Comparison of Three Domestications and Wild-Harvested Plants for Nutraceutical Properties and Sensory Profiles in Five Wild Edible Herbs: Is Domestication Possible?
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth, and Experimental Conditions
2.2. Determination of the Total Phenolic and Flavonoid Content
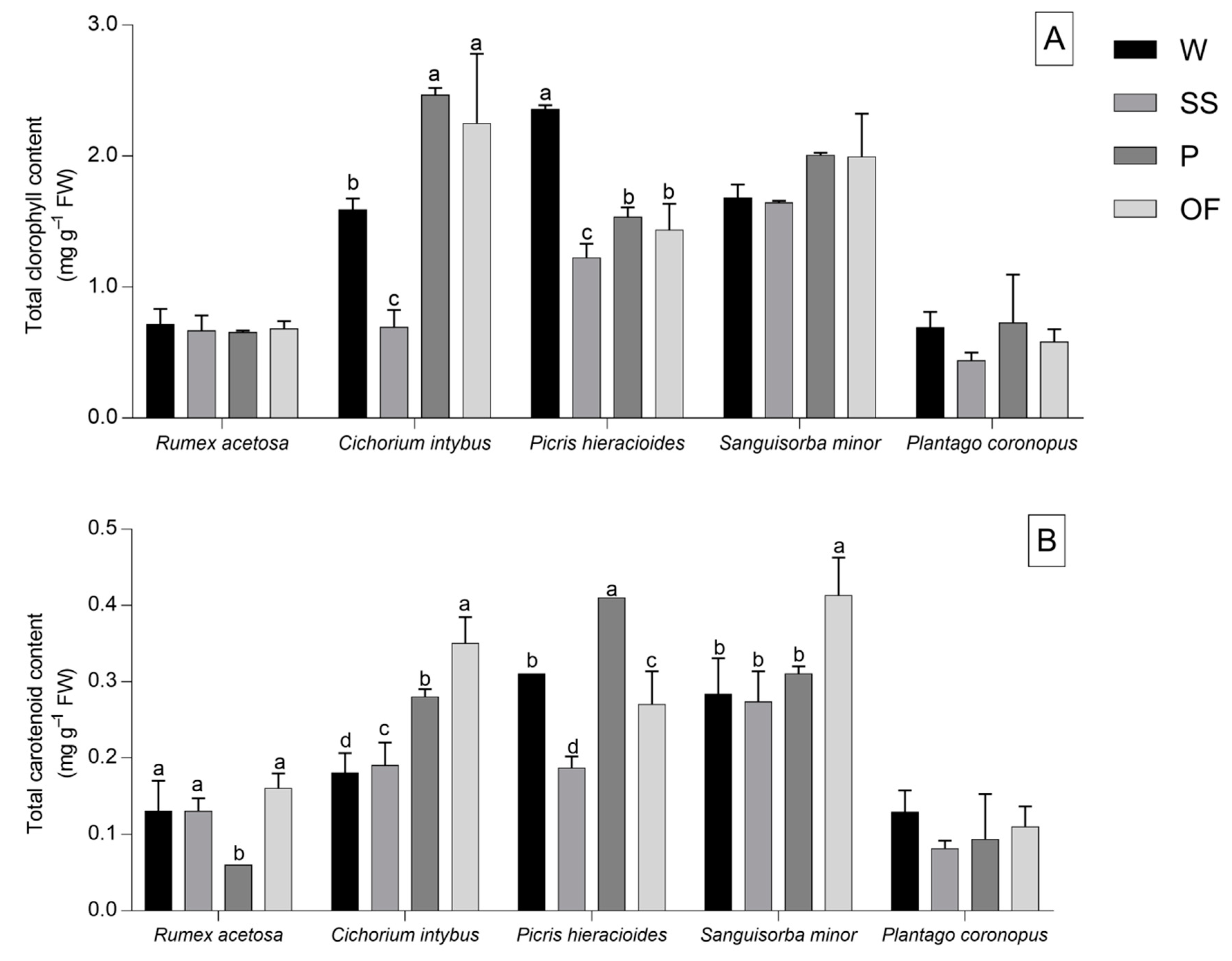
2.3. Chlorophyll and Carotenoid Analysis
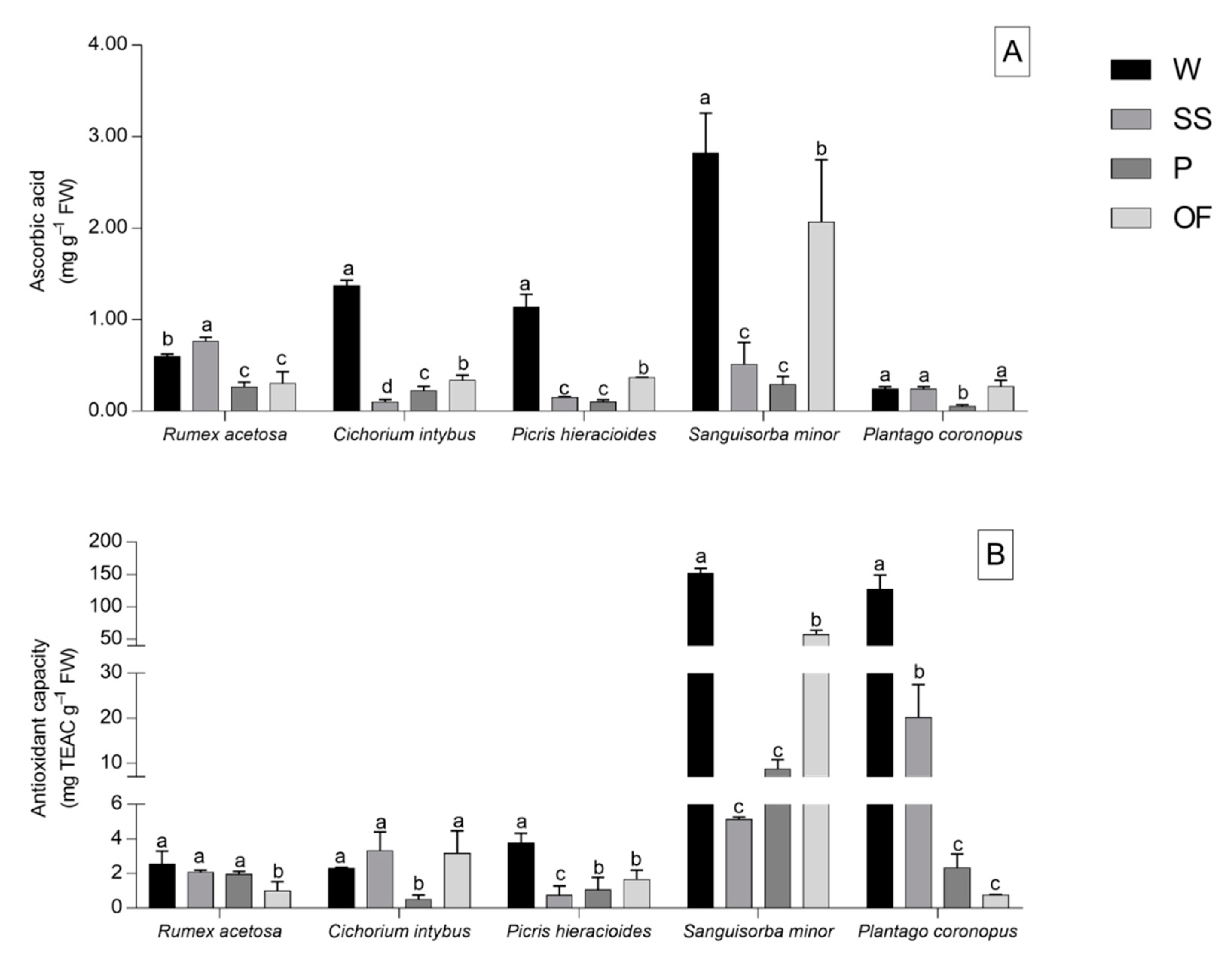
2.4. Ascorbic Acid Assay
2.5. 2,2-Diphenyl-1-picrylhydrazyl Hydrate (DPPH) Free Radical Scavenging Assay
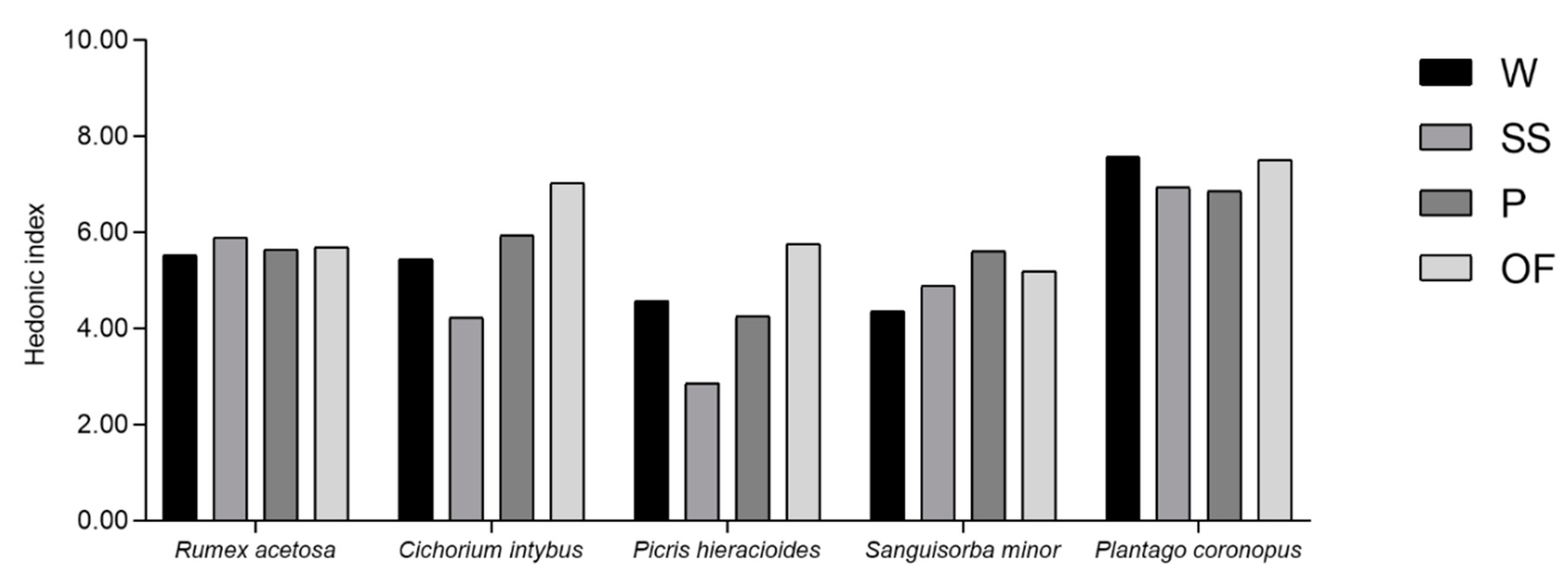
2.6. Sensory Profile
2.7. Statistical Analysis
3. Results
3.1. Total Phenolic (TP) and Total Flavonoid (TF) Content
3.2. Chlorophyll and Carotenoid Content
3.3. Ascorbic Acid and DPPH Free Radical Scavenging
3.4. Sensory Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schmeda-Hirschmann, G.; Jiménez-Aspee, F.; Theoduloz, C.; Ladio, A. Patagonian berries as native food and medicine. J. Ethnopharmacol. 2019, 241, 111979. [Google Scholar] [CrossRef]
- Bokov, D.O.; Luferov, A.N.; Bessonov, V.V. Ethno-pharmacological review on the wild edible medicinal plant, Lilium martagon L. Tropic. J. Pharm. Res. 2009, 18, 1559–1564. [Google Scholar]
- Lockett, C.T.; Calvert, C.C.; Grivetti, L.E. Energy and micronutrient composition of dietary and medicinal wild plants consumed during drought. Study of rural Fulani, Northeastern Nigeria. Int. J. Food Sci. Nutr. 2000, 51, 195–208. [Google Scholar]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Iyda, J.H.; Fernandes, Â.; Calhelha, R.C.; Alves, M.J.; Ferreira, F.D.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Nutritional composition and bioactivity of Umbilicus rupestris (Salisb.) Dandy: An underexploited edible wild plant. Food Chem. 2019, 295, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C. Strawberry-tree, blackthorn and rose fruits: Detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J. Profile of bioactive compounds in the morphological parts of wild Fallopia japonica (Houtt) and Fallopia sachalinensis (F. Schmidt) and their antioxidative activity. Molecules 2019, 24, 1436. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Benabderrahim, M.A.; Guasmi, F.; Elfalleh, W.; Triki, T.; Zammouri, T.; Ferchichi, A. Phenolic profiling, sugar composition and antioxidant capacity of arta (Calligonum comosum L.), a wild Tunisian desert plant. Ind. Crop. Prod. 2019, 130, 436–442. [Google Scholar] [CrossRef]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C.F.R. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef]
