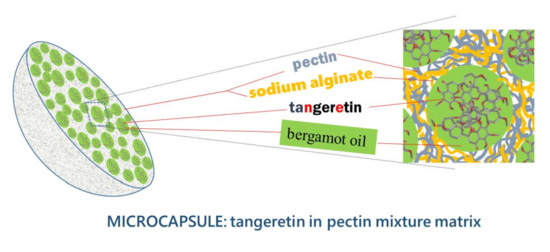
Microencapsulation of Tangeretin in a Citrus Pectin Mixture Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pectin Extraction
2.3. Tangeretin Extraction
2.4. Preparation of Tangeretin Microcapsules
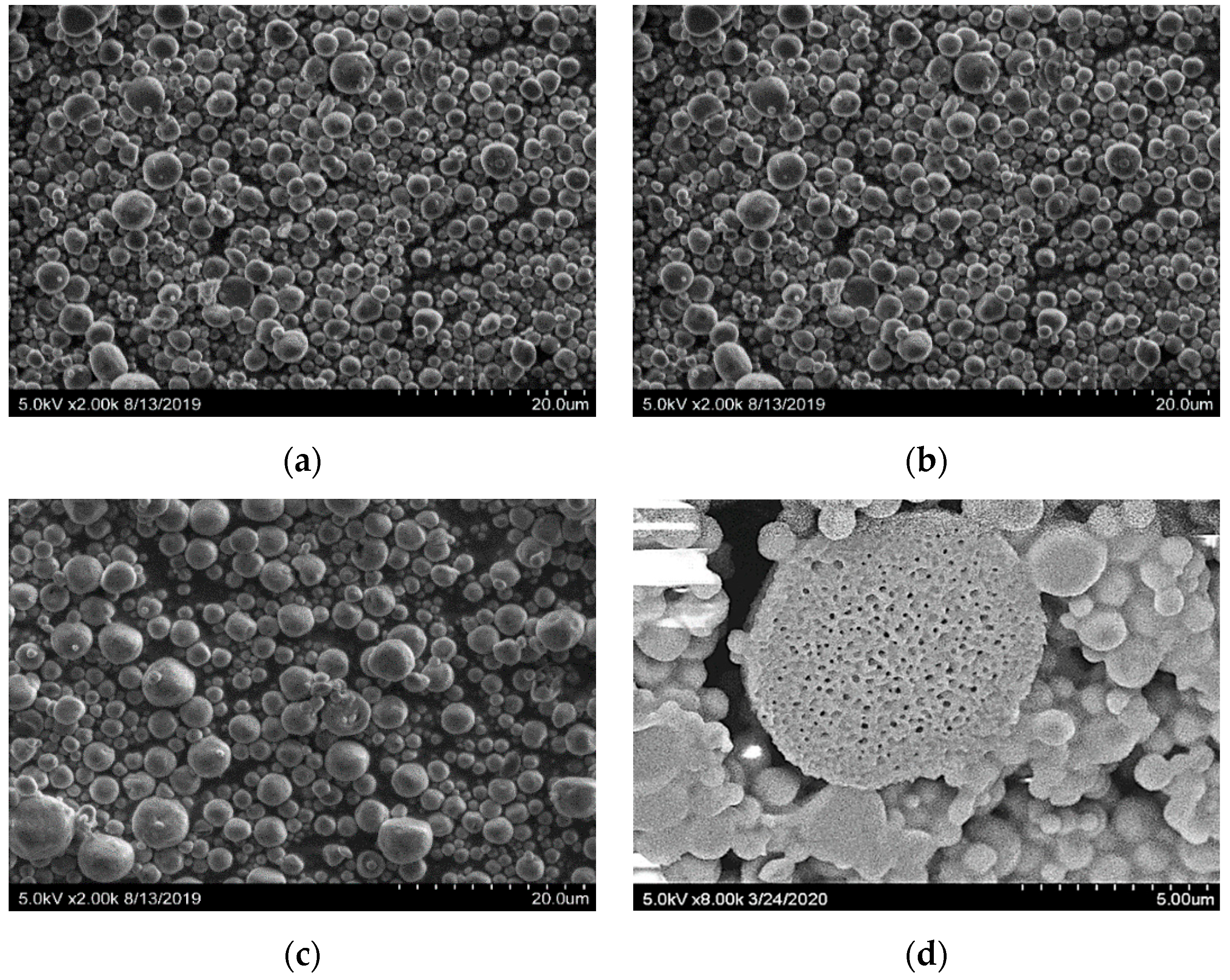
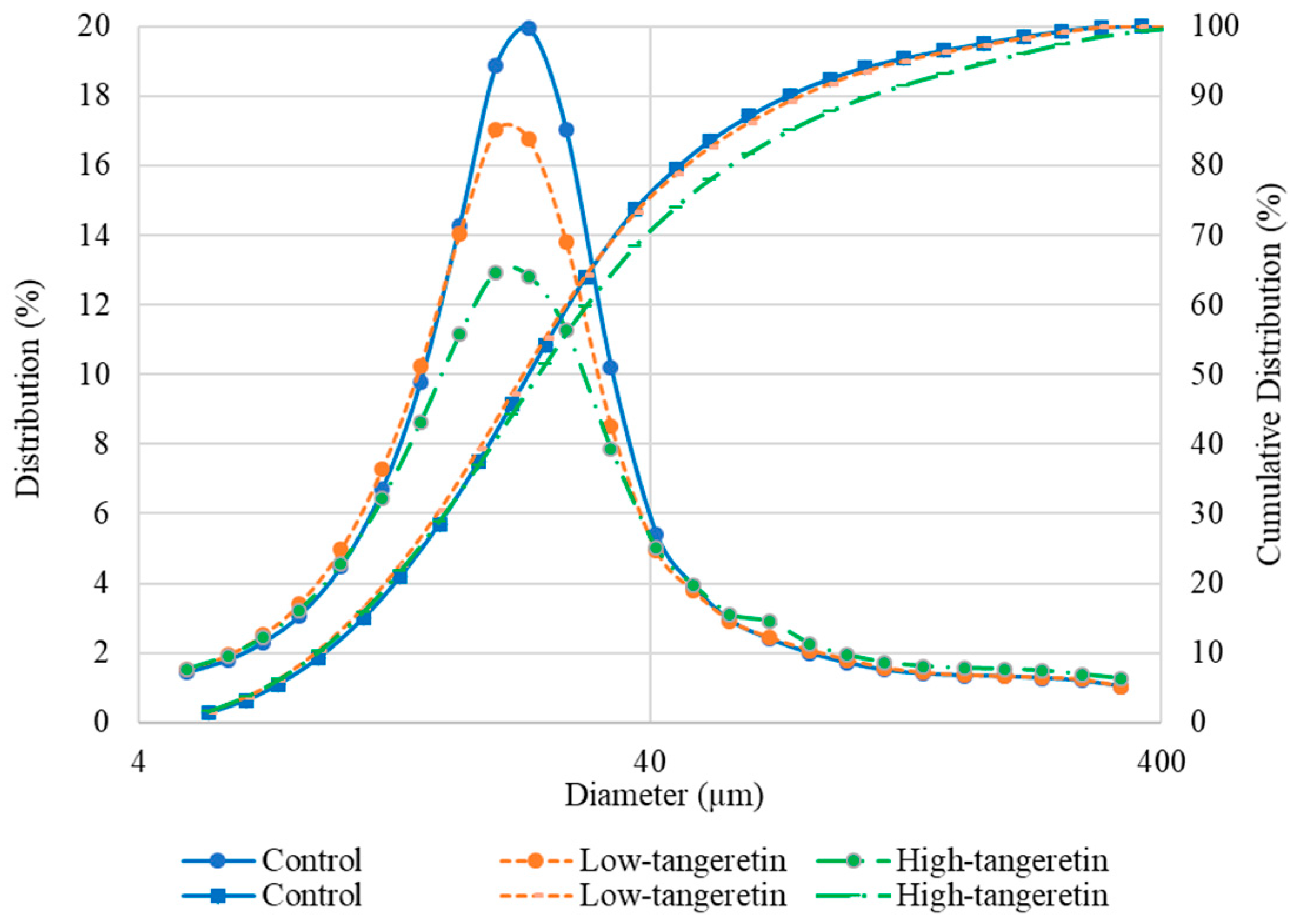
2.5. Morphology and Particle Size Distribution of the Microcapsules
2.6. Physical Properties of the Microcapsule Powder
2.6.1. Moisture Content
2.6.2. Bulk Density
2.6.3. Dissolution Time
2.6.4. Hygroscopicity
2.7. Retention Efficiency and Encapsulation Efficiency of Tangeretin in the Microcapsule Powder
2.7.1. Total Tangeretin of the Microcapsule Powder
2.7.2. Surface Tangeretin of the Microcapsule Powder
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Particle Size Distribution of the Microcapsules
3.2. Physical Properties of the Microcapsule Powder
3.3. Retention Efficiency and Encapsulation Efficiency of Tangeretin in the Microcapsule Powder
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manthey, J.A.; Grohmann, K.; Montanari, A.; Ash, K.; Manthey, C.L. Polymethoxylated flavones derived from citrus suppress tumor necrosis factor-alpha expression by human monocytes. J. Nat. Prod. 1999, 62, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Ghasemipour Afshar, E. Tangeretin: A mechanistic review of its pharmacological and therapeutic effects. J. Basic Clin. Physiol. Pharmacol. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, C.; Chen, J.; Tian, G.; McClements, D.J.; Xiao, H.; Zheng, J. Encapsulation of Polymethoxyflavones in Citrus Oil Emulsion-Based Delivery Systems. J. Agric. Food Chem. 2017, 65, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Batenburg, A.M.; de Joode, T.; Gouka, R.J. Characterization and modulation of the bitterness of polymethoxyflavones using sensory and receptor-based methods. J. Agric. Food Chem. 2016, 64, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Li, D.; Song, R.; Shah, B.R.; Li, B.; Li, Y. Enhancement of physical stability and bioaccessibility of tangeretin by soy protein isolate addition. Food Chem. 2017, 221, 760–770. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, J.; McClements, D.J.; Xiao, H. Tangeretin-loaded protein nanoparticles fabricated from zein/beta-lactoglobulin: Preparation, characterization, and functional performance. Food Chem. 2014, 158, 466–472. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Levy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Bai, J. Effect of spray-drying temperature on physicochemical, antioxidant and antimicrobial properties of pectin/sodium alginate microencapsulated carvacrol. Food Hydrocoll. 2020, 100, 1–7. [Google Scholar] [CrossRef]
- Shahidi, F.; Han, X.Q. Encapsulation of food ingredients. Crit. Rev. Food Sci. Nutr. 1993, 33, 501–547. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Zheng, T.; Liu, Q.; Zhu, J.; Huang, Q. Evaluation of Oral Bioaccessibility of Aged Citrus Peel Extracts Encapsulated in Different Lipid-Based Systems: A Comparison Study Using Different in Vitro Digestion Models. J. Agric. Food Chem. 2020, 68, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Nakamura, T.; Uchida, A.; Ogawa, K.; Yuminoki, K.; Hashimoto, N.; Hiza, A.; Tsukaguchi, Y.; Asakawa, T.; Kan, T.; et al. Physicochemical and biopharmaceutical characterization of amorphous solid dispersion of nobiletin, a citrus polymethoxylated flavone, with improved hepatoprotective effects. Eur. J. Pharm. Sci. 2013, 49, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, K.; Kumar, P. Optimizing microencapsulation of alpha-tocopherol with pectin and sodium alginate. J. Food Sci. Technol. 2018, 55, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cameron, R.G.; Bai, J. Microencapsulation and antimicrobial activity of carvacrol in a pectin-alginate matrix. Food Hydrocoll. 2019, 92, 69–73. [Google Scholar] [CrossRef]
- Liu, L.S.; Kost, J.; Yan, F.; Spiro, R.C. Hydrogels from biopolymer hybrid for biomedical, food, and functional food applications. Polymers 2012, 4, 997–1011. [Google Scholar] [CrossRef]
- Drusch, S. Sugar beet pectin: A novel emulsifying wall component for microencapsulation of lipophilic food ingredients by spray-drying. Food Hydrocoll. 2007, 21, 1223–1228. [Google Scholar] [CrossRef]
- Ogonczyk, D.; Siek, M.; Garstecki, P. Microfluidic formulation of pectin microbeads for encapsulation and controlled release of nanoparticles. Biomicrofluidics 2011, 5, 13405. [Google Scholar] [CrossRef]
- Jantrawut, P.; Boonsermsukcharoen, K.; Thipnan, K.; Chaiwarit, T.; Hwang, K.M.; Park, E.S. Enhancement of antibacterial activity of orange oil in pectin thin film by microemulsion. Nanomaterials 2018, 8, 545. [Google Scholar] [CrossRef]
