Preparation and Characterization of Soy Isoflavones Nanoparticles Using Polymerized Goat Milk Whey Protein as Wall Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
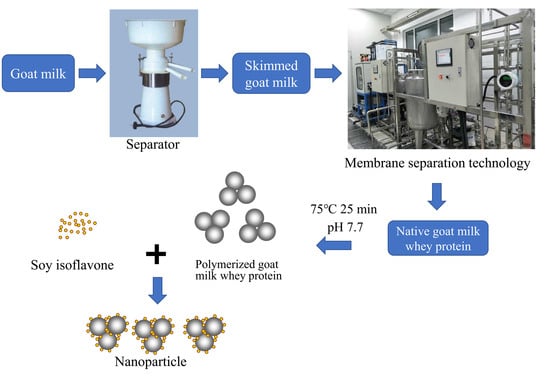
2.2. Nanoparticle Preparation
2.2.1. Preparation of Goat Milk Whey Protein Concentrate
2.2.2. Preparation of Polymerized Goat Milk Whey Protein
2.2.3. Preparation of Soy Isoflavones Solution
2.2.4. PGWP-SIF Nanoparticle Preparation
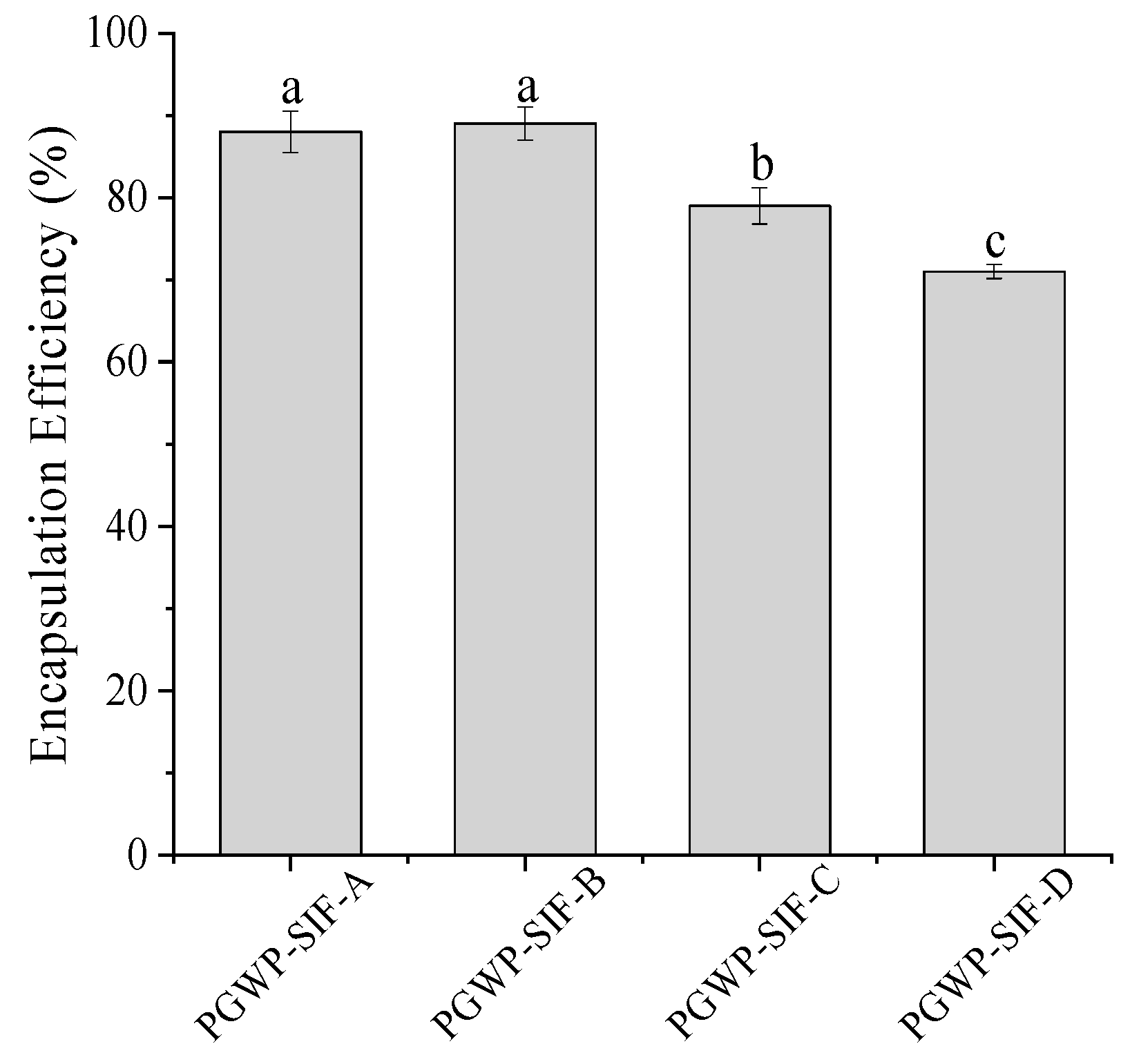
2.3. Encapsulation Efficiency Determination
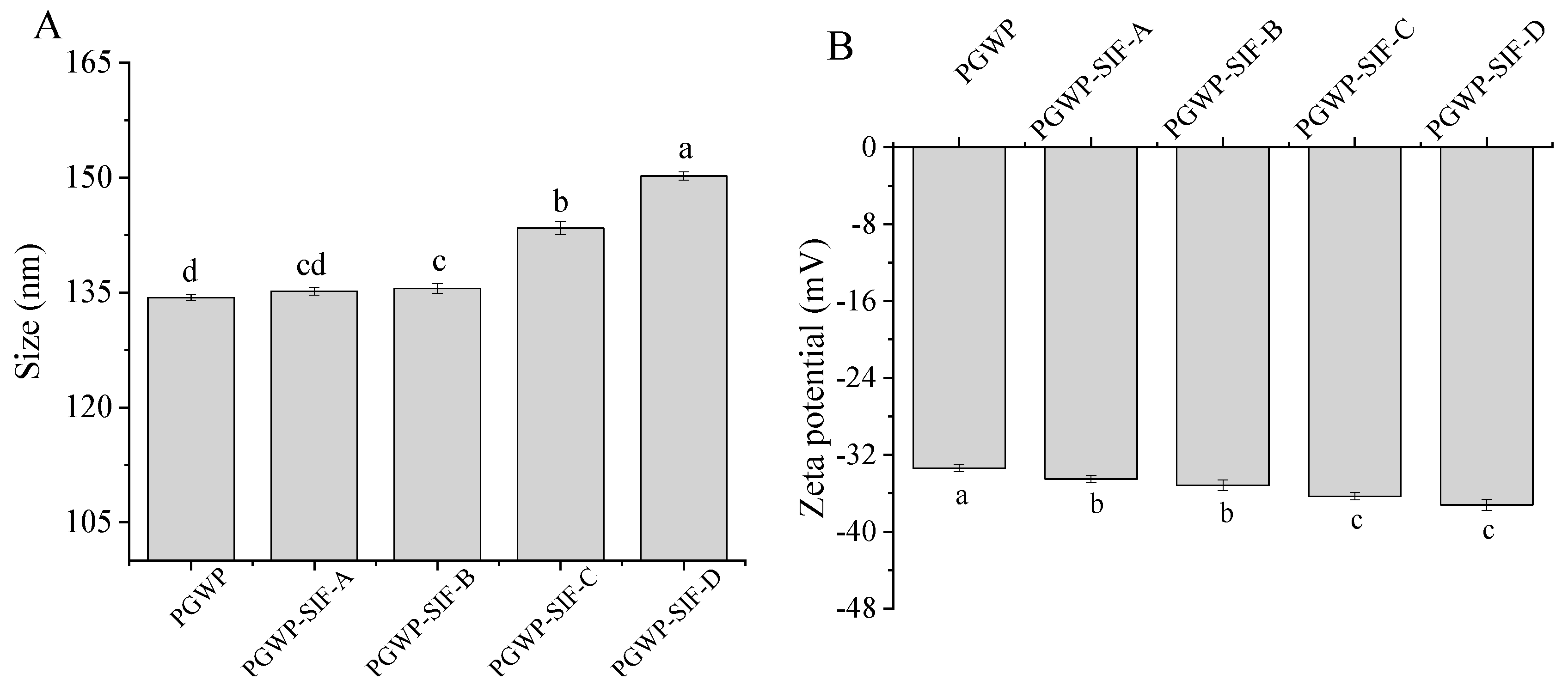
2.4. Particle Size and Zeta Potential Analysis
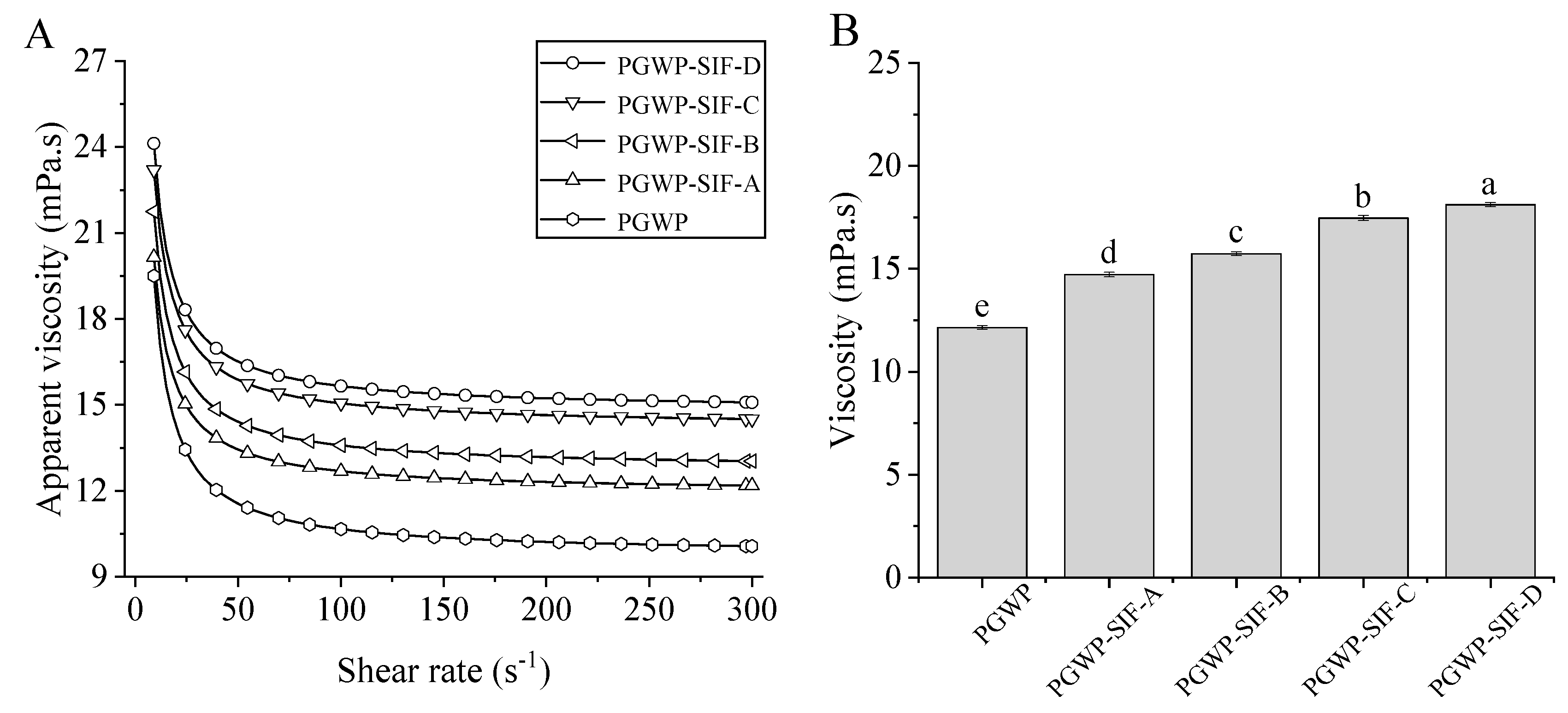
2.5. Rheological Properties Measurement
2.6. Fourier Transform Infrared (FT-IR) Spectroscopy
2.7. Fluorescence Spectroscopy
2.8. Differential Scanning Calorimetry (DSC)
2.9. Transmission Electron Microscopy (TEM)
2.10. Statistical Analysis
3. Results
3.1. Encapsulation Efficiency
3.2. Particle Size and Zeta Potential
3.3. Rheological Properties
3.4. FT-IR Spectra
3.5. Fluorescence Spectra
3.5.1. Inherent Fluorescence
3.5.2. Stern–Volmer Analysis of Quenching Data
3.5.3. Thermodynamic Parameters
3.5.4. Synchronous Fluorescence Spectra
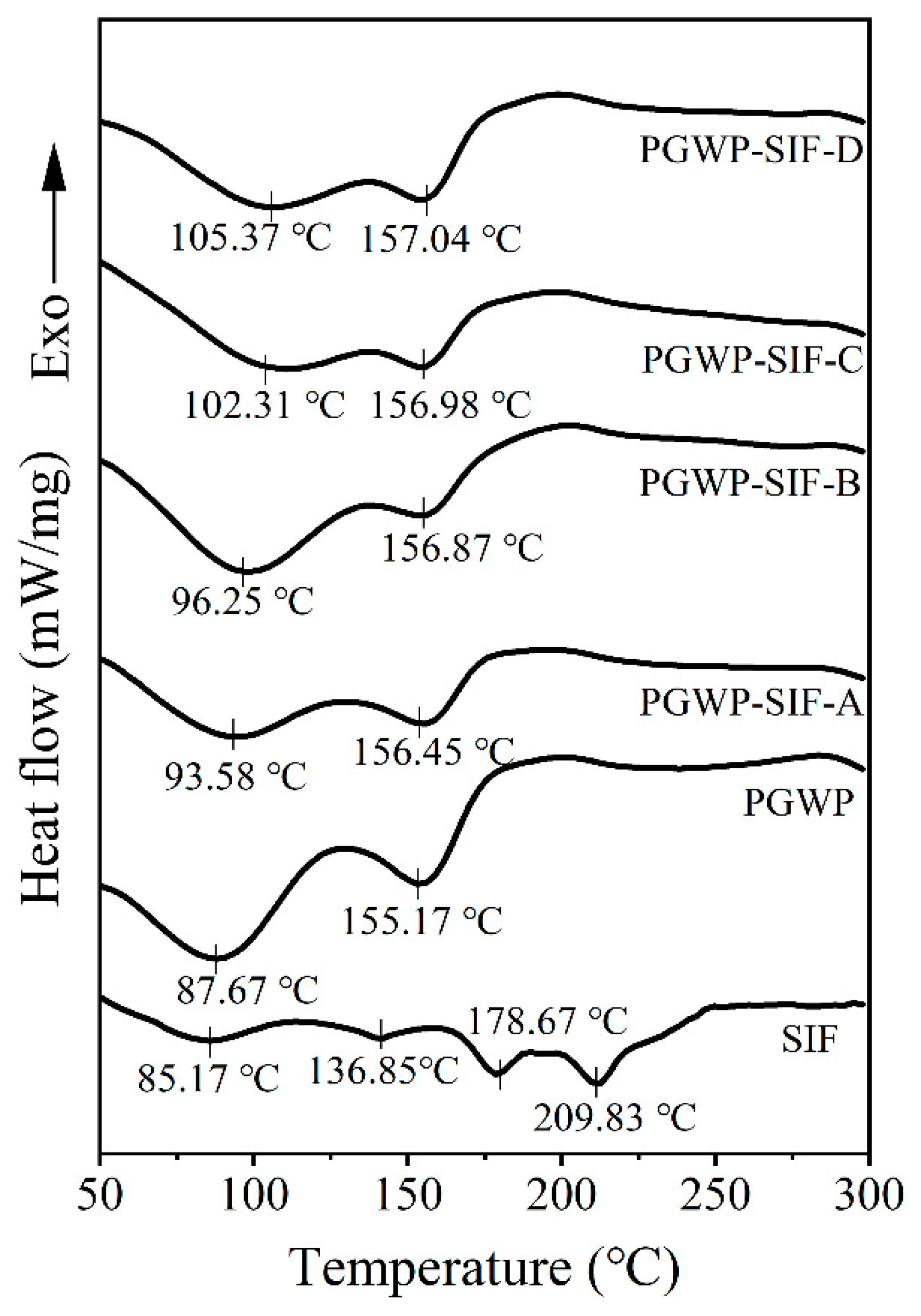
3.6. Differential Scanning Calorimetry (DSC)
3.7. Microstructure
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dong, X.; Xu, W.; Sikes, R.A.; Wu, C. Combination of low dose of genistein and daidzein has synergistic preventive effects on isogenic human prostate cancer cells when compared with individual soy isoflavone. Food Chem. 2013, 141, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 423S–430S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marini, H.R.