Milk Processing Affects Structure, Bioavailability and Immunogenicity of β-lactoglobulin
Abstract
1. Introduction
2. β-Lactoglobulin Secretion, Structure, and Genetic Variants
2.1. β-Lactoglobulin is a Member of the Lipocalin Family
2.2. β-Lactoglobulin is Post-Translationally Modified and Secreted
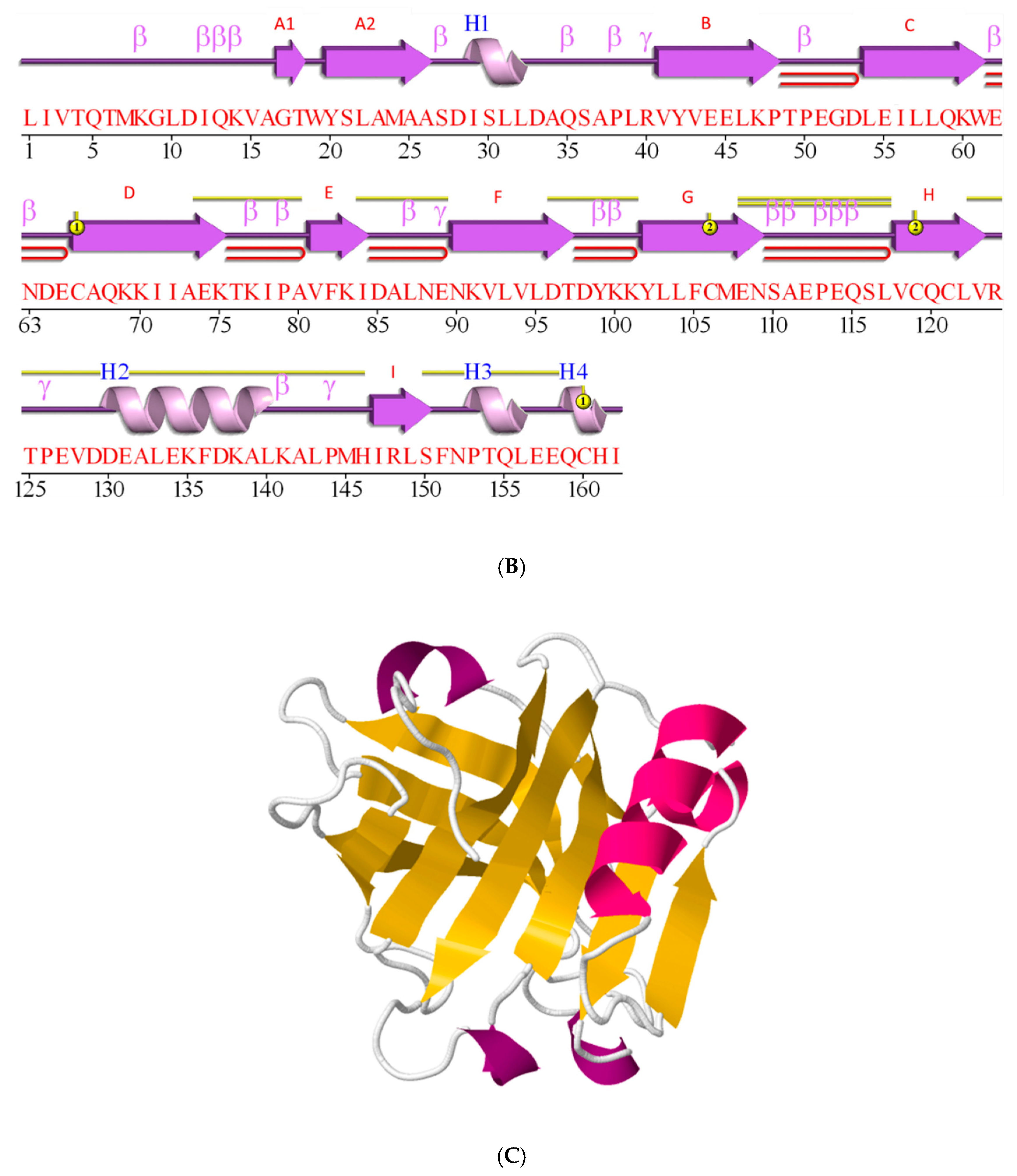
2.3. β-Lactoglobulin Fold is a β-Sheet Rich, Non-Covalently Coupled Homodimer
2.4. β-Lactoglobulin Knows as Many as Ten Genetic Variants
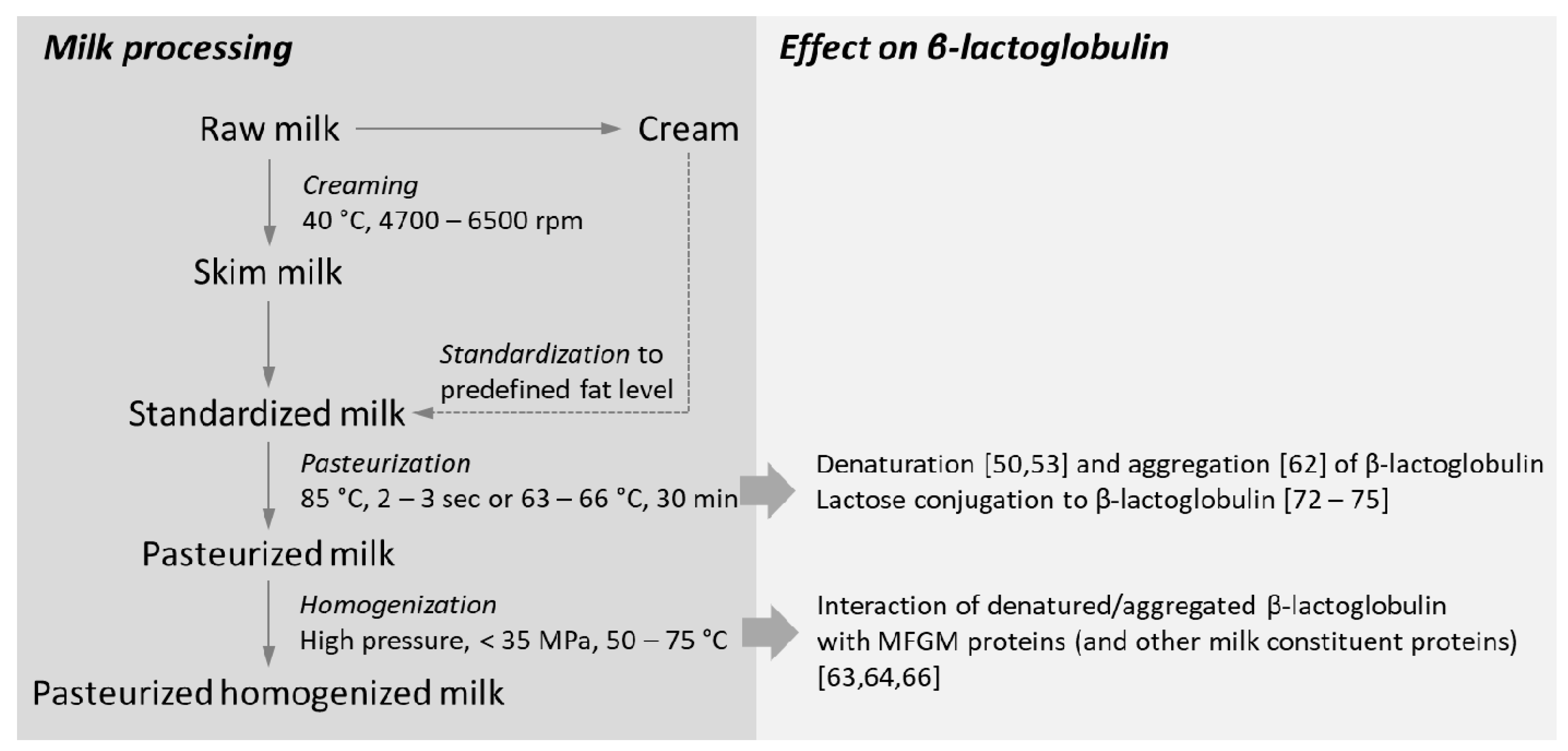
3. Processing of Milk on β-Lactoglobulin Folding and Structure
3.1. Impact of Heat-Processing on β-Lactoglobulin Structure
3.1.1. Denaturation and Molten Globule State
3.1.2. Partially Denatured β-Lactoglobulin is Prone to Aggregation
3.2. Homogenization Induces Disruption of the Milk Fat Globule Membrane
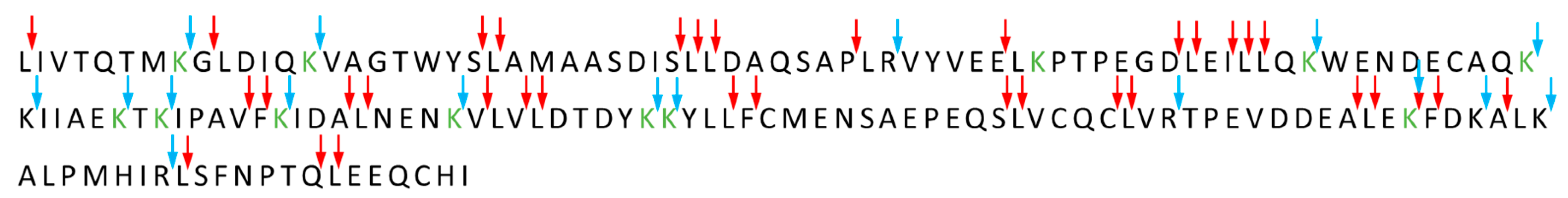
3.3. Amino Acid Modifications
3.3.1. The Maillard Reaction
3.3.2. Heating Affects the Degree of Lactose Conjugation to β-Lactoglobulin
3.3.3. Effects of β-Lactoglobulin Glycation on Structure and Aggregation
4. A Special Type of Fold: β-Sheet Motif Spherical Particles and Amyloids
5. Interaction of β-Lactoglobulin with other Milk Proteins
5.1. Interaction of β-Lactoglobulin with α-Lactalbumin
5.2. Interaction of β-Lactoglobulin with Caseins
5.3. Interaction of β-Lactoglobulin with MFGM Proteins
6. Relation between β-Lactoglobulin Fold and Digestion and the Gastro-Intestinal Immune System
6.1. Protein Digestion and Absorption in the Gastro-Intestinal Tract—An Overview
6.2. Native β-Lactoglobulin is Largely Resistant to Gastric Digestion
6.3. Digestion and Absorption of Denatured and Aggregated Proteins in the Gastrointestinal Tract
6.4. Digestion of Lactosylated β-Lactoglobulin
7. Response of the Gastro-Intestinal Immune System to Heat-Treated β-Lactoglobulin
7.1. Mucus Layer as the First Line of Physical Defense
7.2. Gut-Associated Lymphoid Tissue and Peyer’s Patches
7.3. Uptake of β-Lactoglobulin by Microfold Cells
7.4. Response of Gastrointestinal Lymphocytes to β-Lactoglobulin
7.4.1. Peyer’s Patch Lymphocytes
7.4.2. Lamina Propria-Associated Lymphocytes
7.5. Human Leukocyte Response to β-Lactoglobulin after Absorption
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef]
- Newcomer, M.; Jones, T.; Aqvist, J.; Sundelin, J.; Eriksson, U.; Rask, L.; Peterson, P. The three-dimensional structure of retinol-binding protein. EMBO J. 1984, 3, 1451–1454. [Google Scholar] [CrossRef]
- Papiz, M.Z.; Sawyer, L.; Eliopoulos, E.E.; North, A.C.T.; Findlay, J.B.C.; Sivaprasadarao, R.; Jones, T.A.; Newcomer, M.E.; Kraulis, P.J. The structure of β-lactoglobulin and its similarity to plasma retinol-binding protein. Nature 1986, 324, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Godovac-Zimmermann, J.; Conti, A.; Liberatori, J.; Braunitzer, G. Homology between the primary structures of β-lactoglobulins and human retinol binding protein: Evidence for a similar biological function? Biol. Chem. Hoppe Seyler 1985, 366, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S.; Brew, K. Homology and structure-function correlations between α-1-acid glycoprotein and serum retinol-binding protein and its relatives. FASEB J. 1987, 1, 209–214. [Google Scholar] [CrossRef] [PubMed]
- .Flower, D.R. The lipocalin protein family—Structure and function. Biochem. J. 1996, 318, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bocskei, Z.; Groom, C.R.; Flower, D.R.; Wright, C.E.; Phillips, S.E.V.; Cavaggioni, A.; Findlay, J.B.C.; North, A.C.T. Pheromone binding to two rodent urinary proteins revealed by X-ray crystallography. Nature 1992, 360, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Holden, H.M.; Rypniewski, W.R.; Law, J.H.; Rayment, I. The molecular-structure of insecticyanin from the tobacco hornworm manduca-sexta l at 2.6 Å resolution. EMBO J. 1987, 6, 1565–1570. [Google Scholar] [CrossRef]
- Chaudhuri, B.N.; Kleywegt, G.J.; Bjorkman, J.; Lehman-McKeeman, L.D.; Oliver, J.D.; Jones, A.T. The structures of alpha (2u)-globulin and its complex with a hyaline droplet inducer. Acta Crystallogr. Sect. D Biol. Crystallogr. 1999, 55, 753–762. [Google Scholar] [CrossRef]
- Loch, J.I.; Polit, A.; Bonarek, P.; Olszweska, D.; Kurpiewska, K.; Dziedzicka-Wasylewska, M.; Lewiński, K. Structural and thermodynamic studies of binding saturated fatty acids to bovine β-lactoglobulin. Int. J. Biol. Macromol. 2012, 50, 1095–1102. [Google Scholar] [CrossRef]
- Kontopidis, G.; Holt, C.; Sawyer, L. β-Lactoglobulin: Binding properties, structure, and function. J. Dairy Sci. 2004, 87, 785–796. [Google Scholar] [CrossRef]
- Muresan, S.; van der Bent, A.; de Wolf, F.A. Interaction of β-lactoglobulin with small hydrophobic ligands as monitored by fluorometry and equilibrium dialysis: Nonlinear quenching effects related to protein—Protein association. J. Agric. Food Chem. 2001, 49, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Keppler, J.K.; Sönnichsen, F.D.; Lorenzen, P.C.; Scharz, K. Differences in heat stability and ligand binding among β-lactoglobulin genetic variants A, B and C using 1H NMR and fluorescence quenching. Biochim. Biophys. Acta 2014, 1844, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Magdaleno, G.; Bello, M.; Portillo-Téllez, M.C.; Rodríguez-Romero, A.; García-Hernández, E. Ligand binding and self-association cooperativity of β-lactoglobulin. J. Mol. Recognit. 2013, 26, 67–75. [Google Scholar] [CrossRef]
- Ge, J.; Yue, X.; Wang, S.; Chi, J.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Nanocomplexes composed of chitosan derivatives and β-lactoglobulin as a carrier for anthocyanins: Preparation, stability and bioavailability in vitro. Food Res. Int. 2019, 116, 336–345. [Google Scholar] [CrossRef]
- Świątek, S.; Komorek, P.; Turner, G.; Jachimska, B. β-Lactoglobulin as a potential carrier for bioactive molecules. Bioelectrochemistry 2019, 126, 137–145. [Google Scholar] [CrossRef]