- Vaucher, A.; Dias, P.C.M.; Coimbra, P.T.; Costa, I.; Marreto, R.N.; Dellamora-Ortiz, G.M.; De Freitas, O.; Ramos, M.F.S. Microencapsulation of fish oil by casein-pectin complexes and gum arabic microparticles: Oxidative stabilisation. J. Microencapsul. 2019, 36, 459–473. [Google Scholar] [CrossRef]
- Noh, J.; Kim, J.; Kim, J.S.; Chung, Y.S.; Chang, S.T.; Park, J. Microencapsulation by pectin for multi-components carriers bearing both hydrophobic and hydrophilic active agents. Carbohydr. Polym. 2018, 182, 172–179. [Google Scholar] [CrossRef]
- Koo, S.Y.; Cha, K.H.; Song, D.G.; Chung, D.; Pan, C.H. Microencapsulation of peppermint oil in an alginate–pectin matrix using a coaxial electrospray system. Int. J. Food Sci. Tech. 2014, 49, 733–739. [Google Scholar] [CrossRef]
- Arab, M.; Hosseini, S.M.; Nayebzadeh, K.; Khorshidian, N.; Yousefi, M.; Razavi, S.H.; Mortazavian, A.M. Microencapsulation of microbial canthaxanthin with alginate and high methoxyl pectin and evaluation the release properties in neutral and acidic condition. Int. J. Biol. Macromol. 2019, 121, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.G.; Chau, H.K.; Manthey, J.A. Continuous process for enhanced release and recovery of pectic hydrocolloids and phenolics from citrus biomass. J. Chem. Technol. Biotechnol. 2016, 91, 2597–2606. [Google Scholar] [CrossRef]
- Kurowska, E.M.; Manthey, J.A. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. J. Agric. Food Chem. 2004, 52, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E.; Kalemba, D.; Adamiec, J.; Piatkowski, M. Microencapsulation of Nigella sativa oleoresin by spray drying for food and nutraceutical applications. Food Chem. 2016, 204, 326–333. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G.; Kazakis, N.A. Influence of spray drying conditions on tomato powder properties. Dry. Technol. 2004, 22, 1129–1151. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.A.; Plotto, A.; Manthey, J.A.; Duan, Y.; Bai, J. Effects of thermal processing and pulp filtration on physical, chemical and sensory properties of winter melon juice. J. Sci. Food Agric. 2017, 97, 543–550. [Google Scholar] [CrossRef]