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast Safety and Efficacy of Genistein Aglycone for Postmenopausal Bone Loss: A Follow-Up Study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, H.; Xia, X.; Pu, N.; Yu, Z.; Nabih, M.; Zhu, Y.; Zhang, S.; Jiang, L. Preparation and physicochemical characterization of soy isoflavone (SIF) nanoparticles by a liquid antisolvent precipitation method. Adv. Powder Technol. 2019, 30, 1522–1530. [Google Scholar] [CrossRef]
- Wang, S.; Shao, G.; Yang, J.; Liu, J.; Wang, J.; Zhao, H.; Yang, L.; Liu, H.; Zhua, D.; Li, Y.; et al. The production of gel beads of soybean hull polysaccharides loaded with soy isoflavone and their pH-dependent release. Food Chem. 2020, 313, 126095. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.B.; Barac, M.; Vrvić, M.; Ristić, N.; Macej, O.; Stanojevic, S.; Kostić, A. The distributions of major whey proteins in acid wheys obtained from caprine/bovine and ovine/bovine milk mixtures. Int. Dairy J. 2011, 21, 831–838. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Pimentel, T.; Ferrão, L.; Almada, C.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.; Nascimento, J.; Silva, M.; et al. Sheep Milk: Physicochemical Characteristics and Relevance for Functional Food Development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef]