- Mikropoulou, E.; Vougogiannopoulou, K.; Kalpoutzakis, E.; Sklirou, A.; Skaperda, Z.; Houriet, J.; Wolfender, J.L.; Trougakos, I.P.; Kouretas, D.; Halabalaki, M.; et al. Phytochemical composition of the decoctions of Greek edible greens (chórta) and evaluation of antioxidant and cytotoxic properties. Molecules 2018, 23, 1541. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Savo, V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: A review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Savo, V.; Giulia, C.; Maria, G.P.; David, R. Folk phytotherapy of the Amalfi coast (Campania, Southern Italy). J. Ethnopharmacol. 2011, 135, 376–392. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Savo, V.; Bonsangue, G.; Gargano, M.L.; Venturella, G.; La Bella, S. Ethnobotanical investigation on wild medicinal plants in the Monti Sicani Regional Park (Sicily, Italy). J. Ethnopharmacol. 2014, 153, 568–586. [Google Scholar] [CrossRef]
- Campisi, J.; Andersen, J.K.; Kapahi, P.; Melov, S. Cellular senescence: A link between cancer and age-related degenerative disease? Semin. Cancer Biol. 2011, 21, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Dueker, S.R.; Clifford, A.J.; Grivetti, L.E. Carotenoid values of selected plant foods common to southern Burkina Faso, West Africa. Ecol. Food Nutr. 1996, 35, 43–58. [Google Scholar] [CrossRef]
- Grauso, L.; Emrick, S.; de Falco, B.; Lanzotti, V.; Bonanomi, G. Common dandelion: A review of its botanical, phytochemical and pharmacological profiles. Phytochem. Rev. 2019, 18, 1115–1132. [Google Scholar] [CrossRef]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional uses, phytochemistry, pharmacology, and toxicology. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Durazzo, A.; Azzini, E.; Lazzè, M.C.; Raguzzini, A.; Pizzala, R.; Maiani, G. Italian wild rocket [Diplotaxis tenuifolia (L.) DC.]: Influence of agricultural practices on antioxidant molecules and on cytotoxicity and antiproliferative effects. Agriculture 2013, 3, 285–298. [Google Scholar] [CrossRef]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean wild edible plants: Weeds or “new functional crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Real, C.; Prohens, J.; Rodriguez-Burruezo, A.; Adalid-Martínez, A.M.; López-Gresa, M.P.; Fita, A. Wild edible fool’s watercress, a potential crop with high nutraceutical properties. PeerJ 2019, 7, e6296. [Google Scholar] [CrossRef] [PubMed]
- Bello, O.M.; Fasinu, P.S.; Bello, O.E.; Ogbesejana, A.B.; Adetunji, C.O.; Dada, A.O.; Ibitoye, O.S.; Aloko, S.; Oguntoye, O.S. Wild vegetable Rumex acetosa Linn.: Its ethnobotany, pharmacology and phytochemistry—A review. S. Afr. J. Bot. 2019, 125, 149–160. [Google Scholar] [CrossRef]
- Tardío, J.; De Sánchez-Mata, M.C.; Morales, R.; Molina, M.; García-Herrera, P.; Fernández-Ruiz, V.; Cámara, M.; Pardo-De-Santayana, M.; Matallana-González, M.C.; Ruiz-Rodríguez, B.M.; et al. Ethnobotanical and food composition monographs of selected Mediterranean wild edible plants. In Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; de Sánchez-Mata, M.C., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; pp. 273–470. ISBN 978-1-4939-3327-3. [Google Scholar]
- Judžentienė, A.; Būdienė, J. Volatile constituents from aerial parts and roots of Cichorium intybus L. (chicory) grown in Lithuania. Chemija 2008, 19, 25–28. [Google Scholar]
