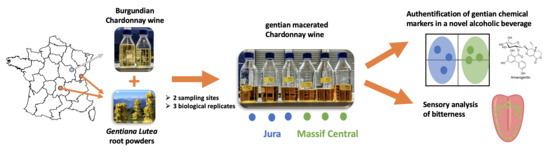
How Chemical and Sensorial Markers Reflect Gentian Geographic Origin in Chardonnay Wine Macerated with Gentiana lutea Roots?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Analytical Methods
2.2.1. Bitter Content
2.2.2. Mineral Composition
2.2.3. Analysis of Volatile Compounds by HS–SPME–GCMS
2.2.4. Gentian Macerated Wines Enological Parameters
2.3. Sensory Analysis
2.4. Statistical Analysis
3. Results
3.1. Chemical Markers of Gentian Root Origin Present in Gentian Root Powders
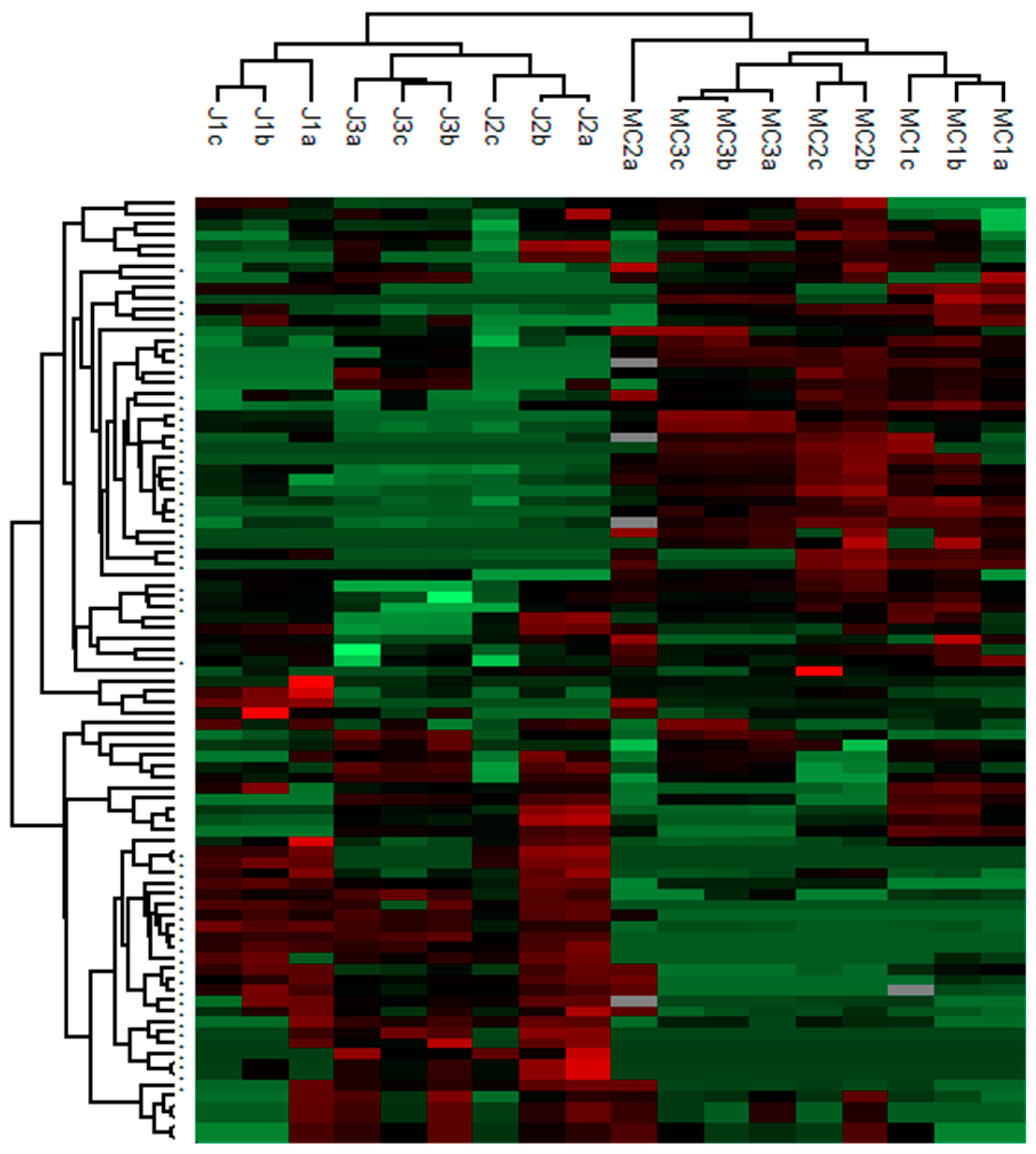
3.2. Chemical Markers of Gentian Root Origin Present in Gentian Macerated Wines
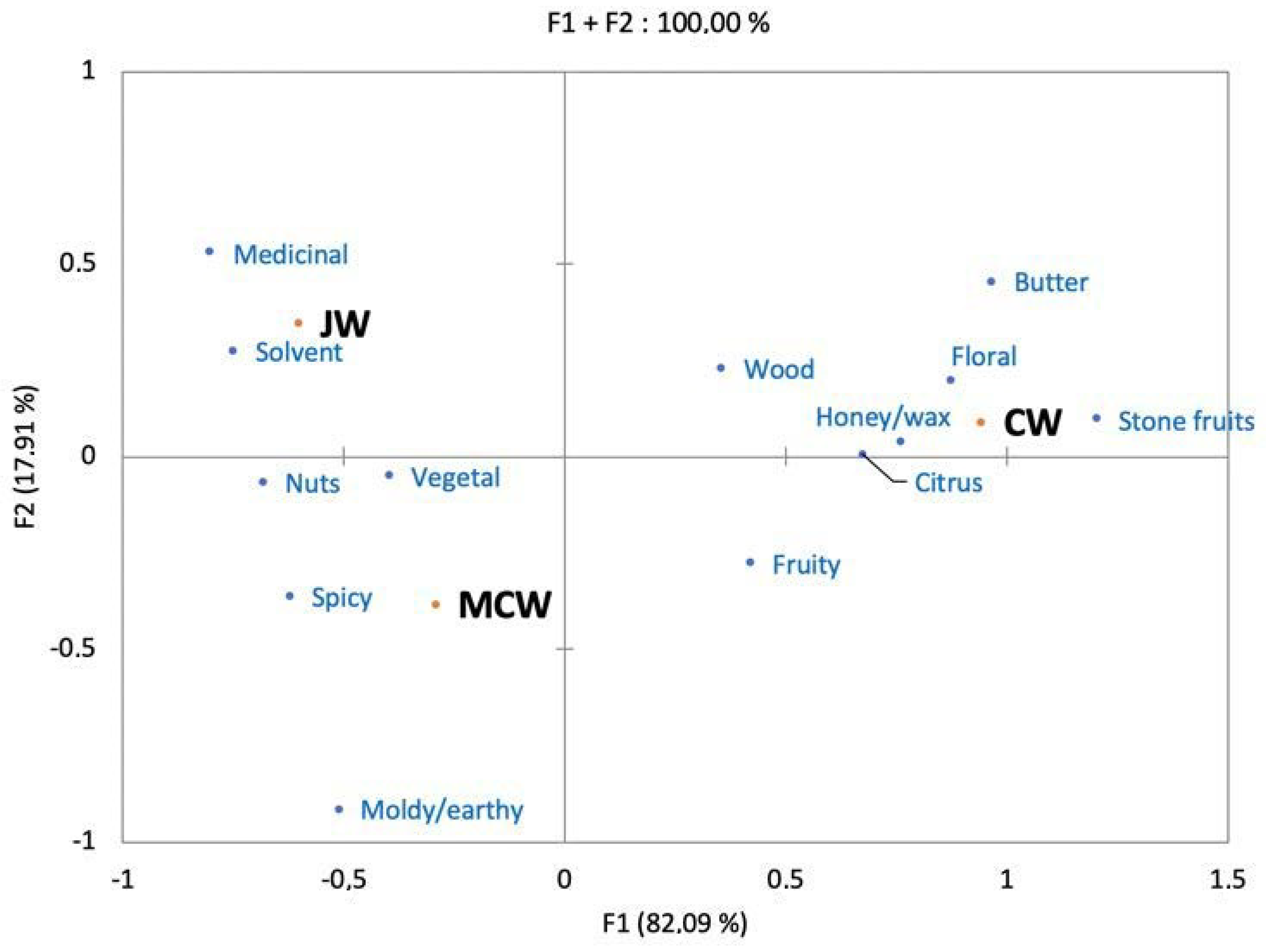
3.3. Sensory Characterization of the Wines
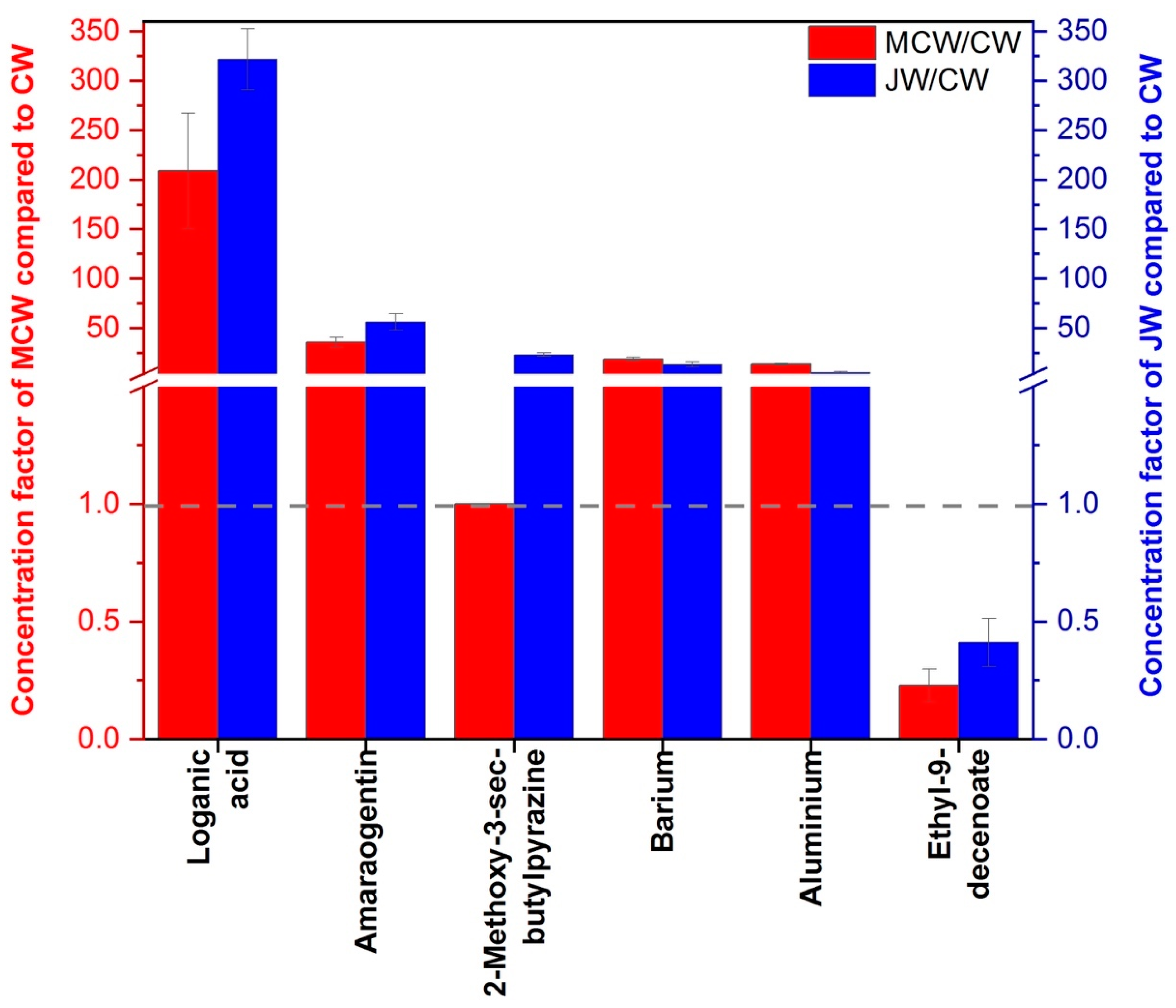
4. Discussion of Chemically Differentiated Compounds between Two Gentian Sampling Sites and Sensory Properties in Macerated Wines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noble, A.C. Bitterness and astringency in wine in Bitterness in Foods and Beverages. In Developments in Food Science; Rousset, R.L., Ed.; Elsevier Science: New York, NY, USA, 1990; pp. 145–158. [Google Scholar]
- Smith, A.K.; June, H.; Noble, A.C. Effects of viscosity on the bitterness and astringency of grape seed tannin. Food Qual. Prefer. 1996, 7, 161–166. [Google Scholar] [CrossRef]