- Sanmartín, B.; Díaz, O.; Rodriguez-Turienzo, L.; Cobos, Á. Properties of heat-induced gels of caprine whey protein concentrates obtained from clarified cheese whey. Small Rumin. Res. 2015, 123, 142–148. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Assadpour, E.; Esfanjani, A.F. Nano-encapsulation of olive leaf phenolic compounds through WPC–pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 2016, 82, 816–822. [Google Scholar] [CrossRef]
- Sun, X.; Wang, C.; Guo, M. Interactions between whey protein or polymerized whey protein and soybean lecithin in model system. J. Dairy Sci. 2018, 101, 9680–9692. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Guo, M.; Sun, X.; Killpartrick, A.; Guo, M. Preparation and Characterization of Whey Protein Isolate-DIM Nanoparticles. Int. J. Mol. Sci. 2019, 20, 3917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Zhou, X.; Wang, H.; Sun, X.; Guo, M. Interactions between β-Lactoglobulin and 3,3′-Diindolylmethane in Model System. Molecules 2019, 24, 2151. [Google Scholar] [CrossRef] [Green Version]
- Fang, T.; Shen, X.; Hou, J.; Guo, M. Effects of polymerized whey protein prepared directly from cheese whey as fat replacer on physiochemical, texture, microstructure and sensory properties of low-fat set yogurt. LWT 2019, 115, 108268. [Google Scholar] [CrossRef]
- Chen, L.; Subirade, M. Alginate–whey protein granular microspheres as oral delivery vehicles for bioactive compounds. Biomaterials 2006, 27, 4646–4654. [Google Scholar] [CrossRef]