- Al-Snafi, A.E. Medical importance of Cichorium intybus–A review. IOSR J. Pharm. 2016, 6, 41–56. [Google Scholar]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Gillbank, L. The weed that was not: Picris hieracioides (Asteraceae) in Australia. Muelleria 2014, 32, 39–51. [Google Scholar]
- Escarré, J.; Lepart, J.; Sans, X.; Sentuc, J.J.; Gorse, V. Effects of herbivory on the growth and reproduction of Picris hieracioides in the Mediterranean region. J. Veg. Sci. 1999, 10, 101–110. [Google Scholar] [CrossRef]
- Nishimura, K.; Miyase, T.; Ueno, A.; Noro, T.; Kuroyanagi, M.; Fukushima, S. Sesquiterpene lactones from Picris hieracioides L. var. japonica Regel. I. Chem. Pharm. Bull. 1986, 34, 2518–2521. [Google Scholar] [CrossRef][Green Version]
- Uchiyama, T.; Nishimura, K.; Miyase, T.; Ueno, A. Terpenoid glycosides from Picris hieracioides. Phytochemistry 1990, 29, 2947–2951. [Google Scholar] [CrossRef]
- Ghirardini, M.P.; Carli, M.; Del Vecchio, N.; Rovati, A.; Cova, O.; Valigi, F.; Agnetti, G.; Macconi, M.; Adamo, D.; Traina, M.; et al. The importance of a taste. A comparative study on wild food plant consumption in twenty-one local communities in Italy. J. Ethnobiol. Ethnomed. 2007, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, C.; Landi, M.; Rocchetti, G.; Miras-Moreno, M.B.; Lucini, L.; Incrocci, L.; Pardossi, A.; Guidi, L. Hydroponically grown Sanguisorba minor Scop.: Effects of cut and storage on fresh-cut produce. Antioxidants 2019, 8, 631. [Google Scholar] [CrossRef] [PubMed]
- Hickman, J.C. (Ed.) The Jepson Manual: Higher Plants of California; University of California Press: Berkeley, CA, USA, 1993; p. 1400. [Google Scholar]
- Karkanis, A.; Vellios, E.; Thomaidis, T.; Bilalis, D.; Efthimiadou, A.; Travlos, I. Phytochemistry and biological properties of burnet weed (Sanguisorba spp.): A review. Not. Sci. Biol. 2014, 6, 395–398. [Google Scholar] [CrossRef]
- Villellas, J.; Ehrlén, J.; Olesen, J.M.; Braza, R.; García, M.B. Plant performance in central and northern peripheral populations of the widespread Plantago coronopus. Ecography 2013, 36, 136–145. [Google Scholar] [CrossRef]
- Koyro, H.W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Jdey, A.; Falleh, H.; Jannet, S.B.; Hammi, K.M.; Dauvergne, X.; Ksouri, R.; Magné, C. Phytochemical investigation and antioxidant, antibacterial and anti-tyrosinase performances of six medicinal halophytes. S. Afr. J. Bot. 2017, 112, 508–514. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Dewanto, V.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta (BBA) Biogenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Zinnai, A. Effect of the baking process on artisanal sourdough bread-making: A technological and sensory evaluation. Agrochimica 2016, 60, 222–234. [Google Scholar]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Ying, X.; Deng, S.; Andrich, G. The influence of packaging on the time evolution of red wine. Agro Food Ind. Hi-Tech 2017, 28, 60–63. [Google Scholar]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed cake as a tool for the improvement of nutraceutical and sensorial features of sourdough bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef]
