Preliminary Study of Australian Pinot Noir Wines by Colour and Volatile Analyses, and the Pivot© Profile Method Using Wine Professionals
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Wines
2.1.1. Basic Oenological Attributes
2.1.2. Vineyard Sites
2.2. Pivot© Profile
2.3. Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry
2.3.1. Esters, Alcohols and Fatty Acids
2.3.2. C13-Norisoprenoids
2.4. Chromatic Components and Colour Structure Analysis
2.5. Data Analysis
3. Results and Discussion
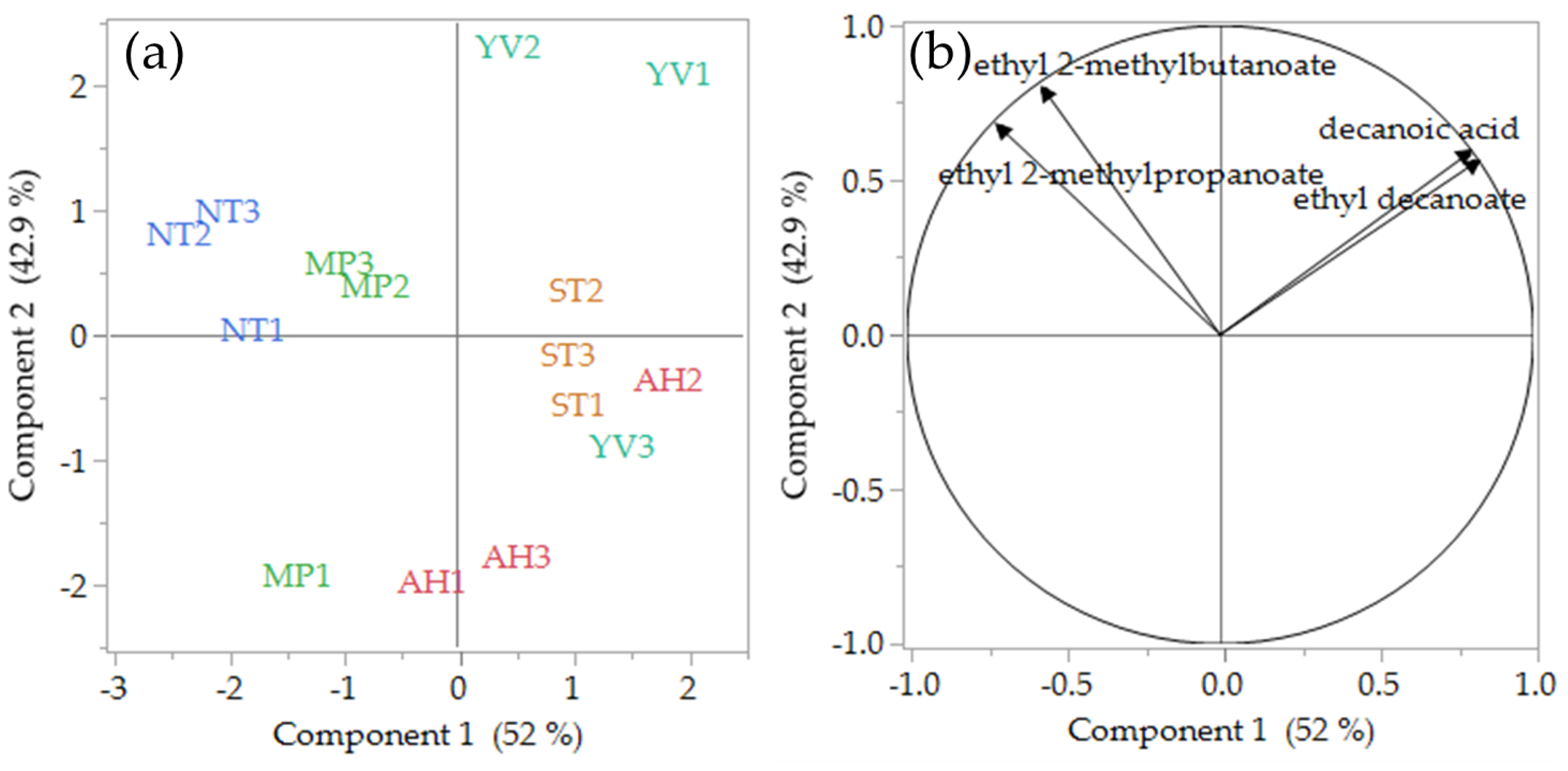
3.1. Volatile Analysis
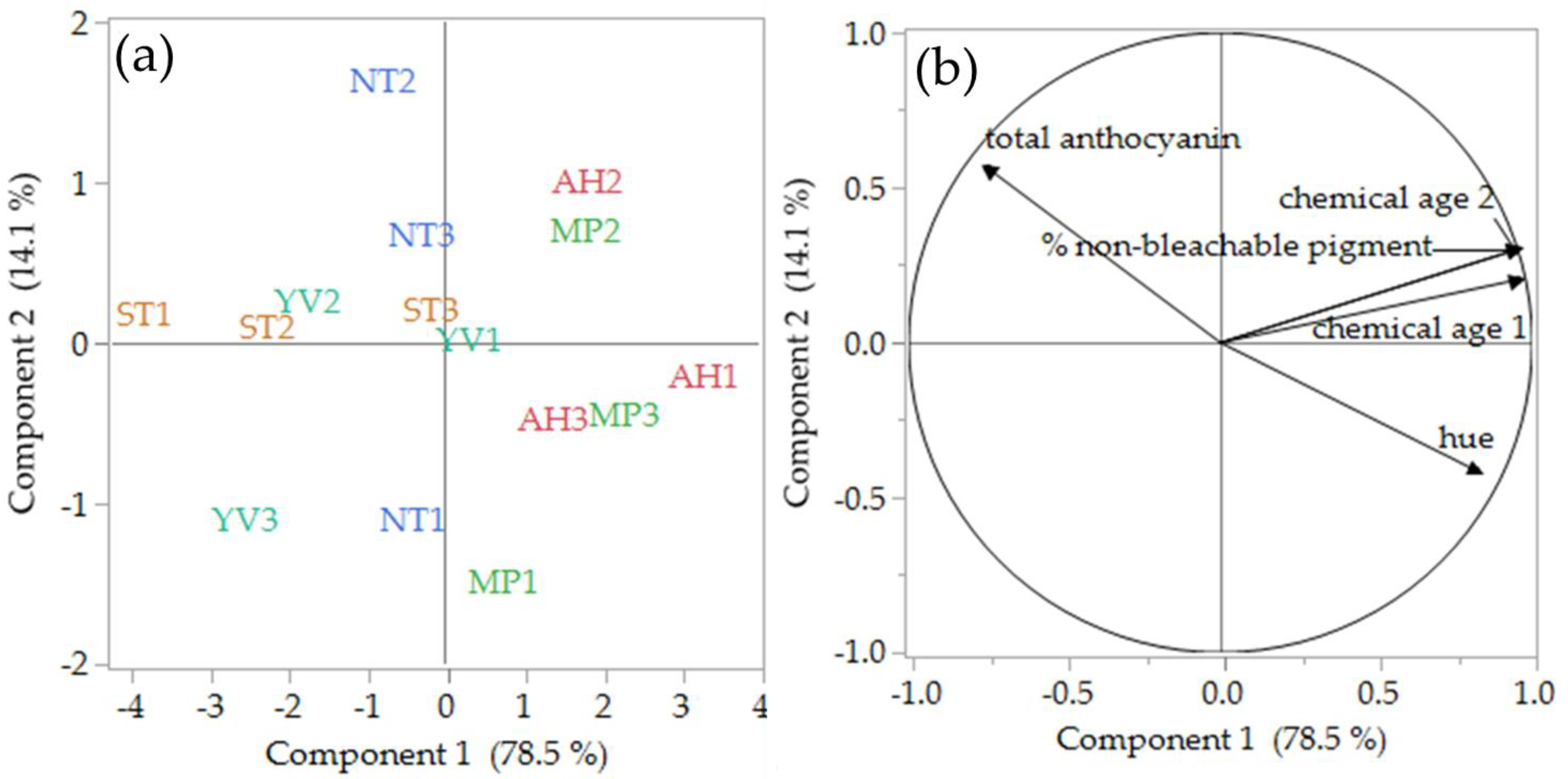
3.2. Chromatic and Colour Structure Analysis
3.3. Principal Component Analysis
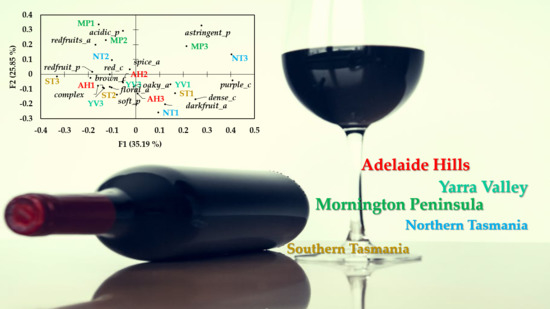
3.4. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roullier-Gall, C.; Lucio, M.; Noret, L.; Schmitt-Kopplin, P.; Gougeon, R.D. How subtle is the “terroir” effect? Chemistry-related signatures of two “climats de Bourgogne”. PLoS ONE 2014, 9, e97615. [Google Scholar] [CrossRef] [PubMed]
- Rutan, T.; Herbst-Johnstone, M.; Pineau, B.; Kilmartin, P.A. Characterization of the aroma of Central Otago Pinot noir wines using sensory reconstitution studies. Am. J. Enol. Viticult. 2014, 65, 424–434. [Google Scholar] [CrossRef]