- Salem, A.; Ramadan, A.R.; Shoeib, T. Entrapment of β-carotene and zinc in whey protein nanoparticles using the pH cycle method: Evidence of sustained release delivery in intestinal and gastric fluids. Food Biosci. 2018, 26, 161–168. [Google Scholar] [CrossRef]
- Al-Hanish, A.; Stanić, D.; Mihailovic, J.; Prodic, I.; Minić, S.; Stojadinovic, M.; Radibratovic, M.; Milčić, M.; Veličković, T. Ćirković Noncovalent interactions of bovine α-lactalbumin with green tea polyphenol, epigalocatechin-3-gallate. Food Hydrocoll. 2016, 61, 241–250. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, H.; Bao, W.; Zou, G. Studies on the interaction between docetaxel and human hemoglobin by spectroscopic analysis and molecular docking. J. Photochem. Photobiol. B Biol. 2011, 105, 126–132. [Google Scholar] [CrossRef]
- Ranamukhaarachchi, S.A.; Peiris, R.H.; Moresoli, C. Fluorescence spectroscopy and principal component analysis of soy protein hydrolysate fractions and the potential to assess their antioxidant capacity characteristics. Food Chem. 2017, 217, 469–475. [Google Scholar] [CrossRef]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of binding interaction between β-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef]
- Gorji, E.G.; Rocchi, E.; Schleining, G.; Bender, D.; Furtmüller, P.G.; Piazza, L.; Iturri, J.; Toca-Herrera, J.L. Characterization of resveratrol–milk protein interaction. J. Food Eng. 2015, 167, 217–225. [Google Scholar] [CrossRef]
- Patel, A.R.; Hu, Y.; Tiwari, J.K.; Velikov, K.P. Synthesis and characterisation of zein–curcumin colloidal particles. Soft Matter 2010, 6, 6192–6199. [Google Scholar] [CrossRef]
