NMR-Based Metabolomic Comparison of Brassica oleracea (Var. italica): Organic and Conventional Farming
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sample Preparation and Metabolites Extraction
2.3. NMR Spectroscopy
2.4. Statistics
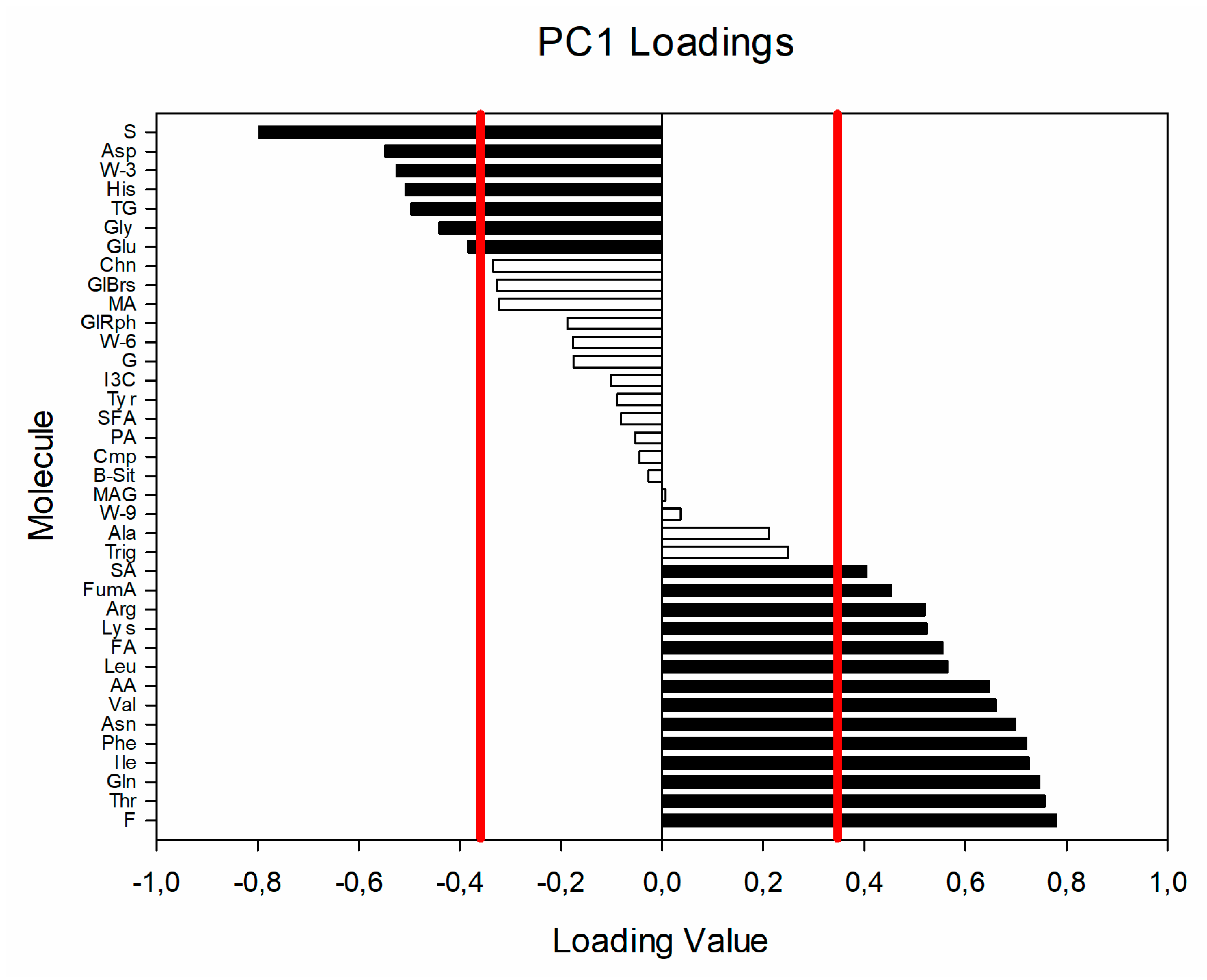
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals: Beyond the Diet Before the Drugs. Curr. Bioact. Compd. 2014, 10, 1–12. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Cicero, N. Development of Food Chemistry, Natural Products, and Nutrition Research: Targeting New Frontiers. Foods 2020, 9, 482. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations, Data from 2014. 2017. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 May 2020).