- Bonarek, P.; Loch, J.I.; Tworzydło, M.; Cooper, D.R.; Milto, K.; Wróbel, P.; Kurpiewska, K.; Lewiński, K. Structure-based design approach to rational site-directed mutagenesis of β-lactoglobulin. J. Struct. Biol. 2020, 210, 107493. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Behe, M.J.; Enyeart, J.A. Binding of p-nitrophenyl phosphate and other aromatic compounds by β-lactoglobulin. J. Dairy Sci. 1987, 70, 252–258. [Google Scholar] [CrossRef]
- Gaye, P.; Denamur, R. Preferential synthesis of β-lactoglobulin by the bound polyribosomes of the mammary gland. Biochem. Biophys. Res. Commun. 1970, 41, 266–272. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Mizukami, T.; Sasaki, R.; Chiba, H. Pre-β-lactoglobulin synthesis by mRNA from bovine mammary gland. Agric. Biol. Chem. 1978, 42, 2185–2186. [Google Scholar]
- Bairoch, A.; Apweiler, R. The SWISS-PROT protein sequence database and its supplement TREMBL in 2000. Nucleic Acids Res. 2000, 28, 45–48. [Google Scholar] [CrossRef]
- Ferranti, P.; Mamone, G.; Picariello, G.; Addeo, F. The “dark side” of β-lactoglobulin: Unedited structural features suggest unexpected functions. J. Chrom. A 2011, 1218, 3423–3431. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Eigel, W.N.; Keenan, T.W. Lactose and major milk proteins are present in secretory vesicle-rich fractions from lactating mammary gland. Proc. Natl. Acad. Sci. USA 1978, 75, 5020–5024. [Google Scholar] [CrossRef] [PubMed]
- Mepham, T.B.; Gayer.; Martin, P.; Mercier, J.C. Biosynthesis of milk protein. In Advanced Dairy Chemistry I. Proteins, 2nd ed.; Fox, P.F., Ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1992; pp. 491–543. [Google Scholar]
- Hennighausen, L.; Robinson, G.W.; Wagner, K.U.; Liu, X. Prolactin signaling in mammary gland development. J. Biol. Chem. 1997, 272, 7567–7569. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.H. The preparation of a crystalline globulin from the albumin fraction of cow’s milk. J. Biol. Chem. 1934, 104, 359–372. [Google Scholar]
- Boye, J.I.; Ma, C.Y.; Ismail, A. Thermal stability of beta-lactoglobulins A and B: Effect of SDS, urea, cysteine and N-ethylmaleimide. J. Dairy Res. 2004, 71, 207–215. [Google Scholar] [CrossRef]
- Brownlow, S.; Cabral, J.H.M.; Cooper, R.; Flower, D.R.; Yewdall, S.J.; Polikarpov, I.; North, A.C.T.; Sawyer, L. Bovine beta-lactoglobulin at 1.8 angstrom resolution—Still an enigmatic lipocalin. Structure 1997, 5, 481–495. [Google Scholar] [CrossRef]
- Hoffmann, M.A.M.; Sala, G.; Olieman, C.; de Kruif, K.G. Molecular mass distributions of heat-induced beta-lactoglobulin aggregates. J. Agric. Food Chem. 1997, 45, 2949–2957. [Google Scholar] [CrossRef]
- Qin, B.Y.; Creamer, L.K.; Baker, E.N.; Jameson, G.B. 12-Bromododecanoic acid binds inside the calyx of bovine beta-lactoglobulin. FEBS Lett. 1998, 438, 272–278. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Swaisgood, H.E. Chemistry of milk proteins. In Developments in Dairy Chemistry; Fox, P.F., Ed.; Applied Science Publishers: London, UK, 1982; pp. 1–59. [Google Scholar]
- Aschaffenburg, R.; Drewry, R. Occurrence of different beta-lactoglobulins in cow’s milk. Nature 1955, 176, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Braunitzer, G.; Chen, R.; Schrank, B.; Stangl, A. The sequence of beta-lactoglobulin (Author’s transl). Hoppe Seyler’s Z. Physiol. Chem. 1973, 354, 867–878. [Google Scholar] [CrossRef]
- Gough, P.; Jenness, R. Heat denaturation of β-lactoglobulins A and B. J. Dairy Sci. 1962, 45, 1033–1039. [Google Scholar] [CrossRef]
- Imafidon, G.I.; Ng-Kwai-Hang, K.F.; Harwalkar, V.R.; Ma, C.Y. Effect of genetic polymorphism on the thermal stability of β-lactoglobulin and κ-casein mixture. J. Dairy Sci. 1991, 74, 1791–1802. [Google Scholar] [CrossRef]
- Manderson, G.A.; Hardman, M.J.; Creamer, L.K. Effect of heat treatment on the conformation and aggregation of β-lactoglobulin A, B, and C. J. Agric. Food Chem. 1998, 46, 5052–5061. [Google Scholar] [CrossRef]
- Creamer, L.K.; Nilsson, H.C.; Paulsson, M.A.; Coker, C.J.; Hill, J.P.; Jiménez-Flores, R. Effect of genetic variation on the tryptic hydrolysis of bovine β-lactoglobulin A, B, and C. J. Dairy Sci. 2004, 87, 4023–4032. [Google Scholar] [CrossRef]
- Bell, J.A.; Beck, M.D.; Huebner, R.J. Epidemiologic studies of Q fever in Southern California. J. Am. Med. Assoc. 1950, 142, 868–872. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Chapter 10. Milk and Dairy Products. In Food Chemistry, 4th ed.; Springer: Berlin, Germany, 2009; pp. 517–518. [Google Scholar]
- Mercadante, D.; Melton, L.; Norris, G.; Loo, T.; Williams, M.; Dobson, R.; Jameson, G. Bovine β-lactoglobulin is dimeric under imitative physiological conditions: Dissociation equilibrium and rate constants over the pH range of 2.5–7.5. Biophys. J. 2011, 103, 303–312. [Google Scholar] [CrossRef]
- Crowther, J.M.; Allison, J.R.; Smolenski, G.A.; Hodgkinson, A.J.; Jameson, G.B.; Dobson, R.C.J. The self-association and thermal denaturation of caprine and bovine β-lactoglobulin. Eur. Biophys. J. 2018, 47, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, K.; Era, S.; Hoshino, M.; Forge, V.; Goto, Y.; Batt, C. Solution structure and dynamics of bovine β-lactoglobulin A. Protein Sci. 1999, 8, 2541–2545. [Google Scholar] [CrossRef]
- Uhrínová, S.; Smith, M.H.; Jameson, G.B.; Uhrín, D.; Sawyer, L.; Barlow, P.N. Structural changes accompanying pH-induced dissociation of the beta-lactoglobulin dimer. Biochemistry 2000, 39, 3565–3574. [Google Scholar] [CrossRef]
- Seo, J.A.; Hédoux, A.; Guinet, Y.; Paccou, L.; Affouard, F.; Lerbret, A.; Descamps, M. Thermal denaturation of beta-lactoglobulin and stabilization mechanism by trehalose analyzed from raman spectroscopy investigations. J. Phys. Chem. B 2010, 114, 6675–6684. [Google Scholar] [CrossRef] [PubMed]
- Townend, R.; Herskovits, T.T.; Timasheff, S.N.; Gorbunoff, M.T. The state of amino acid residues in β-lactoglobulin. Arch. Biochem. Biophys. 1969, 129, 567–580. [Google Scholar] [CrossRef]