- Bringas-Lantigua, M.; Expósito-Molina, I.; Reineccius, G.A.; López-Hernández, O.; Pino, J.A. Influence of spray-dryer air temperatures on encapsulated mandarin oil. Dry. Technol. 2011, 29, 520–526. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Mundra, S.; Ramalingam, C.; Kumar, A. Fabrication of food grade vitamin E nanoemulsion by low energy approach, characterization and its application. Int. J. Food Prop. 2016, 19, 700–708. [Google Scholar] [CrossRef]
- Correa-Filho, L.C.; Lourenco, M.M.; Moldao-Martins, M.; Alves, V.D. Microencapsulation of beta-carotene by spray drying: Effect of wall material concentration and drying inlet temperature. Int. J. Food Sci. 2019, 2019, 8914852. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Rosenberg, M.; Rosenberg, Y.; Zhang, J. Microencapsulation of a model oil in wall system consisting of wheat proteins isolate (WHPI) and lactose. Appl. Sci. 2018, 8, 1944. [Google Scholar] [CrossRef]
- Delfiya, D.S.A.; Thangavel, K.; Natarajan, N.; Kasthuri, R.; Kailappan, R. Microencapsulation of turmeric oleoresin by spray drying and in vitro release studies of microcapsules. J. Food Process Eng. 2015, 38, 37–48. [Google Scholar] [CrossRef]
- Sheu, T.Y.; Rosenberg, M. Microencapsulation by spray-drying ethyl caprylate in whey-protein and carbohydrate wall systems. J. Food Sci. 1995, 60, 98–103. [Google Scholar] [CrossRef]
- Nochos, A.; Douroumis, D.; Bouropoulos, N. In vitro release of bovine serum albumin from alginate/HPMC hydrogel beads. Carbohydr. Polym. 2008, 74, 451–457. [Google Scholar] [CrossRef]
- Chegini, G.R.; Ghobadian, B. Effect of spray-drying conditions on physical properties of orange juice powder. Dry. Technol. 2005, 23, 657–668. [Google Scholar] [CrossRef]
- Jumah, R.Y.; Tashtoush, B.; Shaker, R.R.; Zraiy, A.F. Manufacturing parameters and quality characteristics of spray dried jameed. Dry. Technol. 2000, 18, 967–984. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, S.J. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef]
- Serro, A.P.; Colaco, R.; Saramago, B. Adhesion forces in liquid media: Effect of surface topography and wettability. J. Colloid Interface Sci. 2008, 325, 573–579. [Google Scholar] [CrossRef]
- Kiaei Pour, P.; Alemzadeh, I.; Vaziri, A.S.; Beiroti, A. Potential effects of alginate-pectin biocomposite on the release of folic acid and their physicochemical characteristics. J. Food Sci. Technol. 2020, 57, 3363–3370. [Google Scholar] [CrossRef]