- Nagy, K.; Courtet-Compondu, M.-C.; Williamson, G.; Rezzi, S.; Kussmann, M.; Rytz, A. Non-covalent binding of proteins to polyphenols correlates with their amino acid sequence. Food Chem. 2012, 132, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, S.D.; Von Staszewski, M.; Pilosof, A.M. Green tea polyphenols-whey proteins nanoparticles: Bulk, interfacial and foaming behavior. Food Hydrocoll. 2015, 50, 108–115. [Google Scholar] [CrossRef]
- Mantovani, R.A.; Fattori, J.; Michelon, M.; Cunha, R. Formation and pH-stability of whey protein fibrils in the presence of lecithin. Food Hydrocoll. 2016, 60, 288–298. [Google Scholar] [CrossRef]
- Von Staszewski, M.; Jara, F.L.; Ruiz, A.; Jagus, R.J.; De Carvalho, J.E.; Pilosof, A.M. Nanocomplex formation between β-lactoglobulin or caseinomacropeptide and green tea polyphenols: Impact on protein gelation and polyphenols antiproliferative activity. J. Funct. Foods 2012, 4, 800–809. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P. Response surface methodology and multivariate analysis of equilibrium headspace concentration of orange beverage emulsion as function of emulsion composition and structure. Food Chem. 2009, 115, 324–333. [Google Scholar] [CrossRef]
- Yang, C.; Wang, B.; Wang, J.; Xia, S.; Wu, Y. Effect of pyrogallic acid (1,2,3-benzenetriol) polyphenol-protein covalent conjugation reaction degree on structure and antioxidant properties of pumpkin (Cucurbita sp.) seed protein isolate. LWT 2019, 109, 443–449. [Google Scholar] [CrossRef]
