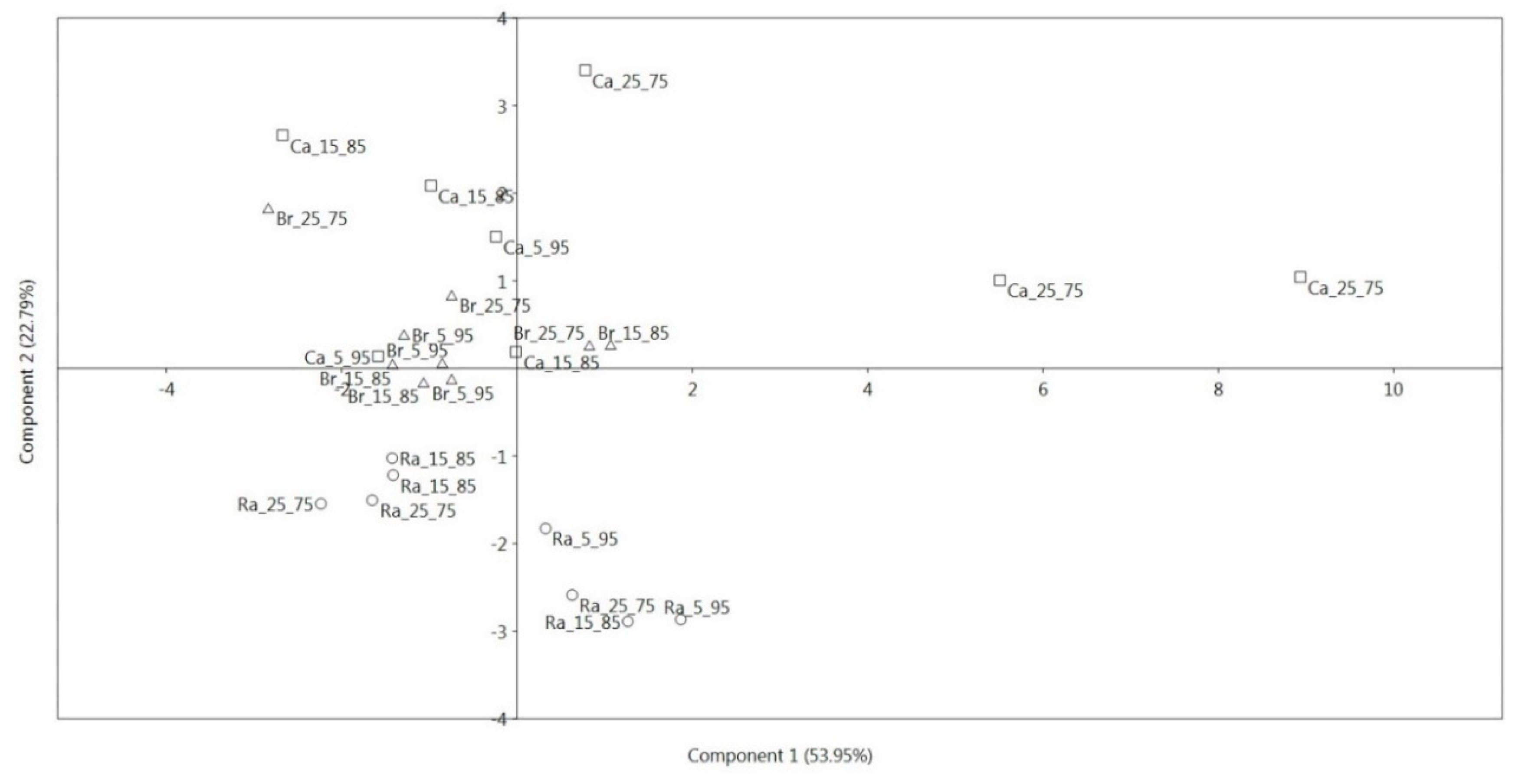
3.2. Principal Component Analysis
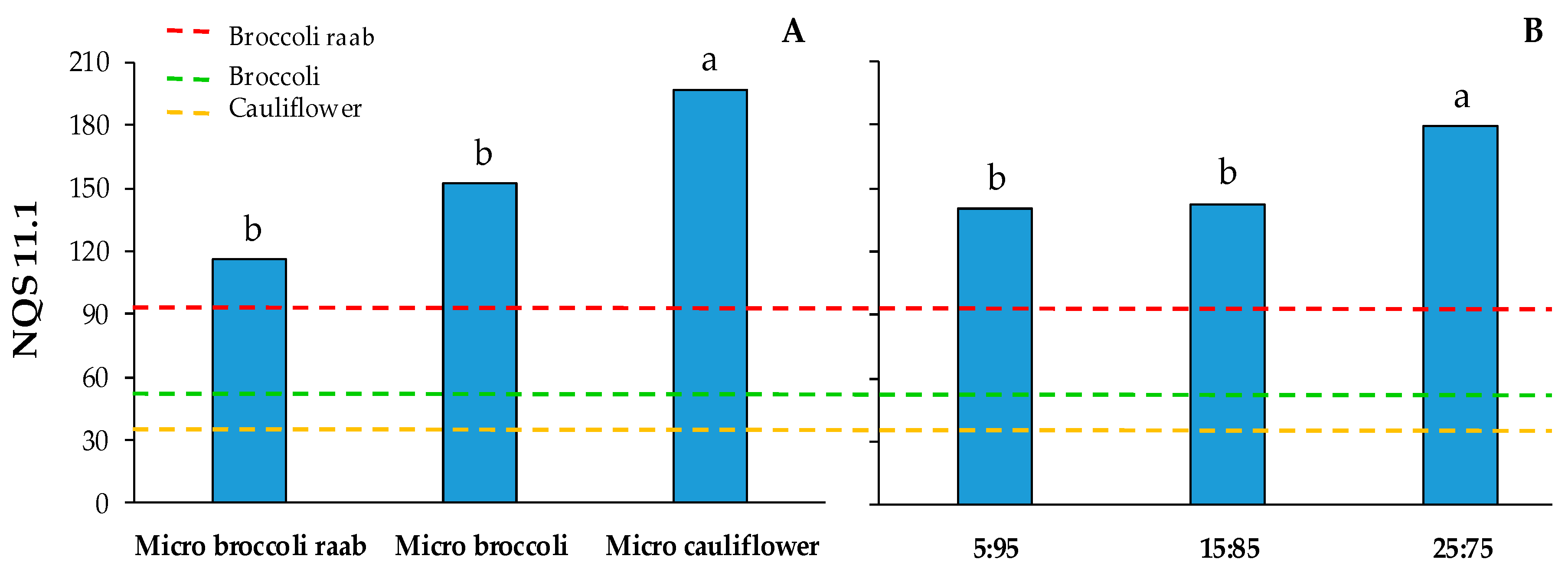
3.3. Percent of Nutritional Contribution and Nutrient Quality Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Berendsen, A.A.M.; Kramer, C.S.; de Groot, L.C. The Newly Developed Elderly Nutrient-Rich Food Score Is a Useful Tool to Assess Nutrient Density in European Older Adults. Front. Nutr. 2019, 6, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef] [Green Version]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for vitamin A. EFSA J. 2015, 13, 4028. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. EFSA J. 2015, 13, 4149. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for calcium. EFSA J. 2015, 13, 4101. [Google Scholar] [CrossRef] [Green Version]
- Marian, M.; Sacks, G. Micronutrients and older adults. Nutr. Clin. Pract. 2009, 24, 179–195. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Gattullo, C.E.; Calasso, M.; Terzano, R.; Allegretta, I.; Leoni, B.; Caponio, F.; Santamaria, P. Nutritional characterization and shelf-life of packaged microgreens. Food Funct. 2018, 9, 5629–5640. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Xiao, Z.; Lin, L.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling Polyphenols in Five Brassica Species Microgreens by UHPLC-PDA-ESI/HRMSn. J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [Green Version]
- Lenzi, A.; Orlandini, A.; Bulgari, R.; Ferrante, A.; Bruschi, P. Antioxidant and Mineral Composition of Three Wild Leafy Species: A Comparison Between Microgreens and Baby Greens. Foods 2019, 8, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Santamaria, P.; Caponio, F. Setup of an Extraction Method for the Analysis of Carotenoids in Microgreens. Foods 2020, 9, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020, 91. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic Constitution, Phytochemical and Macronutrient Content in Three Species of Microgreens as Modulated by Natural Fiber and Synthetic Substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef] [Green Version]