- Kemp, S.E.; Hort, J.; Hollowood, T. Descriptive Analysis in Sensory Evaluation, 1st ed.; Wiley Balckwell: Hoboken, NJ, USA, 2018. [Google Scholar]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef]
- Gutiérrez-Velázquez, M.V.; Almaraz-Abarca, N.; Herrera-Arrieta, Y.; Ávila-Reyes, J.A.; González-Valdez, L.S.; Torres-Ricario, R.; Uribe-Soto, J.N.; Monreal-García, H.M. Comparison of the phenolic contents and epigenetic and genetic variability of wild and cultivated watercress (Rorippa nasturtium var. aquaticum L.). Electron. J. Biotechnol. 2018, 34, 9–16. [Google Scholar] [CrossRef]
- Mongelli, A.; Rodolfi, M.; Ganino, T.; Marieschi, M.; Caligiani, A.; Dall’Asta, C.; Bruni, R. Are Humulus lupulus L. ecotypes and cultivars suitable for the cultivation of aromatic hop in Italy? A phytochemical approach. Ind. Crop. Prod. 2016, 83, 693–700. [Google Scholar] [CrossRef]
- Kaur, K.; Grewal, S.K.; Gill, P.S.; Singh, S. Comparison of cultivated and wild chickpea genotypes for nutritional quality and antioxidant potential. J. Food Sci. Technol. 2019, 56, 1864–1876. [Google Scholar] [CrossRef]
- Spina, M.; Cuccioloni, M.; Sparapani, L.; Acciarri, S.; Eleuteri, A.M.; Fioretti, E.; Angeletti, M. Comparative evaluation of flavonoid content in assessing quality of wild and cultivated vegetables for human consumption. J. Sci. Food Agric. 2008, 88, 294–304. [Google Scholar] [CrossRef]
- Innocenti, M.; Gallori, S.; Giaccherini, C.; Ieri, F.; Vincieri, F.F.; Mulinacci, N. Evaluation of the phenolic content in the aerial parts of different varieties of Cichorium intybus L. J. Agric. Food Chem. 2005, 53, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Pellegrini, N.; Brenna, O.V.; Del Rio, D.; Frasca, G.; Brighenti, F.; Tumino, R. Antioxidant characterization of some Sicilian edible wild greens. J. Agric. Food Chem. 2005, 53, 9465–9471. [Google Scholar] [CrossRef] [PubMed]
- Heimler, D.; Isolani, L.; Vignolini, P.; Tombelli, S.; Romani, A. Polyphenol content and antioxidative activity in some species of freshly consumed salads. J. Agric. Food Chem. 2007, 55, 1724–1729. [Google Scholar] [CrossRef]
- Abbad, A.; Belaqziz, R.; Bekkouche, K.; Markouk, M. Influence of temperature and water potential on laboratory germination of two Moroccan endemic thymes: Thymus maroccanus Ball. and Thymus broussonetii Boiss. Afr. J. Agric. Res. 2011, 6, 4740–4745. [Google Scholar]
- Davis, D.R. Declining fruit and vegetable nutrient composition: What is the evidence? HortScience 2009, 44, 15–19. [Google Scholar] [CrossRef]
- Di Gioia, F.; Avato, P.; Serio, F.; Argentieri, M.P. Glucosinolate profile of Eruca sativa, Diplotaxis tenuifolia and Diplotaxis erucoides grown in soil and soilless systems. J. Food Compos. Anal. 2018, 69, 197–204. [Google Scholar] [CrossRef]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Antioxidant phytochemicals in lettuce grown in high tunnels and open field. Hortic. Environ. Biotechnol. 2011, 52, 133. [Google Scholar] [CrossRef]