- Moeiniafshari, A.-A.; Zarrabi, A.; Bordbar, A.-K. Exploring the interaction of naringenin with bovine beta-casein nanoparticles using spectroscopy. Food Hydrocoll. 2015, 51, 1–6. [Google Scholar] [CrossRef]
- Ghalandari, B.; Divsalar, A.; Saboury, A.A.; Haertlé, T.; Parivar, K.; Bazl, R.; Eslami-Moghadam, M.; Amanlou, M. Spectroscopic and theoretical investigation of oxali–palladium interactions with?-lactoglobulin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 1038–1046. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Q.H.; Wang, Z.; Zhao, J. Study of the interaction of carbamazepine with bovine whey albumin by fluorescence quenching method. Anal. Sci. 2006, 22, 435–438. [Google Scholar] [CrossRef] [Green Version]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Seedher, N.; Agarwal, P. Complexation of fluoroquinolone antibiotics with human serum albumin: A fluorescence quenching study. J. Lumin. 2010, 130, 1841–1848. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, B.; Jiang, L.; Regenstein, J.M.; Jiang, N.; Poias, V.; Zhang, X.; Qi, B.; Li, A.-L.; Wang, Z.; et al. Interaction of soybean protein isolate and phosphatidylcholine in nanoemulsions: A fluorescence analysis. Food Hydrocoll. 2019, 87, 814–829. [Google Scholar] [CrossRef]
- Xu, J.; Hao, M.; Sun, Q.; Tang, L. Comparative studies of interaction of β-lactoglobulin with three polyphenols. Int. J. Biol. Macromol. 2019, 136, 804–812. [Google Scholar] [CrossRef]