- Kella, N.K.D.; Kinsella, J.E. Structural stability of β-lactoglobulin in the presence of kosmotropic salts, a kinetic and a thermodynamic study. Int. J. Pept. Protein Res. 1988, 32, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Ptitsyn, O.B. Molten globule and protein folding. Adv. Protein Chem. 1995, 47, 83–229. [Google Scholar] [PubMed]
- .Judy, E.; Kishore, N. A look back at the molten globule state of proteins: Thermodynamic aspects. Biophys. Rev. 2019, 11, 365–375. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saha, S.; Biswas, A.; Kundu, M.; Ghosh, L.; Das, K.P. Structural changes of β-lactoglobulin during thermal unfolding and refolding—An FT-IR and circular dichroism study. Prot. J. 2005, 24, 27–35. [Google Scholar] [CrossRef]
- Kella, N.K.D.; Kinsella, J.E. Enhanced thermostability of β-lactoglobulin at low pH. A possible mechanism. Biochem. J. 1988, 225, 113–118. [Google Scholar] [CrossRef]
- Surewicz, W.K.; Mantsch, H.H.; Chapman, D. Determination of protein secondary structure by fourier transform infrared spectroscopy: A critical assessment. Biochemistry 1993, 32, 389–394. [Google Scholar] [CrossRef]
- De Jongh, H.H.J.; Gröneveld, T.; de Groot, J. Mild isolation procedure discloses new protein structural properties of β-lactoglobulin. J. Dairy Sci. 2001, 84, 562–571. [Google Scholar] [CrossRef]
- Lefèvre, T.; Subirade, M. Structural and interaction properties of β-lactoglobulin as studied by FTIR spectroscopy. Int. J. Food Sci. Technol. 1999, 34, 419–428. [Google Scholar] [CrossRef]
- Qi, X.L.; Holt, C.; McNulty, D.; Clarke, D.T.; Brownlow, S.; Jones, G.R. Effect of temperature on the secondary structure of β-lactoglobulin at pH 6.7, as determined by CD and IR spectroscopy: A test of the molten globule hypothesis. Biochem. J. 1997, 324, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, W.H. Heat denaturation of bovine β-lactoglobulins and relevance of disulfide aggregation. J. Dairy Sci. 1968, 51, 323–329. [Google Scholar] [CrossRef]
- Watanabe, K.; Klostermeyer, H. Heat-induced changes in sulphydryl and disulfide levels of β-lactoglobulin A and the formation of polymers. J. Dairy Res. 1976, 43, 411–418. [Google Scholar] [CrossRef]
- Hillier, R.M.; Lyster, R.L.J.; Cheeseman, G.C. Gelation of reconstituted whey powders by heat. J. Sci. Food Agric. 1980, 31, 1152–1157. [Google Scholar] [CrossRef]
- Shimada, K.; Cheftel, J.C. Sulfhydryl group/disulfide bond interchange reactions during heat-induced gelation of whey protein isolate. J. Agric. Food Chem. 1989, 37, 161–168. [Google Scholar] [CrossRef]
- Liu, T.X.; Relkin, P.; Launay, B. Thermal denaturation of heat-induced gelation properties of β-lactoglobulin. Effects of some chemical parameters. Thermochim. Acta 1994, 246, 387–403. [Google Scholar] [CrossRef]
- McSwiney, M.; Singh, H.; Campanella, O.H. Thermal aggregation and gelation of bovine β-lactoglobulin. Food Hydrocoll. 1994, 8, 441–453. [Google Scholar] [CrossRef]
- McSwiney, M.; Singh, H.; Campanella, O.H.; Creamer, L.K. Thermal gelation and denaturation of bovine β-lactoglobulins A and B. J. Dairy Res. 1994, 61, 221–232. [Google Scholar] [CrossRef]
- Iametti, S.; Cairoli, S.; De Gregori, B.; Bonomi, F. Modification of high order structures upon heating of β-lactoglobulin: Dependence on the protein concentration. J. Agric. Food Chem. 1995, 43, 53–58. [Google Scholar] [CrossRef]
- Roefs, S.P.F.M.; De Kruif, C.G. A model for the denaturation and aggregation of β-lactoglobulin. Eur. J. Biochem. 1994, 226, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Michalski, M.C.; Januel, C. Does homogenization affect the human health properties of cow’s milk? Trends Food Sci. Technol. 2006, 17, 423–437. [Google Scholar] [CrossRef]
- Lee, S.J.; Sherbon, J.W. Chemical changes in bovine milk fat globule membrane caused by heat treatment and homogenization of whole milk. J. Dairy Res. 2002, 69, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.X.; Ren, D.; Xiao, Y.; Tomasula, P.M. Effect of homogenization and pasteurization on the structure and stability of whey protein in milk. J. Dairy Sci. 2015, 98, 2884–2897. [Google Scholar] [CrossRef] [PubMed]
- Tunick, M.H.; Ren, D.X.; Van Hekken, D.L.; Bonnaillie, L.; Paul, M.I.; Kwoczak, R.; Tomasula, P.M. Effect of heat and homogenization on in vitro digestion of milk. J. Dairy Sci. 2016, 99, 4124–4139. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, G.A. Relating food emulsion structure and composition to the way it is processed in the gastrointestinal tract and physiological responses: What are the opportunities? Food Biophys. 2010, 5, 258–283. [Google Scholar] [CrossRef]
- Munch, G.; Schicktanz, D.; Behme, A.; Gerlach, M.; Riederer, P.; Palm, D.; Schinzel, R. Amino acid specificity of glycation and protein-AGE crosslinking reactivities determined with a dipeptide SPOT library. Nat. Biotechnol. 1999, 17, 1006–1010. [Google Scholar] [CrossRef]
- Hodge, J.E. Chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Mossine, V.V.; Glinsky, G.V.; Feather, M.S. The preparation and characterization of some Amadori compounds (1-amino-1-deoxy-D-fructose derivatives) derived from a series of aliphatic ω-amino acids. Carbohydr. Res. 1994, 262, 257–270. [Google Scholar] [CrossRef]
- Röper, H.; Röper, S.; Heyns, K.; Meyer, B.N.M.R. spectroscopy of N-(1-deoxy-D-fructos-1-yl)-L amino acids (“fructose-amino acids”). Carbohydr. Res. 1983, 116, 183–195. [Google Scholar] [CrossRef]
- Fogliano, V.; Monti, S.M.; Visconti, A.; Randazzo, G.; Facchiano, A.M.; Colonna, G.; Ritieni, A. Identification of a β-lactoglobulin lactosylation site. Biochim. Biophys. Acta 1998, 1388, 295–304. [Google Scholar] [CrossRef]
- Meltretter, J.; Wüst, J.; Pischetsrieder, M. Modified peptides as indicators for thermal and nonthermal reactions in processed milk. J. Agric. Food Chem. 2014, 62, 10903–10915. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, A.; Buhler, S.; Faccini, A.; Sforza, S.; Tedeschi, T. Thermally-induced lactosylation of whey proteins: Identification and synthesis of lactosylated β-lactoglobulin epitope. Molecules 2020, 25, 1294. [Google Scholar] [CrossRef]