- Glabasnia, A.; Hofmann, T. Sensory-Directed Identification of Taste-Active Ellagitannins in American (Quercus alba L.) and European Oak Wood (Quercus robur L.) and Quantitative Analysis in Bourbon Whiskey and Oak-Matured Red Wines. J. Agric. Food Chem. 2006, 54, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kong, L.; Xue, R.; Wang, W.; Xia, X. Bitterness in alcoholic beverages: The profiles of perception, constituents, and contributors. Trends Food Sci. Technol. 2020, 96, 222–232. [Google Scholar] [CrossRef]
- Bauer, R.; Cowan, D.A.; Crouch, A. Acrolein in Wine: Importance of 3-Hydroxypropionaldehyde and Derivatives in Production and Detection. J. Agric. Food Chem. 2010, 58, 3243–3250. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.C. Why Do Wines Taste Bitter and Feel Astringent? In Chemistry of Wine Flavor; American Chemical Society: Washington, DC, USA, 1998; pp. 156–165. [Google Scholar]
- Gougeon, L.; da Costa, G.; Guyon, F.; Richard, T. 1H NMR metabolomics applied to Bordeaux red wines. Food Chem. 2019, 301, 125257. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Sokolowsky, M.; Fischer, U. Evaluation of bitterness in white wine applying descriptive analysis, time-intensity analysis, and temporal dominance of sensations analysis. Anal. Chim. Acta 2012, 732, 46–52. [Google Scholar] [CrossRef]
- Morata, A.; Vaquero, C.; Palomero, F.; Loira, I.; Bañuelos, M.A.; Suárez-Lepe, J.A. 2-Technology of Vermouth Wines. In Alcoholic Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 35–63. [Google Scholar]