- Bobone, S.; Van De Weert, M.; Stella, L. A reassessment of synchronous fluorescence in the separation of Trp and Tyr contributions in protein emission and in the determination of conformational changes. J. Mol. Struct. 2014, 1077, 68–76. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Wang, P.-P.; Luo, Z.-G.; Peng, X.-C. Encapsulation of Vitamin E and Soy Isoflavone Using Spiral Dextrin: Comparative Structural Characterization, Release Kinetics, and Antioxidant Capacity during Simulated Gastrointestinal Tract. J. Agric. Food Chem. 2018, 66, 10598–10607. [Google Scholar] [CrossRef]
- Ghayour, N.; Hosseini, S.M.H.; Eskandari, M.H.; Esteghlal, S.; Nekoei, A.-R.; Gahruie, H.H.; Tatar, M.; Naghibalhossaini, F. Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocoll. 2019, 87, 394–403. [Google Scholar] [CrossRef]
- Fang, Y.; Dalgleish, D.G. Conformation of β-lactoglobulin studied by FTIR: Effect of pH, temperature, and adsorption to the oil-water interface. J. Colloid Interface Sci. 1997, 196, 292–298. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Oxidative stability and in vitro digestion of menhaden oil emulsions with whey protein: Effects of EGCG conjugation and interfacial cross-linking. Food Chem. 2018, 265, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Mandeville, J.S.; Tajmir-Riahi, H.A. Nanocomplexes of dendrimers with bovine whey albumin. Biomacromolecules 2010, 11, 465–472. [Google Scholar] [CrossRef] [PubMed]
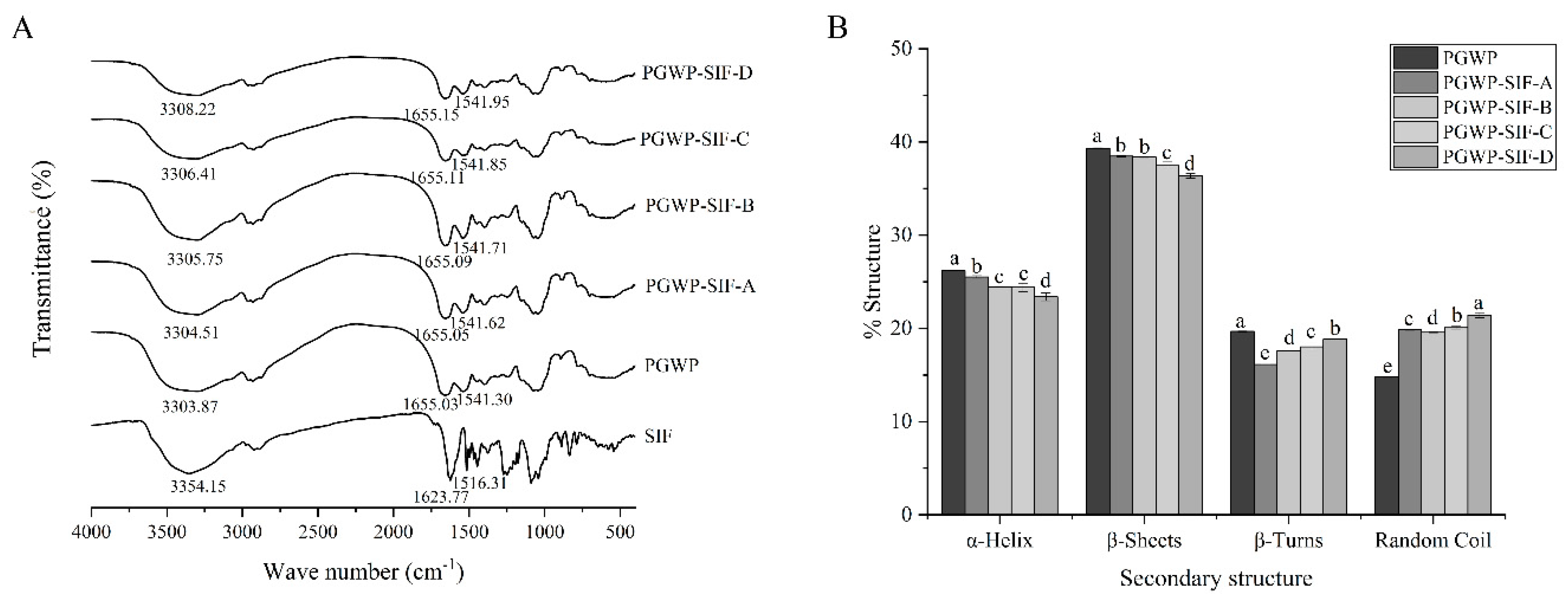
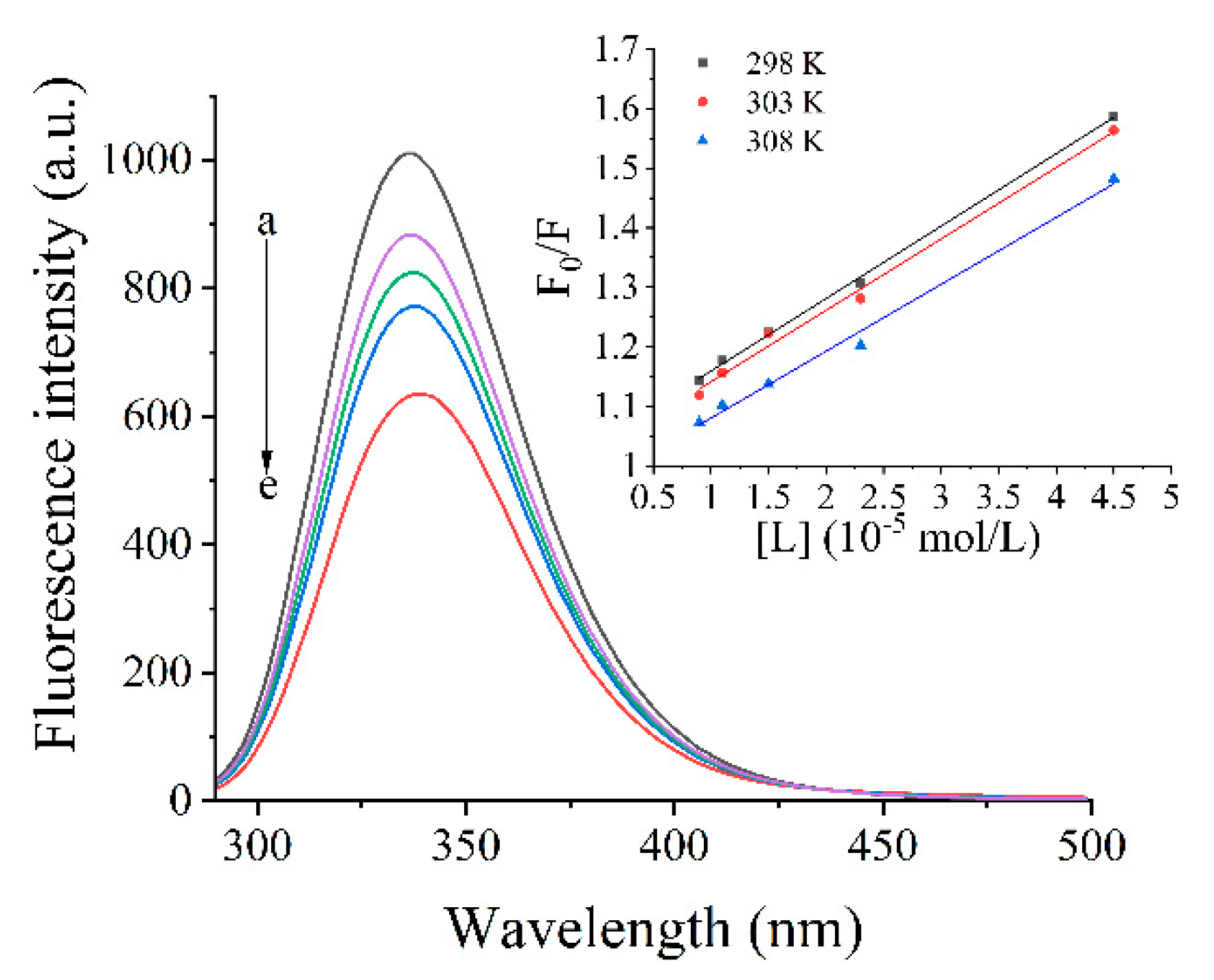