- Brands, C.M.J.; van Boekel, M.A.J.S. Reactions of monosaccharides during heating of sugar—Casein systems: Building of a reaction network model. J. Agric. Food Chem. 2001, 49, 4667–4675. [Google Scholar] [CrossRef]
- Czerwenka, C.; Maier, I.; Pittner, F.; Lindner, W. Investigation of the lactosylation of whey proteins by liquid chromatography—Mass spectrometry. J. Agric. Food Chem. 2006, 54, 8874–8882. [Google Scholar] [CrossRef]
- Morgan, F.; Bouhallab, S.; Molle, D.; Henry, G.; Maubois, J.L.; Leonil, J. Lactolation of β-lactoglobulin monitored by electrospray ionisation mass spectrometry. Int. Dairy J. 1998, 8, 95–98. [Google Scholar] [CrossRef]
- Fenaille, F.; Morgan, F.; Parisod, V.; Tabet, J.C.; Guy, P.A. Solid-state glycation of β-lactoglobulin by lactose and galactose: Localization of the modified amino acids using mass spectrometric techniques. J. Mass Spectrom. 2004, 39, 16–28. [Google Scholar] [CrossRef]
- Morgan, F.; Molle, D.; Henry, G.; Venien, A.; Leonil, J.; Peltre, G.; Levieux, D.; Maubois, J.L.; Bouhallab, S. Glycation of bovine β-lactoglobulin: Effect on the protein structure. Int. J. Food Sci. Technol. 1999, 34, 429–435. [Google Scholar] [CrossRef]
- Brands, S.M.J.; van Boekel, M.A.J.S. Kinetic modelling of reactions in heated monosaccharide-casein systems. J. Agric. Food Chem. 2002, 50, 6725–6739. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Nagai, H.; Watanabe, Y.; Ochi, K.; Takagi, T. Heat-induced aggregation of recombinant erythropoietin in the intact and deglycosylated states as monitored by gel permeation chromatography combined with a low-angle laser light scattering technique. J. Biochem. 1992, 112, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.J.; Rabinowitz, M.L. Preparation and characterization of a dextran-trypsin conjugate. J. Biol. Chem. 1976, 251, 1081–1087. [Google Scholar] [PubMed]
- Meldgaard, M.; Svendsen, I. Different effects of N-glycosylation on the thermostability of highly homologous bacterial (1,3-1,4)-β-glucanases secreted from yeast. Microbiology 1994, 140, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Eufemi, M.; Turano, C.; Giartosio, A. Influence of the carbohydrate moiety on the stability of glycoproteins. Biochemistry 1996, 35, 7299–7307. [Google Scholar] [CrossRef]
- Broersen, K.; Voragen, A.G.; Hamer, R.J.; De Jongh, H.H. Glycoforms of beta-lactoglobulin with improved thermostability and preserved structural packing. Biotechnol. Bioeng. 2004, 86, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Medrano, A.; Abirached, C.; Panizzolo, L.; Moyna, P.; Anon, M.C. The effect of glycation on foam and structural properties of β-lactoglobulin. Food Chem. 2009, 113, 127–133. [Google Scholar] [CrossRef]
- Mulsow, B.; Jacob, M.; Henle, T. Studies on the impact of glycation on the denaturation of whey proteins. Eur. Food Res. Technol. 2009, 228, 643–649. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, Q. Thermal aggregation properties of whey protein glycated with various saccharides. Food Hydrocol. 2013, 32, 87–96. [Google Scholar] [CrossRef]
- Broersen, K.; Elshof, M.; De Groot, J.; Voragen, A.G.; Hamer, R.J.; De Jongh, H.H. Aggregation of beta-lactoglobulin regulated by glucosylation. J. Agric. Food Chem. 2007, 55, 2431–2437. [Google Scholar] [CrossRef]
- Van Teeffelen, A.M.; Broersen, K.; de Jongh, H.H. Glucosylation of beta-lactoglobulin lowers the heat capacity change of unfolding; a unique way to affect protein thermodynamics. Protein Sci. 2005, 14, 2187–2194. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Xu, D.; Su, G.; Li, B.; Li, C. Heat-induced amyloid-like aggregation of β-lactoglobulin affected by glycation by α-dicarbonyl compounds in a model study. J. Sci. Food Agric. 2020, 100, 607–613. [Google Scholar] [CrossRef]
- Pinto, M.D.; Bouhallab, S.; De Carvalho, A.F.; Henry, G.; Putaux, J.L.; Leonil, J. Glucose slows down the heat-induced aggregation of beta-lactoglobulin at neutral pH. J. Agric. Food Chem. 2012, 60, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, L.; Xu, D.; Sheng, B.; Chen, J.; Li, B.; Zhang, X. Heat-induced amyloid-like aggregation of β-lactoglobulin regulated by glycation: A comparison of five kinds of reducing saccharides. Int. J. Biol. Macromol. 2018, 120, 302–309. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, Q.X. Glycation of whey protein to provide steric hindrance against thermal aggregation. J. Agric. Food Chem. 2012, 60, 9754–9762. [Google Scholar] [CrossRef] [PubMed]
- Dave, A.C.; Loveday, S.M.; Anema, S.G.; Jameson, G.B.; Singh, H. Glycation as a tool to probe the mechanism of β-lactoglobulin nanofibril self-assembly. J. Agric. Food Chem. 2014, 62, 3269–3278. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013, 501, 45–51. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Ann. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Chiti, F.; Taddei, N.; Bucciantini, M.; White, P.; Ramponi, G.; Dobson, C.M. Mutational analysis of the propensity for amyloid formation by a globular protein. EMBO J. 2000, 19, 1441–1449. [Google Scholar] [CrossRef]
- Dobson, C.M. The structural basis of protein folding and its links with human disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Fink, A.L. Conformational constraints for amyloid fibrillation: The importance of being unfolded. Biochim. Biophys. Acta 2004, 1698, 131–153. [Google Scholar] [CrossRef]
- Schokker, E.P.; Singh, H.; Pinder, D.N.; Creamer, L.K. Heat-induced aggregation of β-lactoglobulin AB at pH 2.5 as influenced by ionic strength and protein concentration. Int. Dairy J. 2000, 10, 233–240. [Google Scholar] [CrossRef]