- Pennington, J.A.T.; Fisher, R.A. Food component profiles for fruit and vegetable subgroups. J. Food Comp. Anal. 2010, 23, 411–418. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Guzman, I.; Yousef, G.G.; Brown, A.F. Simultaneous extraction and quantitation of carotenoids, chlorophylls, and tocopherols in Brassica vegetables. J. Agric. Food Chem. 2012, 60, 7238–7244. [Google Scholar] [CrossRef]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhaeuser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219. [Google Scholar] [CrossRef]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Beck, T.K.; Jensen, S.; Bjoern, G.K.; Kidmose, U. The masking effect of sucrose on perception of bitter compounds in Brassica vegetables. J. Sens. Stud. 2014, 29, 190–200. [Google Scholar] [CrossRef]
- Groenbaek, M.; Jensen, S.; Neugart, S.; Schreiner, M.; Kidmose, U.; Lakkenborg Kristensen, H. Influence of cultivar and fertilizer approach on curly kale (Brassica oleracea L. var. sabellica). Genetic diversity reflected in agronomic characteristics and phytochemical concentration. J. Agric. Food Chem. 2014, 62, 11393–11402. [Google Scholar]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Veeranki, O.; Bhattacharya, A.; Tang, L.; Marshall, J.; Zhang, Y. Cruciferous vegetables, isothiocyanates, and prevention of bladder cancer. Curr. Pharmacol. Rep. 2015, 1, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pujante, P.J.; Borja-Martínez, M.; Pedreño, M.Á.; Almagro, L. Biosynthesis and bioactivity of glucosinolates and their production in plant in vitro cultures. Planta 2017, 246, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients from Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Klopsch, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep. 2017, 7, 40807. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Errico, A.; Petruk, G.; Monti, D.M.; Barone, A.; Rigano, M.M. Bioactive Compounds in Brassicaceae Vegetables with a Role in the Prevention of Chronic Diseases. Molecules 2017, 23, 15. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar]
- Quirante-Moya, S.; García-Ibañez, P.; Quirante-Moya, F.; Villaño, D.; Moreno, D.A. The Role of Brassica Bioactives on Human Health: Are We Studying It the Right Way? Molecules 2020, 25, 1591. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal. 2018, 12, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Kim, J.S. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef]
- Zhang, N.-Q.; Ho, S.C.; Mo, X.-F.; Lin, F.-Y.; Huang, W.-Q.; Luo, H.; Huang, J.; Zhang, C.-X. Glucosinolate and isothiocyanate intakes are inversely associated with breast cancer risk: A case–control study in China. Br. J. Nutr. 2018, 119, 957–964. [Google Scholar] [CrossRef]
- Gründemann, C.; Huber, R. Chemoprevention with isothiocyanates—From bench to bedside. Cancer Lett. 2018, 414, 26–33. [Google Scholar] [CrossRef]
- Traka, M.H.; Melchini, A.; Coode-Bate, J.; Al Kadhi, O.; Saha, S.; Defernez, M.; Troncoso-Rey, P.; Kibblewhite, H.; O’Neill, C.M.; Bernuzzi, F.; et al. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention-results from the Effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1133–1144. [Google Scholar] [CrossRef]
- Burčul, F.; Generalić Mekinić, I.; Radan, M.; Rollin, P.; Blažević, I. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef]
- Oliviero, T.; Verkerk, R.; Dekker, M. Reply to “Dietary glucosinolates and risk of type 2 diabetes in 3 prospective cohort studies”. Am. J. Clin. Nutr. 2018, 108, 425. [Google Scholar] [CrossRef]