- Gomez-Rodriguez, G.H.; Lopez-Mata, M.A.; Valbuena-Gregorio, E.; Melchor, R.G.V.; Campos-Garcia, J.C.; Silva-Beltran, N.P.; Quihui-Cota, L.; Ruiz-Cruz, S.; Juarez, J. Microencapsulation of carvacrol using pectin/aloe-gel as a novel wound dressing films. Curr. Top. Med. Chem. 2018, 18, 1261–1268. [Google Scholar] [CrossRef]
- Mok, H.; Park, T.G. Water-free microencapsulation of proteins within PLGA microparticles by spray drying using PEG-assisted protein solubilization technique in organic solvent. Eur. J. Pharm. Biopharm. 2008, 70, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Arana-Sanchez, A.; Estarron-Espinosa, M.; Obledo-Vazquez, E.N.; Padilla-Camberos, E.; Silva-Vazquez, R.; Lugo-Cervantes, E. Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated in beta-cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
| Samples | Moisture Content (%) | Bulk Density (g cm−3) | Dissolution Time (min) | Hygroscopicity (g H2O 100 g−1) |
|---|---|---|---|---|
| Control (no tangeretin) | 4.47 ± 0.75 | 0.31 ± 0.01 | 5.03 ± 0.12 | 2.70 ± 0.30 |
| Low tangeretin | 4.63 ± 0.12 | 0.31 ± 0.01 | 4.97 ± 0.06 | 3.03 ± 0.31 |
| High tangeretin | 4.70 ± 0.20 | 0.31 ± 0.01 | 4.93 ± 0.32 | 2.80 ± 0.36 |
| Samples | Retention Efficiency (%) | Encapsulation Efficiency (%) |
|---|---|---|
| Low tangeretin | 71.05 b | 75.64 a |
| High tangeretin | 98.92 a | 55.51 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Cameron, R.G.; Manthey, J.A.; Hunter, W.B.; Bai, J. Microencapsulation of Tangeretin in a Citrus Pectin Mixture Matrix. Foods 2020, 9, 1200. https://doi.org/10.3390/foods9091200
Sun X, Cameron RG, Manthey JA, Hunter WB, Bai J. Microencapsulation of Tangeretin in a Citrus Pectin Mixture Matrix. Foods. 2020; 9(9):1200. https://doi.org/10.3390/foods9091200
Chicago/Turabian StyleSun, Xiuxiu, Randall G. Cameron, John A. Manthey, Wayne B. Hunter, and Jinhe Bai. 2020. "Microencapsulation of Tangeretin in a Citrus Pectin Mixture Matrix" Foods 9, no. 9: 1200. https://doi.org/10.3390/foods9091200
APA StyleSun, X., Cameron, R. G., Manthey, J. A., Hunter, W. B., & Bai, J. (2020). Microencapsulation of Tangeretin in a Citrus Pectin Mixture Matrix. Foods, 9(9), 1200. https://doi.org/10.3390/foods9091200