- Schueuermann, C.; Silcock, P.; Bremer, P. Front-face fluorescence spectroscopy in combination with parallel factor analysis for profiling of clonal and vineyard site differences in commercially produced Pinot Noir grape juices and wines. J. Food Compos. Anal. 2018, 66, 30–38. [Google Scholar] [CrossRef]
- Schueuermann, C.; Bremer, P.; Silcock, P. PTR-MS volatile profiling of Pinot noir wines for the investigation of differences based on vineyard site. J. Mass Spectrom. 2017, 52, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.; Fuentealba, C.; Yáñez, L.; Bravo, M.; Quiroz, W.; Lukacsy, G.; Carot, J. Chemometric approaches for the zoning of Pinot Noir wines from the Casablanca Valley, Chile. Food Chem. 2011, 127, 1842–1847. [Google Scholar] [CrossRef]
- Schueuermann, C.; Khakimov, B.; Engelsen, S.B.; Bremer, P.; Silcock, P. GC-MS metabolite profiling of extreme Southern Pinot Noir wines: Effects of vintage, barrel maturation, and fermentation dominate over vineyard site and clone selection. J. Agric. Food Chem. 2016, 64, 2342–2351. [Google Scholar] [CrossRef]
- Guinard, J.X.; Cliff, M. Descriptive analysis of Pinot noir wines from Carneros, Napa, and Sonoma. Am. J. Enol. Viticult. 1987, 38, 211–215. [Google Scholar]