- Renna, M.; Di Gioia, F.; Leoni, B.; Mininni, C.; Santamaria, P. Culinary Assessment of Self-Produced Microgreens as Basic Ingredients in Sweet and Savory Dishes. J. Culin. Sci. Technol. 2017, 15, 126–142. [Google Scholar] [CrossRef]
- Renna, M.; Castellino, M.; Leoni, B.; Paradiso, V.M.; Santamaria, P. Microgreens production with low potassium content for patients with impaired kidney function. Nutrients 2018, 10, 675. [Google Scholar] [CrossRef] [Green Version]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.-M. Blue and Red LED Illumination Improves Growth and Bioactive Compounds Contents in Acyanic and Cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Luo, Y.; Lester, G.E.; Kou, L.; Yang, T.; Wang, Q. Postharvest quality and shelf life of radish microgreens as impacted by storage temperature, packaging film, and chlorine wash treatment. LWT Food Sci. Technol. 2014, 55, 551–558. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.; Ferreira, I.M. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J. Food Compos. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Kamal, K.Y.; Khodaeiaminjan, M.; El-Tantawy, A.A.; Moneim, D.A.; Salam, A.A.; Ash-shormillesy, S.M.A.I.; Attia, A.; Ali, M.A.S.; Herranz, R.; El-Esawi, M.A.; et al. Evaluation of growth and nutritional value of Brassica microgreens grown under red, blue and green LEDs combinations. Physiol. Plant. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral composition and content of 30 varieties. J. Food Compos. Anal. 2016, 49, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 31. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC: Arlington, VA, USA, 2000. [Google Scholar]
- Palmitessa, O.D.; Renna, M.; Crupi, P.; Lovece, A.; Corbo, F.; Santamaria, P. Yield and Quality Characteristics of Brassica Microgreens as Affected by the NH4:NO3 Molar Ratio and Strength of the Nutrient Solution. Foods 2020, 9, 677. [Google Scholar] [CrossRef]
- Mills, H.A.; Jones, J.B.; Wolf, B. Plant Analysis Handbook II: A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing, Incorporated: Athens, GA, USA, 1996; ISBN 18-781-48052. [Google Scholar]