- Mojet, J.; Heidema, J.; Christ-Hazelhof, E. Taste Perception with Age: Generic or Specific Losses in Supra-threshold Intensities of Five Taste Qualities? Chem. Senses 2003, 28, 397–413. [Google Scholar] [CrossRef]
- Kinnamon, S.C. Taste transduction: Linkage between molecular mechanisms and psychophysics. Food Qual. Prefer. 1996, 7, 153–159. [Google Scholar] [CrossRef]
- Behrens, M.; Brockhoff, A.; Batram, C.; Kuhn, C.; Appendino, G.; Meyerhof, W. The Human Bitter Taste Receptor hTAS2R50 Is Activated by the Two Natural Bitter Terpenoids Andrographolide and Amarogentin. J. Agric. Food Chem. 2009, 57, 9860–9866. [Google Scholar] [CrossRef]
- Aberham, A.; Pieri, V.; Croom, E.M.; Ellmerer, E.; Stuppner, H. Analysis of iridoids, secoiridoids and xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC–MS and RP-HPLC. J. Pharm. Biomed. Anal. 2011, 54, 517–525. [Google Scholar] [CrossRef]
- Ando, H.; Hirai, Y.; Fujii, M.; Hori, Y.; Fukumura, M.; Niiho, Y.; Nakajima, Y.; Shibata, T.; Toriizuka, K.; Ida, Y. The chemical constituents of fresh Gentian Root. J. Nat. Med. 2007, 61, 269–279. [Google Scholar] [CrossRef]
- Carnat, A.; Fraisse, D.; Carnat, A.-P.; Felgines, C.; Chaud, D.; Lamaison, J.-L. Influence of drying mode on iridoid bitter constituent levels in gentian root. J. Sci. Food Agric. 2005, 85, 598–602. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Caprioli, G.; Ricciutelli, M.; Maggi, F.; Marín, R.; Vittori, S.; Sagratini, G. Comparative HPLC/ESI-MS and HPLC/DAD study of different populations of cultivated, wild and commercial Gentiana lutea L. Food Chem. 2015, 174, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.D.; Wieser, H. Bitter compounds: Occurrence and structure-activity relationships. Food Rev. Int. 1985, 1, 271–354. [Google Scholar] [CrossRef]
- Gong, T.; Su, X.-T.; Xia, Q.; Wang, J.-G. Gentiana macrophylla Pall (Gentianaceae) extract exerts protective effects against osteoporosis in mice. Trop. J. Pharm. Res. 2018, 17, 429–434. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Hsu, T.-C.; Kuo, W.-W.; Liou, Y.-F.; Lee, S.-D.; Ju, D.-T.; Kuo, C.-H.; Tzang, B.-S. The root extract of Gentiana macrophylla Pall. Alleviates cardiac apoptosis in lupus prone mice. PLoS ONE 2015, 10, e0127440. [Google Scholar] [CrossRef]
- Mirzaee, F.; Hosseini, A.; Jouybari, H.B.; Davoodi, A.; Azadbakht, M. Medicinal, biological and phytochemical properties of Gentiana species. J. Tradit. Complement. Med. 2017, 7, 400–408. [Google Scholar] [CrossRef]
- Niiho, Y.; Yamazaki, T.; Nakajima, Y.; Yamamoto, T.; Ando, H.; Hirai, Y.; Toriizuka, K.; Ida, Y. Gastroprotective effects of bitter principles isolated from Gentian root and Swertia herb on experimentally-induced gastric lesions in rats. J. Nat. Med. 2006, 60, 82–88. [Google Scholar] [CrossRef]