- Tomasino, E.; Harrison, R.; Sedcole, R.; Frost, A. Regional differentiation of New Zealand Pinot noir wine by wine professionals using canonical variate analysis. Am. J. Enol. Viticult. 2013, 64, 357–363. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Thuillier, B.; Valentin, D.; Marchal, R.; Dacremont, C. Pivot© Profile: A new descriptive method based on free description. Food Qual. Pref. 2015, 42, 66–77. [Google Scholar] [CrossRef]
- Pearson, W.; Schmidtke, L.; Francis, L.; Blackman, J.W. An investigation of the Pivot© Profile sensory analysis method using wine experts: Comparison with descriptive analysis and results from two expert panels. Food Qual. Pref. 2020, 83, 103858. [Google Scholar] [CrossRef]
- Shaw, T.B. A climatic analysis of wine regions growing Pinot Noir. J. Wine Res. 2012, 23, 203–228. [Google Scholar] [CrossRef]
- OIV. Distribution of the World’s Grapevine Varieties. 2017. Available online: http://www.oiv.int/public/medias/5888/en-distribution-of-the-worlds-grapevine-varieties.pdf (accessed on 31 January 2020).
- Langlois, J.; Ballester, J.; Campo, E.; Dacremont, C.; Peyron, D. Combining olfactory and gustatory clues in the judgment of aging potential of red wine by wine professionals. Am. J. Enol. Viticult. 2010, 61, 15–22. [Google Scholar]
- Wine Australia. Pinot Noir in Australia. 2019. Available online: https://www.wineaustralia.com/news/market-bulletin/issue-158 (accessed on 31 January 2020).