- Vivaldi, G.A.; Stellacci, A.M.; Vitti, C.; Rubino, P.; Pedrero, F.; Camposeo, S. Nutrient uptake and fruit quality in a nectarine orchard irrigated with treated municipal wastewaters. Desalin. Water Treat. 2017, 71, 312–320. [Google Scholar] [CrossRef]
- USDA CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.12 (accessed on 2 June 2020).
- Ebert, A.W. Sprouts and microgreens for a nutritious diet. Int. J. Rural Dev. 2013, 21, 42–43. [Google Scholar]
- United States Department of Agriculture Broccoli raab, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170381/nutrients (accessed on 15 April 2020).
- United States Department of Agriculture Broccoli, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170379/nutrients (accessed on 15 April 2020).
- United States Department of Agriculture Cauliflower, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169986/nutrients (accessed on 15 April 2020).
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Dietary reference values for potassium. EFSA J. 2016, 14, e04592. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for magnesium. EFSA J. 2015, 13, 4186. [Google Scholar] [CrossRef] [Green Version]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for iron. EFSA J. 2015, 13. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef] [Green Version]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for copper. EFSA J. 2015, 13, 4253. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for manganese. EFSA J. 2013, 11, 3419. [Google Scholar] [CrossRef] [Green Version]
- EFSA NDA Panel. Dietary reference values for sodium. EFSA J. 2019, 17. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Yu, J.; Liao, W.; Zhang, G.; Xie, J.; Lv, J.; Xiao, X.; Yang, B.; Zhou, R.; Bu, R. Moderate ammonium: NItrate alleviates low light intensity stress in mini Chinese cabbage seedling by regulating root architecture and photosynthesis. Sci. Hortic. 2015, 186, 143–153. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration, D. of H. & H. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.54 (accessed on 18 June 2020).