- Elshereef, R.; Budman, H.; Moresoli, C.; Legge, R.L. Fluorescence spectroscopy as a tool for monitoring solubility and aggregation behavior of beta-lactoglobulin after heat treatment. Biotechnol. Bioeng. 2006, 95, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M.; Wang, X.L.; Rao, M.A.; Anema, S.G.; Creamer, L.K.; Singh, H. Tuning the properties of β-lactoglobulin nanofibrils with pH, NaCl and CaCl2. Int. Dairy J. 2010, 20, 571–579. [Google Scholar] [CrossRef]
- Bromley, E.H.C.; Krebs, M.R.H.; Donald, A.M. Mechanisms of structure formation in particulate gels of β-lactoglobulin formed near the isoelectric point. Eur. Phys. J. E 2006, 21, 145–152. [Google Scholar] [CrossRef]
- Langton, M.; Hermansson, A.M. Fine-stranded and particulate gels of β-lactoglobulin and whey protein at varying pH. Food Hydrocoll. 1992, 5, 523–539. [Google Scholar] [CrossRef]
- Krebs, M.R.H.; Devlin, G.L.; Donald, A.M. Amyloid fibril-like structure underlies the aggregate structure across the pH range for β-lactoglobulin. Biophys. J. 2009, 96, 5013–5019. [Google Scholar] [CrossRef]
- Gosal, W.J.; Clark, A.H.; Pudney, D.A.; Ross-Murphy, S.B. Novel amyloid fibrillar networks derived from a globular protein: β-lactoglobulin. Langmuir 2002, 18, 7174–7181. [Google Scholar] [CrossRef]
- Akkermans, C.; Venema, P.; van der Goot, A.J.; Gruppen, H.; Bakx, E.J.; Boom, R.M.; van der Linden, E. Peptides are building blocks of heat-induced fibrillar protein aggregates of beta-lactoglobulin formed at pH 2. Biomacromolecules 2008, 9, 1474–1479. [Google Scholar] [CrossRef]
- Van den Akker, C.C.; Schleeger, M.; Bonn, M.; Koenderink, G.H. Chapter 31: Structural basis for the polymorphism of β-lactoglobulin amyloid-like fibrils. In Bio-Nanoimaging—Protein Misfolding and Aggregation; Uversky, V.N., Luybchenko, Y.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 333–343. [Google Scholar]
- Chen, W.L.; Hwang, M.T.; Liau, C.Y.; Ho, J.C.; Hong, K.C.; Mao, S.J. Beta-lactoglobulin is a thermal marker in processed milk as studied by electrophoresis and circular dichroic spectra. J. Dairy Sci. 2005, 88, 1618–1630. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Berin, M.C.; Arnaboldi, P.; Escalante, C.R.; Dahan, S.; Rauch, J.; Jensen-Jarolim, E.; Mayer, L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy 2008, 63, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.E.; Foegeding, E.A. Interactions of α-lactalbumin and bovine serum albumin with β-lactoglobulin in thermally induced gelation. J. Agric. Food Chem. 1993, 41, 341–346. [Google Scholar] [CrossRef]
- Corredig, M.; Dalgleish, D.G. Effect of temperature and pH on the interactions of whey proteins with casein micelles in skim milk. Food Res. Int. 1996, 29, 49–55. [Google Scholar] [CrossRef]
- Haque, Z.; Kinsella, J.E. Interaction between heated κ-casein and β-lactoglobulin: Predominance of hydrophobic interactions in the initial stage of complex formation. J. Dairy Res. 1988, 55, 67–80. [Google Scholar] [CrossRef]
- Jang, H.D.; Swaisgood, H.E. Disulfide bond formation between thermally denatured β-lactoglobulin and k-casein micelles. J. Dairy Sci. 1990, 74, 900–904. [Google Scholar] [CrossRef]
- Shalabi, S.I.; Wheelock, J.V. The role of α-lactalbumin in the primary phase of chymosin action on heated casein micelles. J. Dairy Res. 1976, 43, 331–335. [Google Scholar] [CrossRef]
- Brew, K.; Grobler, J.A. α-Lactalbumin. In Advanced Dairy Chemistry; Fox, P.F., Ed.; Springer: Boston, MA, USA, 1992; Volume 1, pp. 191–229. [Google Scholar]
- Ramakrishnan, B.; Qasba, P.K. Crystal structure of lactose synthase reveals a large conformational change in its catalytic component, the β1,4-galactosyltransferase-I. J. Mol. Biol. 2001, 310, 205–218. [Google Scholar] [CrossRef]
- Baumy, J.J.; Brule, G. Binding of bivalent-cations to α-lactalbumin and β-lactoglobulin—Effect of pH and ionic strength. Lait 1988, 68, 33–48. [Google Scholar] [CrossRef]
- Relkin, P. Thermal unfolding of β-lactoglobulin, α-lactalbumin and bovine serum albumin. A thermodynamical approach. Crit. Rev. Food Sci. Nutr. 1996, 36, 565–601. [Google Scholar] [CrossRef]
- Jegouic, M.; Grinberg, V.Y.; Guingant, A.; Haertlé, T. Baric oligomerization in α-lactalbumin/β-lactoglobulin mixtures. J. Agric. Food Chem. 1997, 45, 19–22. [Google Scholar] [CrossRef]
- Dickinson, E.; Matsumura, Y. Time-dependent polymerization of β-lactoglobulin through disulfide bonds at the oil-water interface in emulsions. Int. J. Biol. Macromol. 1991, 13, 26–30. [Google Scholar] [CrossRef]
- .Monahan, F.J.; McClements, D.J.; Kinsella, J.E. Polymerization of whey proteins in whey protein-stabilized emulsions. J. Agric. Food Chem. 1993, 41, 1826–1829. [Google Scholar] [CrossRef]
- Bogahawaththa, D.; Chandrapala, J.; Vasiljevic, T. Thermal denaturation of bovine β-lactoglobulin in different protein mixtures in relation to antigenicity. Int. Dairy J. 2019, 19, 89–97. [Google Scholar] [CrossRef]
- Havea, P.; Singh, H.; Creamer, L.K. Characterization of heat-induced aggregates of β-lactoglobulin, α-lactalbumin and bovine serum albumin in a whey protein concentrate environment. J. Dairy Res. 2001, 68, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Schokker, E.P.; Singh, H.; Creamer, L.K. Heat-induced aggregation of β-lactoglobulin A and B with α-lactalbumin. Int. Dairy J. 2000, 10, 843–853. [Google Scholar] [CrossRef]
- Hong, Y.; Creamer, L. Changed protein structures of bovine β-lactoglobulin B and α-lactalbumin as a consequence of heat treatment. Int. Dairy J. 2002, 12, 345–359. [Google Scholar] [CrossRef]
- Bolder, S.G.; Hendrickx, H.; Sagis, L.M.C.; van der Linden, E. Fibril assemblies in aqueous whey protein mixtures. J. Agric. Food Chem. 2006, 54, 4229–4234. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.; Horne, D.S. The hairy casein micelle: Evolution of the concept and its implications for dairy technology. Neth. Milk Dairy J. 1996, 50, 85e111. [Google Scholar]
- Creamer, L.K.; Plowman, J.E.; Liddell, M.J.; Smith, M.H.; Hill, J.P. Micelle stability: Kappa-casein structure and function. J. Dairy Sci. 1998, 81, 3004–3012. [Google Scholar] [CrossRef]