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuwohner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.J.; Sefton, M.A.; Pollnitz, A.P. Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal. Bioanal. Chem. 2005, 381, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics adaptation and validation of methyl cellulose precipitable tannin assay and modified Somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Clarke, R.J. Wine: Flavour Chemistry, 2nd ed.; John Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
- Antalick, G.; Perello, M.C.; de Revel, G. Esters in wines: New insight through the establishment of a database of French wines. Am. J. Enol. Viticult. 2014, 65, 293–304. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Longo, R.; Carew, A.; Sawyer, S.; Kemp, B.; Kerslake, F. A review on the aroma composition of Vitis vinifera L. Pinot noir wines: Origins and influencing factors. Crit. Rev. Food Sci. Nutr. 2020, 1–16, in press. [Google Scholar] [CrossRef]
- Tomasino, E.; Harrison, R.; Breitmeyer, J.; Sedcole, R.; Sherlock, R.; Frost, A. Aroma composition of 2-year-old New Zealand Pinot Noir wine and its relationship to sensory characteristics using canonical correlation analysis and addition/omission tests. Aust. J. Grape Wine Res. 2015, 21, 376–388. [Google Scholar] [CrossRef]
- Fang, Y.; Qian, M. Aroma compounds in oregon pinot noir wine determined by aroma extract dilution analysis (AEDA). Flavour Fragr. J. 2005, 20, 22–29. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache rosé wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Miranda-Lopez, R.; Libbey, L.; Watson, B.; McDaniel, M. Identification of additional odor-active compounds in Pinot noir wines. Am. J. Enol. Viticult. 1992, 43, 90–92. [Google Scholar]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Etievant, P. Wine. In Volatile Compounds in Foods and Beverages; Maarse, H., Ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. 2-methylbutyl acetate in wines: Enantiomeric distribution and sensory impact on red wine fruity aroma. Food Chem. 2017, 237, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics, and color of Cabernet Franc, Merlot, and Pinot Noir wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Viticult. 2001, 52, 67–87. [Google Scholar]
- Carew, A.L.; Smith, P.; Close, D.C.; Curtin, C.; Dambergs, R.G. Yeast effects on Pinot Noir wine phenolics, color, and tannin composition. J. Agric. Food Chem. 2013, 61, 9892–9898. [Google Scholar] [CrossRef]
- Dambergs, R.; Sparrow, A.; Carew, A.; Scrimgeour, N.; Wilkes, E.; Godden, P.; Herderich, M.; Johnson, D. Quality in a cool climate-maceration techniques in Pinot Noir production. Wine Viticult. J. 2012, 27, 20–26. [Google Scholar]
- Dobrei, A.; Poiana, M.A.; Sala, F.; Ghita, A.; Gergen, I. Changes in the chromatic properties of red wines from Vitis vinifera L. cv. Merlot and Pinot Noir during the course of aging in bottle. J. Food. Agric. Environ. 2010, 8, 20–24. [Google Scholar]