- Park, E.; Kim, J.; Yeo, S.; Kim, G.; Ko, E.-H.; Lee, W.S.; Li, Y.W.; Choi, W.C.; Jeong, S.-Y. Antiadipogenic Effects of Loganic Acid in 3T3-L1 Preadipocytes and Ovariectomized Mice. Molecules 2018, 23, 1663. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.; Young, R.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of Intestinal Bitter Sensing in Enteroendocrine Hormone Secretion and Metabolic Control. Front. Endocrinol. 2018, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Arberas, I.; Leiton, M.J.; Domínguez, J.B.; Bueno, J.M.; Ariño, A.; de Diego, E.; Renobales, G.; de Renobales, M. The volatile flavor of fresh Gentiana lutea L. Roots. In Developments in Food Science; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 207–234. [Google Scholar]
- Mustafa, A.M.; Caprioli, G.; Maggi, F.; Vittori, S.; Sagratini, G. Comparative Analysis of the Volatile Profiles from Wild, Cultivated, and Commercial Roots of Gentiana lutea L. by Headspace Solid Phase Microextraction (HS–SPME) Coupled to Gas Chromatography Mass Spectrometry (GC–MS). Food Anal. Methods 2016, 9, 311–321. [Google Scholar] [CrossRef]
- Cambecedes, J.; Garreta, R.; Gire, L.; Morisson, B.; Garcia, J.; Durand, B. Exploiter et Préserver: Vers un Plan de Gestion Durable de la Gentiane Jaune dans les Pyré. Rapport D’étude. CBNPMP. 2018. Available online: https://drive.google.com/file/d/1-0Jr02g6MQ0wsTbPURD8kM2eSpfWbdYm/view (accessed on 23 July 2020).