- Hansen, R.G. An Index of Food Quality. Nutr. Rev. 1973, 31, 1–7. [Google Scholar] [CrossRef]
- Darmon, N.; Darmon, M.; Maillot, M.; Drewnowski, A. A nutrient density standard for vegetables and fruits: Nutrients per calorie and nutrients per unit cost. J. Am. Diet. Assoc. 2005, 105, 1881–1887. [Google Scholar] [CrossRef]
- Drewnowski, A. Concept of a nutritious food: Toward a nutrient density score. Am. J. Clin. Nutr. 2005, 82, 721–732. [Google Scholar] [CrossRef]
- Katz, D.L.; Njike, V.Y.; Rhee, L.Q.; Reingold, A.; Ayoob, K.T. Performance characteristics of NuVal and the Overall Nutritional Quality Index (ONQI). Am. J. Clin. Nutr. 2010, 91, 1102S–1108S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaria, P.; Elia, A.; Papa, G.; Serio, F. Nitrate and ammonium nutrition in chicory and rocket salad plants. J. Plant Nutr. 1998, 21, 1779–1789. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Gonnella, M.; Serio, F.; Santamaria, P. Effects of ammonium and nitrate nutrition on yield and quality in endive. J. Hortic. Sci. Biotechnol. 2008, 83, 64–70. [Google Scholar] [CrossRef]
- Xiao, Z.; Raush, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, L.; Stommel, J.R. Microgreens of Brassicaceae: Genetic diversity of phytochemical concentrations and antioxidant capacity. LWT Food Sci. Technol. 2019, 101, 731–737. [Google Scholar] [CrossRef]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2005, 40, 1–11. [Google Scholar] [CrossRef]
- Weber, C.F. Broccoli Microgreens: A Mineral-Rich Crop That Can Diversify Food Systems. Front. Nutr. 2017, 4, 7. [Google Scholar] [CrossRef]


| Nutrient | DRV | References |
|---|---|---|
| Protein (g) | 62 | [35] |
| Fiber (g) | 25 | [3] |
| Vitamin A (μg RAE) * | 750 | [4] |
| Vitamin E (mg) | 13 | [5] |
| Ca (mg) | 950 | [6] |
| K (mg) | 3500 | [36] |
| Mg (mg) | 350 | [37] |
| Fe (mg) | 11 | [38] |
| Zn (mg) | 11.7 | [39] |
| Cu (mg) | 1.6 | [40] |
| Mn (mg) | 3 | [41] |
| Na (mg) | 2000 | [42] |
| Genotype | NH4:NO3 Molar Ratio | Value | Protein | Fiber | β-Carotene | α-Tocopherol |
|---|---|---|---|---|---|---|
| g 100 g−1 FW | mg 100 g−1 FW | |||||
| Micro broccoli raab | 5:95 | Mean | 2.34 b | 0.49 | 4.05 | 1.84 |
| SD | 0.28 | 0.05 | 1.82 | 0.58 | ||
| 15:85 | Mean | 2.29 b | 0.47 | 3.54 | 1.81 | |
| SD | 0.06 | 0.02 | 0.47 | 0.64 | ||
| 25:75 | Mean | 2.41 b | 0.45 | 3.27 | 2.63 | |
| SD | 0.31 | 0.02 | 0.94 | 0.63 | ||
| Micro broccoli | 5:95 | Mean | 2.19 b | 0.56 | 4.61 | 6.43 |
| SD | 0.10 | 0.02 | 1.14 | 0.20 | ||
| 15:85 | Mean | 2.41 b | 0.44 | 4.92 | 6.37 | |
| SD | 0.30 | 0.07 | 2.24 | 0.50 | ||