- Ma, L.; Liu, G.; Sampson, L.; Willett, W.C.; Hu, F.B.; Sun, Q. Dietary glucosinolates and risk of type 2 diabetes in 3 prospective cohort studies. Am. J. Clin. Nutr. 2018, 107, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Imsic, M.; Franz, P.; Hale, G.; Tomkins, R.B. High nitrogen during growth reduced glucoraphanin and flavonol content in broccoli (Brassica oleracea var. italica) heads. Austr. J. Exp. Agr. 2007, 47, 1498–1505. [Google Scholar] [CrossRef]
- Sciubba, F.; Di Cocco, M.E.; Gianferri, R.; Capuani, G.; De Salvador, F.R.; Fontanari, M.; Gorietti, D.; Delfini, M. Nuclear Magnetic Resonance-Based Metabolic Comparative Analysis of Two Apple Varieties with Different Resistances to Apple Scab Attacks. J. Agric. Food Chem. 2015, 63, 8339–8347. [Google Scholar] [CrossRef]
- Sciubba, F.; Avanzato, D.; Vaccaro, A.; Capuani, G.; Spagnoli, M.; Di Cocco, M.E.; Tzareva, I.N.; Delfini, M. Monitoring of pistachio (Pistacia vera) ripening by high field nuclear magnetic resonance spectroscopy. Nat. Prod. Res. 2017, 31, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, F.; Di Cocco, M.E.; Angori, G.; Spagnoli, M.; De Salvador, F.R.; Engel, P.; Delfini, M. NMR-based metabolic study of leaves of three species of Actinidia with different degrees of susceptibility to Pseudomonas syringae pv actinidiae. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Sciubba, F.; Di Cocco, M.E.; Gianferri, R.; Alise, M.; Santini, A.; Delfini, M.; Lombardi Boccia, G. Stability of Meat protein type I collagen: Influence of pH, ionic strength and phenolic antioxitant. Foods 2020, 4, 480. [Google Scholar] [CrossRef]
- Lucarini, M.; Sciubba, F.; Capitani, D.; Di Cocco, M.E.; D’Evoli, L.; Durazzo, A.; Delfini, M.; Lombardi Boccia, G. Role of catechin on collagen type I stability upon oxidation: A NMR approach. Nat. Prod. Res. 2020, 34, 53–62. [Google Scholar] [CrossRef]
- Miccheli, A.; Ricciolini, R.; Piccolella, E.; Delfini, M.; Conti, F. Modulation of human lymphoblastoid B cell line by phorbol ester and sphingosine. A 31P-NMR study. Biochim. Biophys. Acta. Mol. Cell Res. 1991, 1, 29–35. [Google Scholar] [CrossRef]
- Tomassini, A.; Sciubba, F.; Di Cocco, M.E.; Capuani, G.; Delfini, M.; Aureli, W.; Miccheli, A. 1H NMR-Based Metabolomics Reveals a Pedoclimatic Metabolic Imprinting in Ready-to-Drink Carrot Juices. J. Agric. Food Chem. 2016, 64, 5284–5291. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Joi, K.W. Ammonia, glutamine and asparagine a carbon-nitrogen interface. Can. J. Bot. 1988, 6, 2103–2109. [Google Scholar] [CrossRef]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Yamamoto, S.; Eneji, A.E.; Endo, T.; Honna, T. Urea and manure effect on growth and mineral contents of maize under saline conditions. J. Plant Nutr. 2002, 25, 189–200. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Michalek, S.; Klebel, C.; Treutter, D. Stimulation of phenylpropanoid biosynthesis in apple (Malus domestica borkh.) by abiotic elicitors. Eur. J. Hortic. Sci. 2005, 70, 116–120. [Google Scholar]
- Qudsia, K.; Ishtiaq, H.; Hamid, L.S.; Arshad, J. Antifungal activity of flavonoids isolated from mango (Mangifera indica L.) leaves. Nat. Prod. Res. 2010, 24, 1907–1914. [Google Scholar]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Kanwar, P.; Jha, G. Alterations in plant sugar metabolism: Signatory of pathogen attack. Planta 2019, 249, 305–318. [Google Scholar] [CrossRef]

| Fertilizer Application (t/ha) | ||