- Somers, T.; Verette, E. Phenolic composition of natural wine types. In Wine Analysis; Springer: Berlin/Heidelberg, Germany, 1988; pp. 219–257. [Google Scholar]
- McRae, J.M.; Day, M.P.; Bindon, K.A.; Kassara, S.; Schmidt, S.A.; Schulkin, A.; Kolouchova, R.; Smith, P.A. Effect of early oxygen exposure on red wine colour and tannins. Tetrahedron 2015, 71, 3131–3137. [Google Scholar] [CrossRef]
- Bindon, K.A.; Madani, S.H.; Pendleton, P.; Smith, P.A.; Kennedy. J.A. Factors affecting skin tannin extractability in ripening grapes. J. Agric. Food Chem. 2014, 62, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Kielhorn, S.; Thorngate, J., III. Oral sensations associated with the flavan-3-ols (+)-catechin and (−)-epicatechin. Food Qual. Pref. 1999, 10, 109–116. [Google Scholar] [CrossRef]

| Region | State | Vintage | Alc. % (v/v) | pH | Titratable Acidity (g L−1) |
|---|---|---|---|---|---|
| Adelaide Hills | SA | 2018 | 13.8 ± 0.6 | 3.9 ± 0.2 | 5.14 ± 0.35 |
| Yarra Valley | VIC | 2018 | 13.4 ± 0.3 | 3.7 ± 0.1 | 5.43 ± 0.36 |
| Mornington Peninsula | VIC | 2018 | 13.8 ± 0.3 | 3.7 ± 0.1 | 5.88 ± 0.18 |
| Northern Tasmania | TAS | 2018 | 13.3 ± 0.3 | 3.6 ± 0.2 | 5.83 ± 0.51 |
| Southern Tasmania | TAS | 2018 | 13.6 ± 0.2 | 3.8 ± 0.1 | 5.56 ± 0.28 |
| Region | State | Altitude (m a.s.l.) | GDD (days) 1 | GST (°C) 2 | January (°C) 3 | February (°C) 4 | Rainfall (mm) 5 |
|---|---|---|---|---|---|---|---|
| Adelaide Hills | SA | 340–540 | 1923 ± 117 | 19.1 ± 0.6 | 22.3 ± 0.6 | 21.5 ± 0.6 | 236 ± 33 |
| Yarra Valley | VIC | 150–210 | 1766 ± 27 | 18.3 ± 0.2 | 21.4 ± 0.2 | 20.8 ± 0.2 | 453 ± 17 |
| Mornington Peninsula | VIC | 90–240 | 1697 ± 70 | 18.0 ± 0.3 | 20.7 ± 0.4 | 20.2 ± 0.4 | 348 ± 11 |
| Northern Tasmania | TAS | 25–150 | 1303 ± 29 | 16.1 ± 0.1 | 19.0 ± 0.2 | 17.9 ± 0.2 | 318 ± 24 |
| Southern Tasmania | TAS | <50 | 1210 ± 133 | 15.7 ± 0.6 | 18.4 ± 0.6 | 16.4 ± 0.5 | 317 ± 56 |
| Analyte | Odour Threshold (µg L−1) and Descriptor/s | Adelaide Hills | Yarra Valley | Mornington Peninsula | Northern Tasmania | Southern Tasmania | Odour Activity Value (min–max) | p |
|---|---|---|---|---|---|---|---|---|
| Higher Alcohols | ||||||||
| 2-Methylpropanol (mg L−1) | 40 1 (mg L−1) (solvent) | 69 ± 20 | 66 ± 3 | 75 ± 8 | 100 ± 38 | 78 ± 15 | 1.6–2.5 | ns |
| 3-Methylbutanol (mg L−1) | 30 1 (mg L−1) (solvent) | 168 ± 20 | 183 ± 34 | 176 ± 33 | 254 ± 68 | 197 ± 29 | 5.6–8.5 | ns |
| 2-Methylbutanol (mg L−1) | 1.2 2 (mg L−1) (solvent) | 80 ± 7 | 94 ± 17 | 97 ± 12 | 130 ± 40 | 105 ± 16 | 67–108 | ns |
| 2-Phenylethanol (mg L−1) | 10 1 (mg L−1); 14 3 (mg L−1) (floral) | 21 ± 8 | 22 ± 7 | 24 ± 7 | 29 ± 4 | 19 ± 5 | 1.9–2.9; 1.3–2.1 | ns |
| 1-Hexanol | 8000 1 (cut grass) | 1829 ± 595 | 1629 ± 297 | 1622 ± 770 | 1968 ± 779 | 2350 ± 924 | 0.20–0.29 | ns |