| 25:75 | Mean | 2.41 b | 0.61 | 6.51 | 6.30 | |
| SD | 0.51 | 0.01 | 0.04 | 0.11 | ||
| Micro cauliflower | 5:95 | Mean | 2.44 b | 0.46 | 4.32 | 6.32 |
| SD | 0.23 | 0.13 | 0.94 | 6.59 | ||
| 15:85 | Mean | 2.50 b | 0.62 | 5.33 | 8.37 | |
| SD | 0.32 | 0.19 | 0.88 | 5.41 | ||
| 25:75 | Mean | 4.20 a | 0.89 | 9.08 | 14.93 | |
| SD | 1.14 | 0.26 | 1.63 | 10.28 | ||
| Significance | ||||||
| Genotype (G) | NS | * | ** | * | ||
| Molar ratio (M) | NS | NS | ** | NS | ||
| G × M | * | NS | NS | NS | ||
| Genotype | NH4:NO3 Molar Ratio | Value | Ca | K | Mg | Na | Zn | Fe | Cu | Mn |
|---|---|---|---|---|---|---|---|---|---|---|
| mg 100 g−1 FW | μg 100 g−1 FW | |||||||||
| Micro broccoli raab | 5:95 | Mean | 189.6 | 254.7 | 47.7 b | 11.3 bcd | 1711 | 774 ab | 31.0 | 669 |
| SD | 22.8 | 60.7 | 4.9 | 1.6 | 150 | 57 | 0.9 | 64 | ||
| 15:85 | Mean | 166.5 | 268.5 | 41.7 b | 9 cd | 1591 | 665 ab | 30.9 | 465 | |
| SD | 22.5 | 32.1 | 6.0 | 1.8 | 216 | 154 | 7.2 | 81 | ||
| 25:75 | Mean | 149.9 | 253.0 | 39.3 b | 8.1 d | 1737 | 584 b | 34.0 | 427 | |
| SD | 18.4 | 41.8 | 4.8 | 1.0 | 238 | 24 | 5.4 | 50 | ||
| Micro broccoli | 5:95 | Mean | 157.9 | 216.3 | 38.2 b | 12.3 bc | 1021 | 594 b | 27.3 | 503 |
| SD | 4.9 | 10.4 | 1.7 | 1.0 | 48 | 71 | 0.6 | 19 | ||
| 15:85 | Mean | 165.4 | 258.4 | 40.5 b | 11.6 bcd | 1125 | 721 ab | 26.4 | 424 | |
| SD | 20.2 | 52.7 | 5.1 | 1.8 | 286 | 149 | 4.5 | 57 | ||
| 25:75 | Mean | 143.6 | 251.8 | 38.1 b | 11.5 bcd | 1112 | 596 b | 25.5 | 380 | |
| SD | 23.8 | 80.3 | 5.50 | 0.6 | 286 | 81 | 3.0 | 41 | ||
| Micro cauliflower | 5:95 | Mean | 162.3 | 210.4 | 43.3 b | 13.1 b | 933 | 679 ab | 19.2 | 439 |
| SD | 4.1 | 13.6 | 2.4 | 0.5 | 17 | 13 | 3.4 | 49 | ||
| 15:85 | Mean | 153.6 | 207.3 | 40.7 b | 10.7 bcd | 1002 | 632 b | 19.0 | 346 | |
| SD | 25.4 | 64.6 | 4.3 | 1.1 | 224 | 78 | 2.7 | 40 | ||
| 25:75 | Mean | 212.9 | 322.0 | 60.1 a | 18.0 a | 1566 | 879 a | 28.8 | 481 | |
| SD | 47.4 | 89.4 | 11.2 | 4.4 | 487 | 197 | 10.3 | 103 | ||
| Significance | ||||||||||
| Genotype (G) | NS | NS | * | ** | ** | NS | ** | * | ||
| Molar ratio (M) | NS | NS | NS | NS | NS | NS | NS | * | ||
| G × M | NS | NS | * | * | NS | * | NS | NS | ||
| Genotype | NH4:NO3 Molar Ratio | Protein | Fiber | Vitamin A | Vitamin E |
|---|---|---|---|---|---|
| Micro broccoli raab | 5:95 | 3.21 ± 0.38 | 1.68 ± 0.17 | 38.3 ± 17.2 | 12.1 ± 3.8 |
| 15:85 | 3.14 ± 0.08 | 1.60 ± 0.06 | 33.5 ± 4.4 | 11.8 ± 4.2 | |
| 25:75 | 3.31 ± 0.43 | 1.55 ± 0.06 | 30.9 ± 8.9 | 15.5 ± 4.1 | |
| Micro broccoli | 5:95 | 3.00 ± 0.14 | 1.89 ± 0.03 | 43.5 ± 10.8 | 42.1 ± 1.3 |