- Fabiani, G. Elixirs & Boissons Retrouvés—Collection Carrés Gourmands; Edition Equinoxe: Saint-Rémy-de-Provence, France, 1999. [Google Scholar]
- Zeiner, M.; Cindrić, I.J.; Požgaj, M.; Pirkl, R.; Šilić, T.; Stingeder, G. Influence of soil composition on the major, minor and trace metal content of Velebit biomedical plants. J. Pharm. Biomed. Anal. 2015, 106, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ariño, A.; Arberas, I.; Leiton, M.J.; de Renobales, M.; Dominguez, J.B. The extraction of yellow gentian root (Gentiana lutea L.). Z. Lebensm. Forsch. A 1997, 205, 295–299. [Google Scholar]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Zini, C.A. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Dickinson, E. Mixed biopolymers at interfaces: Competitive adsorption and multilayer structures. Food Hydrocoll. 2011, 25, 1966–1983. [Google Scholar] [CrossRef]
- Dietsch, A.M.; Solomon, N.P.; Steele, C.M.; Pelletier, C.A. The effect of barium on perceptions of taste intensity and palatability. Dysphagia 2014, 29, 96–108. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Day, M.P.; Smith, P.A. Changes in Metal Ion Concentrations in a Chardonnay Wine Related to Oxygen Exposure during Vinification. Molecules 2019, 24, 1523. [Google Scholar] [CrossRef]
- King, E.S.; Osidacz, P.; Curtin, C.; Bastian, S.E.P.; Francis, I.L. Assessing desirable levels of sensory properties in Sauvignon Blanc wines–consumer preferences and contribution of key aroma compounds. Aust. J. Grape Wine Res. 2011, 17, 169–180. [Google Scholar] [CrossRef]
- van Wyngaard, E.; Brand, J.; Jacobson, D.; du Toit, W.J. Sensory interaction between 3-mercaptohexan-1-ol and 2-isobutyl-3-methoxypyrazine in dearomatised Sauvignon Blanc wine. Aust. J. Grape Wine Res. 2014, 20, 178–185. [Google Scholar] [CrossRef]

| Concentration (mg·L−1) | MC | J | p-Value |
|---|---|---|---|
| Gentiopicroside | 724.69 +/− 79.46 | 700.24 +/− 130.25 | 0.795 |
| Amarogentin | 2.05 +/− 1.21 | 2.74 +/− 0.67 | 0.427 |
| Loganic acid | 98.45 +/− 43.89 | 106.13 +/− 31.76 | 0.819 |
| Ca | 1.03 +/− 0.60 | 7.30 +/− 4.21 | 0.003 * |
| Mg | 21.80 +/− 12.59 | 8.00 +/− 4.62 | 0.03 * |
| K | 11.45 +/− 5.22 | 5.51 +/− 0.66 | 0.122 |
| Al | 0.08 +/− 0.02 | 0.05 +/− 0.04 | 0.467 |
| Ba | 0.02 +/− 0.01 | 0.01 +/− 0.01 | 0.205 |
| Sr | 0.0012 +/− 0.0004 | 0.0013 +/− 0.0008 | 0.932 |
| MCW | JW | p−Value | |
|---|---|---|---|
| Enological classical parameters | |||
| Ethanol (%) | 12.05 +/− 0.10 | 12.13 +/− 0.03 | 0.357 |
| Glucose/fructose | 9.0 +/− 0.63 | 7.6 +/− 1.05 | 0.661 |