|---|---|---|
| Urea | Bovine Manure | |
| Conventional | 0.2 | 15 |
| Organic | 0.0 | 28 |
| Molecule | Amount (mg/g) | Change | ||
|---|---|---|---|---|
| Conventional | Organic | |||
| Free Amino acids | Valine | 0.248 ± 0.046 | 0.172 ± 0.016 ** | ↓ |
| Isoleucine | 0.115 ± 0.023 | 0.074 ± 0.003 ** | ↓ | |
| Leucine | 0.124 ± 0.028 | 0.106 ± 0.008 | ||
| Threonine | 0.153 ± 0.021 | 0.122 ± 0.006 ** | ↓ | |
| Alanine | 0.307 ± 0.055 | 0.307 ± 0.024 | ||
| Glutamate | 1.349 ± 0.399 | 1.794 ± 0.245 | ||
| Glutamine | 0.729 ± 0.135 | 0.521 ± 0.028 ** | ↓ | |
| Aspartate | 0.626 ± 0.197 | 0.843 ± 0.066 * | ↑ | |
| Lysine | 0.314 ± 0.066 | 0.254 ± 0.008 * | ↓ | |
| Arginine | 2.852 ± 0.508 | 2.378 ± 0.113 * | ↓ | |
| Asparagine | 1.151 ± 0.234 | 0.631 ± 0.056 ** | ↓ | |
| Glycine | 0.528 ± 0.147 | 0.702 ± 0.128 ** | ↑ | |
| Tyrosine | 0.076 ± 0.010 | 0.100 ± 0.008 ** | ↑ | |
| Histidine | 0.027 ± 0.007 | 0.056 ± 0.010 ** | ↑ | |
| Phenylalanine | 0.273 ± 0.067 | 0.159 ± 0.017 ** | ↓ | |
| Organic acids | Acetate | 0.042 ± 0.009 | 0.026 ± 0.002 ** | ↓ |
| Malate | 2.552 ± 0.452 | 3.186 ± 0.175 * | ↑ | |
| Pyruvate | 0.490 ± 0.209 | 0.650 ± 0.111 | ||
| Succinate | 0.148 ± 0.085 | 0.101 ± 0.012 | ||
| Fumarate | 0.148 ± 0.069 | 0.089 ± 0.020 | ||
| Formate | 0.005 ± 0.001 | 0.004 ± 0.001 | ||
| Carbohydrates | Glucose | 4.552 ± 1.077 | 5.451 ± 0.293 | |
| Fructose | 1.634 ± 0.449 | 1.041 ± 0.083 ** | ↓ | |
| Sucrose | 2.380 ± 0.752 | 6.946 ± 0.487 ** | ↑ | |
| Lipids and sterols | β-Sitosterol | 0.381 ± 0.067 | 0.352 ± 0.042 | |
| Campesterol | 0.117 ± 0.043 | 0.112 ± 0.018 | ||
| Stearic acid | 1.849 ± 0.794 | 1.733 ± 0.271 | ||
| Oleic acid | 1.024 ± 0.474 | 1.163 ± 0.168 | ||
| Linoleic acid | 0.673 ± 0.156 | 0.691 ± 0.071 | ||
| Linolenic acid | 1.310 ± 0.252 | 1.663 ± 0.147 * | ↑ | |
| Monoacylglycerol | 0.356 ± 0.049 | 0.367 ± 0.029 | ||
| Triacylglygerol | 0.305 ± 0.052 | 0.362 ± 0.028 | ||
| Miscellaneous | Choline | 0.337 ± 0.058 | 0.480 ± 0.053 * | ↑ |
| Glucoraphanin | 0.565 ± 0.087 | 0.708 ± 0.027 ** | ↑ | |
| Glucobrassicin | 0.160 ± 0.052 | 0.449 ± 0.105 ** | ↑ | |
| Trigonelline | 0.026 ± 0.004 | 0.027 ± 0.001 | ||
| Indole-3-carbinol | 0.017 ± 0.007 | 0.017 ± 0.003 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, M.; Di Cocco, M.E.; Raguso, V.; Milanetti, F.; Durazzo, A.; Lombardi-Boccia, G.; Santini, A.; Delfini, M.; Sciubba, F. NMR-Based Metabolomic Comparison of Brassica oleracea (Var. italica): Organic and Conventional Farming. Foods 2020, 9, 945. https://doi.org/10.3390/foods9070945
Lucarini M, Di Cocco ME, Raguso V, Milanetti F, Durazzo A, Lombardi-Boccia G, Santini A, Delfini M, Sciubba F. NMR-Based Metabolomic Comparison of Brassica oleracea (Var. italica): Organic and Conventional Farming. Foods. 2020; 9(7):945. https://doi.org/10.3390/foods9070945
Chicago/Turabian StyleLucarini, Massimo, Maria Enrica Di Cocco, Valeria Raguso, Flavia Milanetti, Alessandra Durazzo, Ginevra Lombardi-Boccia, Antonello Santini, Maurizio Delfini, and Fabio Sciubba. 2020. "NMR-Based Metabolomic Comparison of Brassica oleracea (Var. italica): Organic and Conventional Farming" Foods 9, no. 7: 945. https://doi.org/10.3390/foods9070945
APA StyleLucarini, M., Di Cocco, M. E., Raguso, V., Milanetti, F., Durazzo, A., Lombardi-Boccia, G., Santini, A., Delfini, M., & Sciubba, F. (2020). NMR-Based Metabolomic Comparison of Brassica oleracea (Var. italica): Organic and Conventional Farming. Foods, 9(7), 945. https://doi.org/10.3390/foods9070945