- Tattini, M.; Guidi, L.; Morassi-Bonzi, L.; Pinelli, P.; Remorini, D.; Degl’Innocenti, E.; Giordano, C.; Massai, R.; Agati, G. On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phillyrea latifolia to high solar radiation. New Phytol. 2005, 167, 457–470. [Google Scholar] [CrossRef]
- Lo Piccolo, E.; Landi, M.; Pellegrini, E.; Agati, G.; Giordano, C.; Giordani, T.; Lorenzini, G.; Malorgio, F.; Massai, R.; Nali, C.; et al. Multiple consequences induced by epidermally-located anthocyanins in young, mature and senescent leaves of Prunus. Front. Plant Sci. 2018, 9, 917. [Google Scholar] [CrossRef]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Regulated water deficits improve phytochemical concentration in lettuce. J. Am. Soc. Hortic. Sci. 2010, 135, 223–229. [Google Scholar] [CrossRef]
- Baghalian, K.; Abdoshah, S.; Khalighi-Sigaroodi, F.; Paknejad, F. Physiological and phytochemical response to drought stress of German chamomile (Matricaria recutita L.). Plant Physiol. Biochem. 2011, 49, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Beneragama, C.K.; Goto, K. Chlorophyll a:b ratio increases under low-light in shade-tolerant’ Euglena gracilis. Trop. Agric Res. 2010, 22, 12–25. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Keyhaninejad, N.; Richins, R.D.; O’Connell, M.A. Carotenoid content in field-grown versus greenhouse-grown peppers: Different responses in leaf and fruit. HortScience 2012, 47, 852–855. [Google Scholar] [CrossRef]
- Schonhof, I.; Kläring, H.P.; Krumbein, A.; Clausen, W.; Schreiner, M. Effect of temperature increase under low radiation conditions on phytochemicals and ascorbic acid in greenhouse grown broccoli. Agric. Ecosyst. Environ. 2007, 119, 103–111. [Google Scholar] [CrossRef]
- Okonwu, K.; Mensah, S.; Akonye, L. Phytochemical profile of Telfairia occidentalis leaf grown in soilless and soil media using HPLC. J. Agric. Stud. 2017, 5, 20. [Google Scholar]
- Karkanis, A.C.; Fernandes, Â.; Vaz, J.; Petropoulos, S.; Georgiou, E.; Ciric, A.; Sokovic, M.; Oludemi, T.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of Sanguisorba minor Scop. under Mediterranean growing conditions. Food Funct. 2019, 10, 1340–1351. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004, 88, 503–509. [Google Scholar] [CrossRef]
- Isleroglu, H.; Sakin-Yilmazer, M.; Kemerli-Kalbaran, T.; Ueren, A.; Kaymak-Ertekin, F. Kinetics of colour, chlorophyll, and ascorbic acid content in spinach baked in different types of oven. Int. J. Food Prop. 2017, 20, 2456–2465. [Google Scholar] [CrossRef]
- Llorach, R.; Tomás-Barberán, F.A.; Ferreres, F. Lettuce and chicory by products as a source of antioxidant phenolic extracts. J. Agric. Food Chem. 2004, 52, 5109–5116. [Google Scholar] [CrossRef] [PubMed]