- Zittle, C.A.; Thompson, M.P.; Custer, J.H.; Cerbulis, J. κ-Casein-β-lactoglobulin interaction in solution when heated. J. Dairy Sci. 1962, 45, 807–810. [Google Scholar] [CrossRef]
- Dalgleish, D.G. Denaturation and aggregation of serum proteins and caseins in heated milk. J. Agric. Food Chem. 1990, 38, 1996–1999. [Google Scholar] [CrossRef]
- Law, A.J.R.; Banks, J.M.; Horne, D.S.; Leaver, J.; West, I.G. Denaturation of the whey protein in heated milk and their incorporation into Cheddar cheese. Milchwissenschaft 1994, 49, 63–67. [Google Scholar]
- Noh, B.; Creamer, L.K.; Richardson, T. Thermally induced complex formation in an artificial milk system. J. Agric. Food Chem. 1989, 37, 1395–1400. [Google Scholar] [CrossRef]
- Noh, B.; Richardson, T.; Creamer, L.K. Radiolabelling study of the heat-induced interactions between α-lactalbumin, β-lactoglobulin and κ-casein in milk and buffer solutions. J. Food Sci. 1989, 54, 889–893. [Google Scholar] [CrossRef]
- Sawyer, W.H.; Coulter, S.T.; Jenness, R. Role of sulfhydryl groups in the interaction of κ-casein and β-lactoglobulin. J. Dairy Sci. 1963, 46, 564–565. [Google Scholar] [CrossRef]
- Lowe, E.K.; Anema, S.G.; Bienvenue, A.; Boland, M.J.; Creamer, L.K.; Jiménez-Flores, R. Heat-induced redistribution of disulfide bonds in milk proteins. 2. Disulfide bonding patterns between bovine β-lactoglobulin and κ-casein. J. Agric. Food Chem. 2004, 52, 7669–7680. [Google Scholar] [CrossRef]
- Livney, Y.D.; Dalgleish, D.G. Specificity of disulfide bond formation during thermal aggregation in solutions of β-lactoglobulin B and κ-casein A. J. Agric. Food Chem. 2004, 52, 5527–5532. [Google Scholar] [CrossRef]
- Oldfield, D.J.; Singh, H.; Taylor, M.W. Association of β-lactoglobulin and α-lactalbumin with the casein micelles in skim milk heated in an ultra-high temperature plant. Int. Dairy J. 1998, 8, 765–770. [Google Scholar] [CrossRef]
- Cho, Y.; Singh, H.; Creamer, L.K. Heat-induced interactions of beta-lactoglobulin A and kappa-casein B in a model system. J. Dairy Res. 2003, 70, 61–71. [Google Scholar] [CrossRef]
- Anema, S.G. On heating milk, the dissociation of κ-casein from the casein micelles can precede interactions with the denatured whey proteins. J. Dairy Res. 2008, 75, 415–421. [Google Scholar] [CrossRef]
- McKenzie, G.H.; Norton, R.S.; Sawyer, W.H. Heat-induced interaction of β-lactoglobulin and κ-casein. J. Dairy Res. 1971, 38, 343–351. [Google Scholar] [CrossRef]
- Creamer, L.K.; Bienvenue, A.; Nilsson, H.; Paulsson, M.; van Wanroij, M.; Lowe, E.K.; Anema, S.G.; Boland, M.J.; Jiménez-Flores, R. Heat-induced redistribution of disulfide bonds in milk proteins. 1. Bovine beta-lactoglobulin. J. Agric. Food Chem. 2004, 52, 7660–7668. [Google Scholar] [CrossRef] [PubMed]
- Euber, J.R.; Brunner, J.R. Interaction of κ-casein with immobilized β-lactoglobulin. J. Dairy Sci. 1982, 65, 2384–2387. [Google Scholar] [CrossRef]
- Chevalier, F.; Hirtz, C.; Sommerer, N.; Kelly, A.L. Use of reducing/nonreducing two-dimensional electrophoresis for the study of disulfide-mediated interactions between proteins in raw and heated bovine milk. J. Agric. Food Chem. 2009, 57, 5948–5955. [Google Scholar] [CrossRef]
- Donato, L.; Guyomarc’h, F. Formation and properties of the whey protein/κ-casein complexes in heated skim milk—A review. Dairy Sci. Technol. 2009, 89, 3–29. [Google Scholar] [CrossRef]
- Mather, I.H. A review and proposed nomenclature for major proteins of the milk-fat globule membrane. J. Dairy Sci. 2000, 83, 203–247. [Google Scholar] [CrossRef]
- Fong, B.Y.; Norris, C.S.; MacGibbon, A.K.H. Protein and lipid composition of bovine milk-fat-globule membrane. Int. Dairy J. 2007, 17, 275–288. [Google Scholar] [CrossRef]
- Corredig, M.; Dalgleish, D.G. Isolates from industrial buttermilk: Emulsifying properties of materials derived from the milk fat globule membrane. J. Agric. Food Chem. 1997, 45, 4595–4600. [Google Scholar] [CrossRef]
- Damodaran, S. Amino acids, peptides, and proteins. 217-329. In Fennema’s Food Chemistry, 4th ed.; Damodaran, S., Parkin, K.L., Fennema, O.R., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zhang, S.; Vardhanabhuti, B. Effect of initial protein concentration and pH on in vitro gastric digestion of heated whey proteins. Food Chem. 2014, 145, 473–480. [Google Scholar] [CrossRef]
- Ryle, A.P.; Auffret, C.A. The specificity of some pig and human pepsins toward synthetic peptide substrates. Biochem. J. 1979, 179, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.M.G.; Valente-Mesquita, V.L.; Botelho, M.M.; Sawyer, L.; Ferreira, S.T.; Polikarpov, I. Crystal structures of bovine β-lactoglobulin in the orthorhombic space group C2221—Structural differences between genetic variants A and B and features of the Tanford transition. Eur. J. Biochem. 2001, 268, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; McSweeney, P.L.H. Advanced Dairy Chemistry, 3rd ed.; Kluwer Academic: New York, NY, USA; Plenum Publishers: New York, NY, USA, 2013; Volume 1, Part A. [Google Scholar]
- Bossios, A.; Theodoropoulou, M.; Mondoulet, L.; Rigby, N.M.; Papadopoulos, N.G.; Bernard, H.; Adel-Patient, K.; Wal, J.M.; Mills, C.E.N.; Papageorgiou, P. Effect of simulated gastro-duodenal digestion on the allergenic reactivity of beta-lactoglobulin. Clin. Transl. Allergy 2011, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Sélo, I.; Clément, G.; Bernard, H.; Chatel, J.; Créminon, C.; Peltre, G.; Wal, J. Allergy to bovine beta-lactoglobulin: Specificity of human IgE to tryptic peptides. Clin. Exp. Allergy 1999, 29, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Egger, L.; Ménard, O.; Delgado-Andrade, C.; Alvito, P.; Assunção, R.; Balance, S.; Barberá, R.; Brodkorb, A.; Cattenoz, T.; Clemente, A.; et al. The harmonized INFOGEST in vitro digestion method: From knowledge to action. Food Res. Int. 2016, 88, 217–225. [Google Scholar] [CrossRef]
- Kopf-Bolanz, K.A.; Schwander, F.; Gijs, M.; Vergères, G.; Portmann, R.; Egger, L. Impact of milk processing on the generation of peptides during digestion. Int. Dairy J. 2014, 35, 130–138. [Google Scholar] [CrossRef]