| Butanol | 150,000 2 (fusel) | 1789 ± 894 | 1648 ± 259 | 1282 ± 123 | 1302 ± 127 | 2122 ± 1016 | 0.01 | ns |
| Total (mg L−1) | - | 342 ± 56 | 368 ± 61 | 375 ± 61 | 516 ± 151 | 403 ± 67 | - | ns |
| Ethyl Esters | ||||||||
| Ethyl propanoate | 9000 2 (fruity) | 205 ± 40 | 189 ± 56 | 170 ± 18 | 183 ± 18 | 177 ± 35 | 0.02 | ns |
| Ethyl butanoate | 20 1 (acid fruit, apple) | 238 ± 90 | 272 ± 4 | 166 ± 43 | 196 ± 41 | 258 ± 88 | 8.3–12.9 | ns |
| Ethyl hexanoate | 5 1; 14 3 (green apple) | 346 ± 121 | 415 ± 46 | 257 ± 75 | 326 ± 26 | 431 ± 74 | 51–86; 18.3–30.8 | ns |
| Ethyl octanoate | 2 1, 5 3 (sweet, fruity) | 384 ± 114 | 495 ± 27 | 300 ± 117 | 350 ± 16 | 453 ± 83 | 150–247; 60–99 | ns |
| Ethyl decanoate | 200 3 (grape) | 140 ± 61 abc | 218 ± 52 a | 100 ± 38 bc | 89 ± 12 c | 182 ± 35 ab | 0.44–1.09 | <0.05 |
| Ethyl 2-methylpropanoate | 15 1 (sweet, fruity) | 121 ± 18 c | 178 ± 54 bc | 198 ± 28 ab | 255 ± 40 a | 149 ± 30 bc | 8.1–17.0 | <0.01 |
| Ethyl 2-methylbutanoate | 1 1; 18 3 (apple) | 8.4 ± 1.1 b | 14.1 ± 6.3 ab | 14.1 ± 4.4 ab | 18.6 ± 1.0 a | 11.2 ± 0.1 b | 8.4–18.6; 0.47–1.03 | <0.05 |
| Ethyl 3-methylbutanoate | 3 3 (fruity) | 11.8 ± 1.9 | 21.2 ± 9.3 | 20.6 ± 8.3 | 27.7 ± 4.0 | 15.0 ± 0.8 | 3.9–9.2 | ns |
| Total (µg L−1) | - | 1454 ± 447 | 1802 ± 255 | 1226 ± 332 | 1445 ± 158 | 1676 ± 346 | - | ns |
| Acetate Esters | ||||||||
| Ethyl acetate (mg L−1) | 12.2 4 (mg L−1) (fruity) | 98 ± 13 | 102 ± 27 | 102 ± 14 | 88 ± 6 | 91 ± 18 | 7.2–8.3 | ns |
| 2-Methylpropyl acetate | 1600 2 (fruity) | 61 ± 6 | 62 ± 19 | 68 ± 12 | 79 ± 39 | 64 ± 17 | 0.04–0.05 | ns |
| 2-Methylbutyl acetate | 313 5a; 1083 5b (fruity) | 39.5 ± 5.3 | 50 ± 3 | 41.1 ± 5.6 | 58 ± 18 | 47.0 ± 10.8 | 0.13–0.18; 0.04–0.05 | ns |
| 3-Methylbutyl acetate | 300 1 (banana) | 170 ± 27 | 277 ± 67 | 176 ± 42 | 288 ± 127 | 232 ± 92 | 0.57–0.96 | ns |
| Total (mg L−1) | - | 98 ± 13 | 102 ± 27 | 102 ± 14 | 88 ± 6 | 91 ± 18 | - | ns |
| Acids | ||||||||
| 2-Methylbutanoic acid | 2200 2 (cheesy) | 286 ± 11 | 317 ± 120 | 317 ± 58 | 443 ± 104 | 311 ± 45 | 0.13–0.20 | ns |
| 3-Methylbutanoic acid | 33.4 3 (blue cheese) | 386 ± 17 | 448 ± 150 | 433 ± 115 | 643 ± 180 | 436 ± 81 | 11.5–19.2 | ns |
| 2-Methylpropanoic acid | 2300 3 (cheese, rancid) | 1324 ± 184 | 1292 ± 177 | 1439 ± 212 | 2000 ± 573 | 1470 ± 214 | 0.56–0.87 | ns |
| Acetic acid (mg L−1) | 2 1 (mg L−1) (vinegar) | 688 ± 152 | 669 ± 102 | 617 ± 156 | 659 ± 146 | 659 ± 36 | 0.31–0.34 | ns |
| Octanoic acid | 500 3 (cheese) | 1442 ± 517 | 1878 ± 251 | 1085 ± 483 | 1335 ± 58 | 1800 ± 405 | 2.2–3.8 | ns |
| Decanoic acid | 1000 3 (rancid, fat) | 417 ± 147 b | 661 ± 130 a | 355 ± 145 b | 336 ± 22 b | 536 ± 19 ab | 0.34–0.66 | <0.05 |
| Total (mg L−1) | - | 692 ± 153 | 673 ± 103 | 621 ± 157 | 664 ± 147 | 663 ± 37 | - | ns |
| C13-Norisoprenoids | ||||||||