- Žnidarčič, D.; Ban, D.; Šircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011, 129, 1164–1168. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Lefsrud, M.G. Variation in lutein, β-carotene, and chlorophyll concentrations among Brassica oleraceae cultigens and seasons. HortScience 2004, 39, 361–364. [Google Scholar] [CrossRef]
- Jaworska, G.; Kmiecik, W. Effect of the date of harvest on the selected traits of the chemical composition of spinach (Spinacia olearceae L.) and New Zealand spinach (Tetragonia expansa Murr.). Acta Agrar. Silv. 1999, 37, 15–26. [Google Scholar]
- Fallovo, C.; Rouphael, Y.; Cardarelli, M.; Rea, E.; Battistelli, A.; Colla, G. Yield and quality of leafy lettuce in response to nutrient solution composition and growing season. J. Food Agric. Environ. 2009, 7, 456–462. [Google Scholar]
- Tavarini, S.; Degl’Innocenti, E.; Pardossi, A.; Guidi, L. Biochemical aspects in two minimally processed lettuces upon storage. Int. J. Food Sci. Technol. 2007, 42, 214–219. [Google Scholar] [CrossRef]
- Bergquist, S.A.M.; Gertsson, U.E.; Olsson, M.E. Influence of growth stage and postharvest storage on ascorbic acid and carotenoid content and visual quality of baby spinach (Spinacia oleracea L.). J. Sci. Food Agric. 2006, 86, 346–355. [Google Scholar] [CrossRef]


| Quantitative Parameter | p-Value | W | SS | P | OF |
|---|---|---|---|---|---|
| View Attributes | |||||
| Homogeneity of dimensions | ** | 4.9a | 2.8b | 2.4b | 3.6ab |
| Homogeneity of shape | ** | 6.40a | 5.8ab | 4.2b | 4.0b |
| Color intensity | *** | 7.1a | 5.8b | 5.2b | 5.4b |
| Hue (yellow/green) | *** | 7.3a | 6.9a | 4.7b | 5.4b |
| Leaf fuzzy on underside | *** | 1.4a | 0b | 0b | 0b |
| Smell Attributes | |||||
| Odor intensity | *** | 7.5a | 3.6c | 5.6b | 6.8ab |
| Touch/Rheological Sensations | |||||
| Smooth | *** | 5.8b | 8.0a | 7.0a | 8.0a |
| Wrinkled | *** | 3.8a | 2.0ab | 1.6bc | 0c |
| Flabbiness | *** | 2.6a | 1.0ab | 4.0bc | 2.2c |
| Rubbery | *** | 0b | 0.4b | 4.2a | 1.4b |
| Resistance to chewing | ** | 4.0b | 7.8a | 4.2b | 3.0b |
| Taste Attributes/Trigeminal Sensations | |||||
| Acid | ** | 7.8a | 7.2ab | 8.0a | 6.2b |
| Hot | *** | 0b | 1.6a | 0b | 0b |
| Intensity of taste | *** | 8.0a | 8.0a | 7.8a | 4.8b |
| Complexity of taste | ** | 2.1b | 4.6ab | 5.0a | 3.4ab |
| Juiciness | ** | 3.6b | 5.8ab | 6.6a | 6.5a |
| Astringency | * | 2.1ab | 0.4b | 4.2a | 1.4ab |
| Hedonic Parameter | p-Value | W | SS | P | OF |
| Mouthfeel pleasantness | ** | 4.5a | 2.6b | 3.7ab | 2.9ab |
| Quantitative Parameter | p-Value | W | SS | P | OF |
|---|---|---|---|---|---|
| View attributes | |||||
| Homogeneity of dimensions | *** | 5.8a | 3.2b | 3.6b | 2.4b |
| Homogeneity of shape | ** | 5.6ab | 8.0a | 5.0b | 4.0b |
| Color intensity | *** | 6.6c | 9.0a | 7.9b | 8.0b |
| Color regularity | *** | 7.2a | 5.1b | 6.0b | 7.6a |
| Leaf fuzzy on underside | *** | 0.6b | 0b | 3.6a | 0.6b |
| Smell attributes | |||||
| Odor intensity | *** | 7.4a | 7.9a | 2.6c | 5.6b |
| Touch/Rheological sensations | |||||
| Smooth | *** | 7.8a | 8.0a | 3.4b | 8.0a |
| Wrinkled | *** | 0b | 1.0b | 4.8a | 0b |