- Fu, T.J.; Abbott, U.R.; Hatzos, C. Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid-a comparative study. J. Agric. Food Chem. 2002, 50, 7154–7160. [Google Scholar] [CrossRef]
- Schmidt, D.G.; Meijer, R.J.; Slangen, C.J.; van Beresteijn, E.C. Raising the pH of the pepsin-catalysed hydrolysis of bovine whey proteins increases the antigenicity of the hydrolysates. Clin. Exp. Allergy 1995, 25, 1007–1017. [Google Scholar] [CrossRef]
- Mandalari, G.; Adel-Patient, K.; Barkholt, V.; Baro, C.; Bennett, L.; Bublin, M.; Gaier, S.; Graser, G.; Ladics, G.S.; Mierzejewska, D.; et al. In vitro digestibility of β-casein and β-lactoglobulin under simulated human gastric and duodenal conditions: A multi-laboratory evaluation. Regul. Toxicol. Pharmacol. 2009, 55, 372–381. [Google Scholar] [CrossRef]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein digestion of different protein sources using the INFOGEST static digestion model. Food Res. Int. 2020, 130, 108996. [Google Scholar] [CrossRef]
- Peram, M.R.; Loveday, S.M.; Ye, A.; Singh, H. In vitro gastric digestion of heat-induced aggregates of β-lactoglobulin. J. Dairy Sci. 2013, 96, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.J.; Mackie, A.R.; Mills, E.N. Phospholipid interactions protect the milk allergen alpha-lactalbumin from proteolysis during in vitro digestion. J. Agric. Food Chem. 2005, 53, 9810–9816. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: Cham, Switzerland, 2015; pp. 73–81. [Google Scholar]
- Egger, L.; Ménard, O.; Baumann, C.; Duerr, D.; Schlegel, P.; Stoll, P.; Vergères, G.; Dupont, D.; Portmann, R. Digestion of milk proteins: Comparing static and dynamic in vitro digestion systems with in vivo data. Food Res. Int. 2019, 118, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Van Lieshout, G.A.A.; Lambers, T.T.; Bragt, M.C.E.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 1–24. [Google Scholar] [CrossRef]
- Chobert, J.M.; Briand, L.; Grinberg, V.; Haertlé, T. Impact of esterification on the folding and the susceptibility to peptic proteolysis of β-lactoglobulin. Biochim. Biophys. Acta 1995, 1248, 170–176. [Google Scholar] [CrossRef]
- Dalgalarrondo, M.; Dufour, E.; Chobert, J.M.; Bertrand-Harb, C.; Haertlé, T. Proteolysis of β-lactoglobulin and β-casein by pepsin in ethanolic media. Int. Dairy J. 1995, 5, 1–14. [Google Scholar] [CrossRef]
- Guo, M.R.; Fox, P.F.; Flynn, A.; Kindstedt, P.S. Susceptibility of β-lactoglobulin and sodium caseinate to proteolysis by pepsin and trypsin. J. Dairy Sci. 1995, 78, 2336–2344. [Google Scholar] [CrossRef]
- Selvaggini, C.; De Gioia, L.; Cantù, L.; Ghibaudi, E.; Diomede, L.; Passerini, F.; Forloni, G.; Bugiani, O.; Tagliavini, F.; Salmona, M. Molecular characteristics of a protease-resistant, amyloidogenic and neurotoxic peptide homologous to residues 106–126 of the prion protein. Biochem. Biophys. Res. Commun. 1993, 194, 1380–1386. [Google Scholar] [CrossRef]
- Simōes, L.S.; Martins, J.T.; Pinheiro, A.C.; Vicente, A.A.; Ramos, O.L. β-lactoglobulin micro- and nanostructures as bioactive compounds vehicle: In vitro studies. Food Res. Int. 2020, 131, 108979. [Google Scholar] [CrossRef]
- Bateman, L.; Ye, A.; Singh, H. In vitro digestion of β-lactoglobulin fibrils formed by heat treatment at low pH. J. Agric. Food Chem. 2010, 58, 9800–9808. [Google Scholar] [CrossRef]
- Bateman, L.; Ye, A.; Singh, H. Re-formation of fibrils from hydrolysates of β-lactoglobulin fibrils during in vitro gastric digestion. J. Agric. Food Chem. 2011, 59, 9605–9611. [Google Scholar] [CrossRef] [PubMed]
- Giamblanco, N.; Janot, J.M.; Gubbiotti, A.; Chinappi, M.; Balme, S. Characterization of food amyloid protein digestion by conical nanopore. Small Methods 2020, 9, 1900703. [Google Scholar] [CrossRef]
- Keil, B. Specificity of Proteolysis; Springer: Berlin, Germany; New York, NY, USA, 1992; p. 335. [Google Scholar]
- Moughan, P.J.; Rutherfurd, S.M. A new method for determining digestible reactive lysine in foods. J. Agric. Food Chem. 1996, 44, 2202–2209. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Moughan, P.J. Application of a new method for determining digestible reactive lysine to variably heated protein sources. J. Agric. Food Chem. 1997, 45, 1582–1586. [Google Scholar] [CrossRef]
- Rérat, A.; Calmes, R.; Vaissade, P.; Finot, P.A. Nutritional and metabolic consequences of the early Maillard reaction of heat treated milk in the pig. Eur. J. Nutr. 2002, 41, 1–11. [Google Scholar] [CrossRef]
- Desrosiers, T.; Savoie, L.; Bergeron, G.; Parent, G. Estimation of lysine damage in heated whey proteins by furosine determinations in conjunction with the digestion cell technique. J. Agric. Food Chem. 1989, 37, 1385–1391. [Google Scholar] [CrossRef]
- Sanz, M.L.; Corzo-Martínez, M.; Rastall, R.A.; Olano, A.; Moreno, F.J. Characterization and in vitro digestibility of bovine β-lactoglobulin glycated with galactooligosaccharides. J. Agric. Food Chem. 2007, 55, 7916–7925. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Soria, A.C.; Belloque, J.; Villamiel, M.; Moreno, F.J. Effect of glycation on the gastrointestinal digestibility and immunoreactivity of bovine β-lactoglobulin. Int. Dairy J. 2010, 20, 742–752. [Google Scholar] [CrossRef]
- Pinto, M.S.; Léonil, J.; Henry, G.; Cauty, C.; Carvalho, A.F.; Bouhallab, S. Heating and glycation of β-lactoglobulin and β-casein: Aggregation and in vitro digestion. Food Res. Int. 2014, 55, 70–76. [Google Scholar] [CrossRef]
- Zenker, H.E.; van Lieshout, G.A.A.; van Gool, M.P.; Bragt, M.C.E.; Hettinga, K.A. Lysine blockage of milk proteins in infant formula impairs overall protein digestibility and peptide release. Food Funct. 2020, 11, 358–369. [Google Scholar] [CrossRef]