| 15:85 | 3.30 ± 0.42 | 1.50 ± 0.23 | 46.5 ± 21.2 | 41.8 ± 3.3 | |
| 25:75 | 3.31 ± 0.71 | 2.07 ± 0.05 | 61.5 ± 0.4 | 41.2 ± 0.7 | |
| Micro cauliflower | 5:95 | 3.34 ± 0.31 | 1.56 ± 0.44 | 40.8 ± 8.9 | 41.32 ± 43.1 |
| 15:85 | 3.43 ± 0.43 | 2.11 ± 0.65 | 50.3 ± 8.4 | 57.1 ± 35.4 | |
| 25:75 | 5.76 ± 1.56 | 3.30 ± 0.90 | 85.7 ± 15.4 | 97.6 ± 47.2 |
| Genotype | NH4:NO3 Molar Ratio | Ca | K | Mg | Na | Zn | Fe | Cu | Mn |
|---|---|---|---|---|---|---|---|---|---|
| Micro broccoli raab | 5:95 | 17.0 ± 2.0 | 6.2 ± 1.5 | 11.6 ± 1.2 | 0.5 ± 0.1 | 12.4 ± 1.1 | 6.0 ± 0.4 | 1.6 ± 0.1 | 18.9 ± 1.8 |
| 15:85 | 14.9 ± 2.0 | 6.5 ± 0.8 | 10.1 ± 1.5 | 0.4 ± 0.1 | 11.6 ± 1.6 | 5.1 ± 1.2 | 1.6 ± 0.4 | 13.2 ± 2.3 | |
| 25:75 | 13.4 ± 1.6 | 6.1 ± 0.8 | 9.5 ± 1.5 | 0.3 ± 0.1 | 12.6 ± 1.7 | 4.5 ± 0.2 | 1.8 ± 0.3 | 12.1 ± 1.4 | |
| Micro broccoli | 5:95 | 14.1 ± 0.4 | 5.2 ± 0.3 | 9.3 ± 0.4 | 0.5 ± 0.1 | 7.4 ± 0.4 | 4.6 ± 0.5 | 1.5 ± 0.1 | 14.2 ± 0.5 |
| 15:85 | 14.8 ± 1.8 | 6.3 ± 1.3 | 9.8 ± 1.2 | 0.5 ± 0.1 | 8.2 ± 2.1 | 5.6 ± 1.2 | 1.4 ± 0.2 | 12.0 ± 1.6 | |
| 25:75 | 12.8 ± 2.1 | 6.1 ± 1.9 | 9.2 ± 1.3 | 0.5 ± 0.1 | 8.1 ± 2.1 | 4.6 ± 0.6 | 1.4 ± 0.2 | 10.8 ± 1.2 | |
| Micro cauliflower | 5:95 | 14.5 ± 0.4 | 5.1 ± 0.3 | 10.5 ± 0.6 | 0.6 ± 0.1 | 6.8 ± 0.1 | 5.2 ± 0.1 | 1.0 ± 0.2 | 12.4 ± 1.4 |
| 15:85 | 13.7 ± 2.3 | 5.1 ± 1.6 | 9.9 ± 1.3 | 0.5 ± 0.1 | 7.3 ± 1.6 | 5.0 ± 0.6 | 1.0 ± 0.1 | 9.8 ± 1.1 | |
| 25:75 | 19.1 ± 4.2 | 7.8 ± 2.2 | 14.6 ± 3.3 | 0.9 ± 0.2 | 11.4 ± 3.5 | 7.5 ± 1.5 | 1.5 ± 0.5 | 13.6 ± 2.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renna, M.; Stellacci, A.M.; Corbo, F.; Santamaria, P. The Use of a Nutrient Quality Score is Effective to Assess the Overall Nutritional Value of Three Brassica Microgreens. Foods 2020, 9, 1226. https://doi.org/10.3390/foods9091226
Renna M, Stellacci AM, Corbo F, Santamaria P. The Use of a Nutrient Quality Score is Effective to Assess the Overall Nutritional Value of Three Brassica Microgreens. Foods. 2020; 9(9):1226. https://doi.org/10.3390/foods9091226
Chicago/Turabian StyleRenna, Massimiliano, Anna Maria Stellacci, Filomena Corbo, and Pietro Santamaria. 2020. "The Use of a Nutrient Quality Score is Effective to Assess the Overall Nutritional Value of Three Brassica Microgreens" Foods 9, no. 9: 1226. https://doi.org/10.3390/foods9091226
APA StyleRenna, M., Stellacci, A. M., Corbo, F., & Santamaria, P. (2020). The Use of a Nutrient Quality Score is Effective to Assess the Overall Nutritional Value of Three Brassica Microgreens. Foods, 9(9), 1226. https://doi.org/10.3390/foods9091226