| Pungent | * | 0b | 0b | 1.2a | 0b |
| Flabbiness | *** | 4.7b | 6.2a | 4.3b | 4.7b |
| Woody | *** | 2.1a | 0b | 0b | 0b |
| Resistance to chewing | *** | 6.6a | 2.4b | 6.6a | 7.2a |
| Taste attributes/Trigeminal sensations | |||||
| Acid | *** | 2.0b | 2.0b | 5.0a | 2.8b |
| Salty | *** | 3.2a | 0b | 4.4a | 3.9a |
| Intensity of taste | ** | 7.4ab | 6.9b | 8.2a | 6.8b |
| Complexity of taste | ** | 1.4b | 3.0ab | 3.4ab | 4.4a |
| Juiciness | *** | 0.8c | 2.3bc | 2.8b | 5.8a |
| Aftertaste | * | 0.4ab | 0b | 2.2a | 1.4ab |
| Hedonic Parameter | p-value | W | SS | P | OF |
| Visual attractiveness | *** | 7.9a | 4.0c | 6.8b | 8.0a |
| Mouthfeel pleasantness | ** | 3.3b | 4.2ab | 4.8a | 5.3a |
| Persistency | ** | 4.4ab | 3.0b | 5.7a | 6.6a |
| Quantitative Parameter | p-Value | W | SS | P | OF |
|---|---|---|---|---|---|
| View attributes | |||||
| Homogeneity of dimensions | *** | 5,2bc | 6.4ab | 3.3c | 7.4a |
| Homogeneity of shape | *** | 5.2b | 8.0a | 6.4ab | 7.4a |
| Color intensity | ** | 8.1a | 6.5b | 6.9b | 7.1ab |
| Color regularity | *** | 8.1a | 4.0c | 4.8bc | 6.6ab |
| Hue (yellow/green) | *** | 8.3a | 7.0b | 7.2b | 7.0b |
| Leaf fuzzy on upper side | *** | 7.8bc | 7.0c | 9.0a | 8.9ab |
| Leaf fuzzy on underside | *** | 7.9b | 6.0c | 9.0a | 8.8a |
| Touch/Rheological sensations | |||||
| Wrinkled | ** | 04ab | 2.0b | 6.2a | 3.8ab |
| Pungent | *** | 8.8a | 7.0b | 9.0a | 9.0a |
| Flabbiness | *** | 0b | 0b | 0b | 4.4a |
| Woody | *** | 2.6b | 0c | 7.6a | 3.8b |
| Resistance to chewing | ** | 5.4b | 8.6a | 5.4b | 6.8ab |
| Taste attributes/Trigeminal sensations | |||||
| Acid | *** | 4.0a | 0.8b | 5.6a | 3.6a |
| Salty | ** | 4.2a | 1.0b | 4.6a | 3.8ab |
| Bitter | *** | 9.0a | 9.0a | 7.0b | 8.8a |
| Hot | * | 0b | 0b | 0.4ab | 1.8a |
| Intensity of taste | *** | 8.4ab | 9.0a | 6.0c | 7.6b |
| Complexity of taste | *** | 1.2b | 0.8b | 4.2a | 4.8a |
| Juiciness | *** | 1.2b | 1.0b | 3.8a | 5.2a |
| Astringency | *** | 0.2b | 0b | 5.0a | 3.4a |
| Hedonic Parameter | p-value | W | SS | P | OF |
| Visual attractiveness | *** | 6.6a | 2.7b | 3.2b | 3.0b |
| Mouthfeel pleasantness | *** | 3.6bc | 1.0c | 3.8b | 6.8a |
| Persistency | *** | 3.3b | 4.2b | 4.9b | 8.5a |
| Quantitative Parameter | p-Value | W | SS | P | OF |
|---|---|---|---|---|---|
| View Attributes | |||||
| Homogeneity of dimensions | *** | 7.3a | 6.4a | 2.6b | 6.8a |
| Color intensity | * | 7.8a | 7.2ab | 7.0ab | 6.5b |
| Smell Attributes | |||||
| Odor intensity | ** | 5.3b | 5.0b | 6.4ab | 7.8a |
| Touch/Rheological Sensations | |||||
| Smooth | *** | 2.4c | 5.4b | 5.6ab | 8.0a |
| Wrinkled | *** | 0.8b | 5.0a | 4.4a | 0b |
| Pungent | *** | 4.4a | 0b | 0b | 0b |
| Woody | *** | 0b | 3.0a | 2.0a | 2.6a |
| Resistance to chewing | *** | 1.6c | 8.0a | 2.4bc | 4.0b |
| Taste Attributes/Trigeminal Sensations | |||||
| Sweet | *** | 0b | 0b | 1.8a | 1.2ab |
| Bitter | *** | 7.2a | 4.8ab | 3.2bc | 2.2c |
| Intensity of taste | * | 5.8a | 5.0ab | 5.2ab | 3.2b |