| T (K) | Ksv (104 L/mol) | Kq (1012 L/mol) | R2 | Ka (105 L/mol) | n | R2 | ΔH (KJ/mol) | ΔG (KJ/mol) | ΔS (J/mol.K) |
|---|---|---|---|---|---|---|---|---|---|
| 298 | 1.22 ± 0.1 | 1.22 ± 0.1 | 0.9988 | 7.80 ± 0.1 | 1.13 ± 0.02 | 0.9969 | 7.81 ± 0.1 | –33.74 ± 0.1 | 139.42 ± 1.2 |
| 303 | 1.20 ± 0.2 | 1.20 ± 0.2 | 0.9932 | 8.22 ± 0.1 | 0.93 ± 0.01 | 0.9873 | –34.43 ± 0.2 | ||
| 308 | 1.13 ± 0.2 | 1.13 ± 0.2 | 0.9927 | 8.34 ± 0.1 | 0.86 ± 0.02 | 0.9929 | –35.13 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Wang, C.; Cheng, J.; Wang, H.; Jiang, S.; Guo, M. Preparation and Characterization of Soy Isoflavones Nanoparticles Using Polymerized Goat Milk Whey Protein as Wall Material. Foods 2020, 9, 1198. https://doi.org/10.3390/foods9091198
Tian M, Wang C, Cheng J, Wang H, Jiang S, Guo M. Preparation and Characterization of Soy Isoflavones Nanoparticles Using Polymerized Goat Milk Whey Protein as Wall Material. Foods. 2020; 9(9):1198. https://doi.org/10.3390/foods9091198
Chicago/Turabian StyleTian, Mu, Cuina Wang, Jianjun Cheng, Hao Wang, Shilong Jiang, and Mingruo Guo. 2020. "Preparation and Characterization of Soy Isoflavones Nanoparticles Using Polymerized Goat Milk Whey Protein as Wall Material" Foods 9, no. 9: 1198. https://doi.org/10.3390/foods9091198
APA StyleTian, M., Wang, C., Cheng, J., Wang, H., Jiang, S., & Guo, M. (2020). Preparation and Characterization of Soy Isoflavones Nanoparticles Using Polymerized Goat Milk Whey Protein as Wall Material. Foods, 9(9), 1198. https://doi.org/10.3390/foods9091198