| α-Ionone | NA | 1.30 ± 0.03 | 1.26 ± 0.05 | 1.35 ± 0.03 | 1.30 ± 0.04 | 1.21 ± 0.04 | - | ns |
| β-Ionone | 0.09 3 (violet, floral) | 1.23 ± 0.07 | 1.12 ± 0.06 | 1.27 ± 0.09 | 1.19 ± 0.06 | 0.04 ± 0.16 | 0.45–14 | ns |
| β-Damascenone | 0.05 1 (rose, honey) | 1.40 ± 0.35 | 0.96 ± 0.32 | 0.80 ± 0.21 | 1.41 ± 0.45 | 1.17 ± 0.46 | 16–28 | ns |
| Total (µg L−1) | - | 3.93 ± 0.45 | 3.34 ± 0.43 | 3.42 ± 0.33 | 3.90 ± 0.55 | 2.42 ± 1.08 | ns | |
| Attribute | Adelaide Hills | Yarra Valley | Mornington Peninsula | Northern Tasmania | Southern Tasmania | p |
|---|---|---|---|---|---|---|
| Colour density (AU) | 4.57 ± 0.68 | 4.02 ± 0.68 | 3.70 ± 1.06 | 4.66 ± 1.26 | 4.20 ± 0.20 | ns |
| Hue | 0.86 ± 0.07 a | 0.75 ± 0.02 bc | 0.81 ± 0.02 ab | 0.72 ± 0.06 c | 0.72 ± 0.03 c | <0.01 |
| Chemical age 1 | 0.45 ± 0.02 a | 0.34 ± 0.04 c | 0.41 ± 0.02 ab | 0.36 ± 0.02 bc | 0.33 ± 0.04 c | <0.01 |
| Chemical age 2 | 0.16 ± 0.02 a | 0.11 ± 0.03 b | 0.16 ± 0.02 a | 0.14 ± 0.01 ab | 0.11 ± 0.03 b | <0.05 |
| Total anthocyanin (mg L−1) | 98 ± 13 ab | 114 ± 16 a | 76 ± 16 b | 109 ± 23 ab | 131 ± 23 a | <0.05 |
| Non-bleachable pigment (AU) | 1.12 ± 0.22 | 0.80 ± 0.21 | 0.84 ± 0.29 | 0.99 ± 0.32 | 0.81 ± 0.12 | ns |
| Total pigment (AU) | 6.78 ± 0.95 | 7.1± 0.9 | 5.23 ± 1.26 | 7.11 ± 1.70 | 7.89 ± 0.96 | ns |
| (%) Non-bleachable pigment | 16.4 ± 1.6 a | 11.3 ± 2.8 b | 15.9 ± 2.4 a | 13.8 ± 1.3 ab | 10.6 ± 2.9 b | <0.05 |
| Total phenolics (AU) | 34.1 ± 5.3 | 33.1 ± 3.2 | 39.3 ± 11.2 | 32.9 ± 10.4 | 28.0 ± 4.9 | ns |
| Total tannins (g L−1) | 0.78 ± 0.41 | 0.72 ± 0.17 | 1.22 ± 0.63 | 0.87 ± 0.64 | 0.44 ± 0.30 | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, R.; Pearson, W.; Merry, A.; Solomon, M.; Nicolotti, L.; Westmore, H.; Dambergs, R.; Kerslake, F. Preliminary Study of Australian Pinot Noir Wines by Colour and Volatile Analyses, and the Pivot© Profile Method Using Wine Professionals. Foods 2020, 9, 1142. https://doi.org/10.3390/foods9091142
Longo R, Pearson W, Merry A, Solomon M, Nicolotti L, Westmore H, Dambergs R, Kerslake F. Preliminary Study of Australian Pinot Noir Wines by Colour and Volatile Analyses, and the Pivot© Profile Method Using Wine Professionals. Foods. 2020; 9(9):1142. https://doi.org/10.3390/foods9091142
Chicago/Turabian StyleLongo, Rocco, Wes Pearson, Angela Merry, Mark Solomon, Luca Nicolotti, Hanna Westmore, Robert Dambergs, and Fiona Kerslake. 2020. "Preliminary Study of Australian Pinot Noir Wines by Colour and Volatile Analyses, and the Pivot© Profile Method Using Wine Professionals" Foods 9, no. 9: 1142. https://doi.org/10.3390/foods9091142
APA StyleLongo, R., Pearson, W., Merry, A., Solomon, M., Nicolotti, L., Westmore, H., Dambergs, R., & Kerslake, F. (2020). Preliminary Study of Australian Pinot Noir Wines by Colour and Volatile Analyses, and the Pivot© Profile Method Using Wine Professionals. Foods, 9(9), 1142. https://doi.org/10.3390/foods9091142