| Complexity of taste | *** | 2.6b | 8.0a | 6.2a | 2.8b |
| Astringency | ** | 7.0a | 6.0a | 5.0ab | 3.0b |
| Hedonic Parameter | p-Value | W | SS | P | OF |
| Visual attractiveness | ** | 8.1ab | 9.0a | 7.6b | 7.1b |
| Overall pleasantness | * | 3.0ab | 2.6b | 4.5ab | 4.6a |
| Persistency | *** | 2.6c | 4.0b | 5.7a | 3.6bc |
| Quantitative Parameter | p-Value | W | SS | P | OF |
|---|---|---|---|---|---|
| View Attributes | |||||
| Homogeneity of dimensions | *** | 7.2a | 6.0ab | 4.4b | 7.8a |
| Homogeneity of shape | *** | 7.1a | 5.6ab | 3.0b | 4.4b |
| Color intensity | *** | 7.1a | 4.2ab | 5.7b | 6.7a |
| Hue (yellow/green) | *** | 7.4a | 6.0b | 6.2b | 6.6ab |
| Leaf fuzzy on underside | ** | 3.0a | 0.6b | 3.0a | 0.5b |
| Touch/Rheological Sensations | |||||
| Smooth | *** | 0.2c | 7.9a | 5.6b | 7.8a |
| Wrinkled | * | 5.0a | 0.2b | 3.2ab | 1.6ab |
| Pungent | *** | 2.0a | 0b | 0.4b | 0b |
| Woody | *** | 5.8a | 0b | 4.6a | 0b |
| Resistance to chewing | ** | 8.9a | 7.8ab | 5.2b | 6.0b |
| Taste Attributes/Trigeminal Sensations | |||||
| Sweet | *** | 1.0b | 2.6a | 2.0a | 2.6a |
| Acid | ** | 2.8ab | 4.3a | 4,60a | 2.2b |
| Bitter | *** | 2.1a | 0.4b | 3.0a | 0.4b |
| Intensity of taste | ** | 4.2b | 4.0b | 6.2a | 5.9ab |
| Complexity of taste | *** | 3.4b | 4.5b | 7.4a | 5.0ab |
| Juiciness | * | 3.0b | 4.4ab | 6.0ab | 7.0a |
| Hedonic Parameter | p-Value | W | SS | P | OF |
| Mouthfeel pleasantness | ** | 7.4a | 5.1b | 6.2ab | 5.0b |
| Overall pleasantness | *** | 5.2b | 7.9a | 7.3a | 7.6a |
| Persistency | *** | 6.2a | 4.5b | 4.2b | 6.7a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccanti, C.; Landi, M.; Incrocci, L.; Pardossi, A.; Venturi, F.; Taglieri, I.; Ferroni, G.; Guidi, L. Comparison of Three Domestications and Wild-Harvested Plants for Nutraceutical Properties and Sensory Profiles in Five Wild Edible Herbs: Is Domestication Possible? Foods 2020, 9, 1065. https://doi.org/10.3390/foods9081065
Ceccanti C, Landi M, Incrocci L, Pardossi A, Venturi F, Taglieri I, Ferroni G, Guidi L. Comparison of Three Domestications and Wild-Harvested Plants for Nutraceutical Properties and Sensory Profiles in Five Wild Edible Herbs: Is Domestication Possible? Foods. 2020; 9(8):1065. https://doi.org/10.3390/foods9081065
Chicago/Turabian StyleCeccanti, Costanza, Marco Landi, Luca Incrocci, Alberto Pardossi, Francesca Venturi, Isabella Taglieri, Giuseppe Ferroni, and Lucia Guidi. 2020. "Comparison of Three Domestications and Wild-Harvested Plants for Nutraceutical Properties and Sensory Profiles in Five Wild Edible Herbs: Is Domestication Possible?" Foods 9, no. 8: 1065. https://doi.org/10.3390/foods9081065
APA StyleCeccanti, C., Landi, M., Incrocci, L., Pardossi, A., Venturi, F., Taglieri, I., Ferroni, G., & Guidi, L. (2020). Comparison of Three Domestications and Wild-Harvested Plants for Nutraceutical Properties and Sensory Profiles in Five Wild Edible Herbs: Is Domestication Possible? Foods, 9(8), 1065. https://doi.org/10.3390/foods9081065