| Total sugars (g·L−1) | 15.67 +/− 0.73 | 14.3 +/− 1.21 | 0.877 |
| Total acidity (g·L−1 H2SO4) | 3.82 +/− 0.03 | 3.88 +/− 0.09 | 0.572 |
| Volatile acidity (g·L−1 CH3CO2H) | 0.16 +/− 0.02 | 0.15 +/− 0.03 | 0.501 |
| pH | 3.21 +/− 0.01 | 3.17 +/− 0.03 | 0.862 |
| Malic acid (g·L−1) | 0.37 +/− 0.06 | 0.3 +/− 0.06 | 0.518 |
| Density | 0.9955 +/− 0.0003 | 0.9952 +/− 0.0003 | 0.587 |
| Color CIELAB L | 72.85 +/− 1.32 | 71.87 +/− 2.25 | 0.432 |
| a | 18.10 +/− 0.39 | 18.73 +/− 3.32 | 0.191 |
| b | 91.35 +/− 1.69 | 92.52 +/− 4.36 | 0.237 |
| Bitter compounds (mg·L−1) | |||
| Gentiopicroside | 134.42 +/− 97.23 | 447.49 +/− 221.95 | 0.089 |
| Amarogentin | 3.57 +/− 0.50 | 5.63 +/− 0.80 | 0.019 * |
| Loganic acid | 20.87 +/− 5.81 | 32.16 +/− 3.09 | 0.041 * |
| Mineral elements (mg·L−1) | |||
| K | 682.48 +/− 21.80 | 637.05 +/− 8.00 | 0.028 * |
| Mg | 108.33 +/− 5.03 | 102.67 +/− 3.79 | 0.194 |
| Ca | 84.46 +/− 1.03 | 114.39 +/− 7.30 | 0.003 * |
| Al | 6.74 +/− 0.46 | 2.45 +/− 0.74 | 0.001 * |
| Ba | 0.37 +/− 0.04 | 0.27 +/− 0.05 | 0.04 * |
| Sr | 0.41 +/− 0.02 | 0.18 +/− 0.01 | 0.0001 * |
| Sample | Mean for Bitterness | Groups |
|---|---|---|
| JW | 2.57 | A |
| MCW | 2.43 | A |
| CW | 1.00 | B |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biehlmann, M.; Nazaryan, S.; Krauss, E.; Ardeza, M.I.; Flahaut, S.; Figueredo, G.; Ballester, J.; Lafarge, C.; Bou-Maroun, E.; Coelho, C. How Chemical and Sensorial Markers Reflect Gentian Geographic Origin in Chardonnay Wine Macerated with Gentiana lutea Roots? Foods 2020, 9, 1061. https://doi.org/10.3390/foods9081061
Biehlmann M, Nazaryan S, Krauss E, Ardeza MI, Flahaut S, Figueredo G, Ballester J, Lafarge C, Bou-Maroun E, Coelho C. How Chemical and Sensorial Markers Reflect Gentian Geographic Origin in Chardonnay Wine Macerated with Gentiana lutea Roots? Foods. 2020; 9(8):1061. https://doi.org/10.3390/foods9081061
Chicago/Turabian StyleBiehlmann, Manon, Samvel Nazaryan, Emily Krauss, Mike Iron Ardeza, Stéphanie Flahaut, Gilles Figueredo, Jordi Ballester, Céline Lafarge, Elias Bou-Maroun, and Christian Coelho. 2020. "How Chemical and Sensorial Markers Reflect Gentian Geographic Origin in Chardonnay Wine Macerated with Gentiana lutea Roots?" Foods 9, no. 8: 1061. https://doi.org/10.3390/foods9081061
APA StyleBiehlmann, M., Nazaryan, S., Krauss, E., Ardeza, M. I., Flahaut, S., Figueredo, G., Ballester, J., Lafarge, C., Bou-Maroun, E., & Coelho, C. (2020). How Chemical and Sensorial Markers Reflect Gentian Geographic Origin in Chardonnay Wine Macerated with Gentiana lutea Roots? Foods, 9(8), 1061. https://doi.org/10.3390/foods9081061