- Çelebioğlu, H.Y.; Lee, S.; Chronakis, I.S. Interactions of salivary mucins and saliva with food proteins: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Marczynski, M.; Rickert, C.A.; Semerdzhiev, S.A.; Van Dijk, W.R.; Segers-Nolten, I.M.J.; Claessens, M.M.A.E.; Lieleg, O. α-Synuclein penetrates mucin hydrogels despite its mucoadhesive properties. Biomacromolecules 2019, 20, 4332–4344. [Google Scholar] [CrossRef] [PubMed]
- Round, A.N.; Rigby, N.M.; Garcia de la Torre, A.; Macierzanka, A.; Mills, E.N.C.; Mackie, A.R. Lamellar structures of MUC2-rich mucin: A potential role in governing the barrier and lubricating functions of intestinal mucus. Biomacromolecules 2012, 13, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Bajka, B.H.; Rigby, N.M.; Cross, K.L.; Macierzanka, A.; Mackie, A.R. The influence of small intestinal mucus structure on particle transport ex vivo. Colloids Surf. B Biointerfaces 2015, 135, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Golovkina, T.V.; Shlomchik, M.; Hannum, L.; Chervonsky, A. Organogenic role of B lymphocytes in mucosal immunity. Science 1999, 286, 1965–1968. [Google Scholar] [CrossRef]
- Chabot, S.; Wagner, J.S.; Farrant, S.; Neutra, M.R. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J. Immunol. 2006, 176, 4275–4283. [Google Scholar] [CrossRef]
- Rios, D.; Woodl, M.B.; Li, J.; Chassaing, B.; Gewirtz, A.T.; Williams, I.R. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol. 2016, 9, 907–916. [Google Scholar] [CrossRef]
- Santos, L.M.; al-Sabbagh, A.; Londono, A.; Weiner, H.L. Oral tolerance to myelin basic protein induces regulatory TGF-β-secreting T cells in Peyer’s patches of SJL mice. Cell. Immunol. 1994, 157, 439–447. [Google Scholar] [CrossRef]
- Gonnella, P.A.; Chen, Y.; Inobe, J.; Komagata, Y.; Quartulli, M.; Weiner, H.L. In situ immune response in gut-associated lymphoid tissue (GALT) following oral antigen in TCR-transgenic mice. J. Immunol. 1998, 160, 4708–4718. [Google Scholar]
- .Khoury, S.J.; Hancock, W.W.; Weiner, H.L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J. Exp. Med. 1992, 176, 1355–1364. [Google Scholar]
- Jung, C.; Hugot, J.P.; Barreau, F. Peyer’s patches: The immune sensors of the intestine. Int. J. Inflamm. 2010, 2010, 823710. [Google Scholar] [CrossRef]
- Lo, D.; Tynan, W.; Dickerson, J.; Scharf, M.; Cooper, J.; Byrne, D.; Brayden, D.; Higgins, L.; Evans, C.; O’Mahony, D.J. Cell culture modeling of specialized tissue: Identification of genes expressed specifically by follicle-associated epithelium of Peyer’s patch by expression profiling of Caco-2/Raji co-cultures. Int. Immunol. 2004, 16, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Kawano, K.; Nochi, T.; Pontes, G.S.; Fukuda, S.; Ebisawa, M.; Kadokura, K.; Tobe, T.; Fujimura, Y.; Kawano, S.; et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature 2009, 462, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Gebert, A.; Steinmetz, I.; Fassbender, S.; Wendlandt, K.H. Antigen transport into Peyer’s patches: Increased uptake by constant numbers of M cells. Am. J. Pathol. 2004, 164, 65–72. [Google Scholar] [CrossRef]
- Dillon, A.; Lo, D.D. M cells: Intelligent engineering of mucosal immune surveillance. Front. Immunol. 2019, 10, 1499. [Google Scholar] [CrossRef]
- Da Silva, C.; Wagner, C.; Bonnardel, J.; Gorvel, J.P.; Lelouard, H. The Peyer’s patch mononuclear phagocyte system at steady state and during infection. Front. Immunol. 2017, 8, 1254. [Google Scholar] [CrossRef]
- Reboldi, A.; Cyster, J.G. Peyer’s patches: Organizing B-cell responses at the intestinal frontier. Immunol. Rev. 2016, 271, 230–245. [Google Scholar] [CrossRef]
- Nagata, S.; McKenzie, C.; Pender, S.L.; Bajaj-Elliott, M.; Fairclough, P.D.; Walker-Smith, J.A.; Monteleone, G.; MacDonald, T.T. Human Peyer’s patch T cells are sensitized to dietary antigen and display a Th cell type 1 cytokine profile. J. Immunol. 2000, 165, 5315–5321. [Google Scholar] [CrossRef]
- Ebert, E.C.; Roberts, A.I. Lamina propria lymphocytes produce interferon-gamma and develop suppressor activity in response to lactoglobulin. Dig. Dis. Sci. 2001, 46, 661–667. [Google Scholar] [CrossRef]
- Gollob, J.A.; Li, J.; Reinherz, E.L.; Ritz, J. CD2 regulates responsiveness of activated T cells to interleukin 12. J. Exp. Med. 1995, 182, 721–731. [Google Scholar] [CrossRef]
- Ajuebor, M.N.; Das, A.M.; Virág, L.; Flower, R.J.; Szabó, C.; Perretti, M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: Evidence for an inhibitory loop involving endogenous IL-10. J. Immunol. 1999, 162, 1685–1691. [Google Scholar] [PubMed]
- Sadik, C.D.; Kim, N.D.; Luster, A.D. Neutrophils cascading their way to inflammation. Trends Immunol. 2011, 32, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Rusu, D.; Drouin, R.; Pouliot, Y.; Gauthier, S.; Poubelle, P.E. A bovine whey protein extract stimulates human neutrophils to generate bioactive IL-1Ra through a NF-kappaB- and MAPK-dependent mechanism. J. Nutr. 2010, 140, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Rusu, D.; Drouin, R.; Pouliot, Y.; Gauthier, S.; Poubelle, P.E. A bovine whey protein extract can enhance innate immunity by priming normal human blood neutrophils. J. Nutr. 2009, 139, 386–393. [Google Scholar] [CrossRef]
- Dusi, S.; Della Bianca, V.; Grzeskowiak, M.; Rossi, F. Relationship between phosphorylation and translocation to the plasma membrane of p47phox and p67phox and activation of the NADPH oxidase in normal and Ca2+-depleted human neutrophils. Biochem. J. 1993, 290, 173–178. [Google Scholar] [CrossRef]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broersen, K. Milk Processing Affects Structure, Bioavailability and Immunogenicity of β-lactoglobulin. Foods 2020, 9, 874. https://doi.org/10.3390/foods9070874
Broersen K. Milk Processing Affects Structure, Bioavailability and Immunogenicity of β-lactoglobulin. Foods. 2020; 9(7):874. https://doi.org/10.3390/foods9070874
Chicago/Turabian StyleBroersen, Kerensa. 2020. "Milk Processing Affects Structure, Bioavailability and Immunogenicity of β-lactoglobulin" Foods 9, no. 7: 874. https://doi.org/10.3390/foods9070874
APA StyleBroersen, K. (2020). Milk Processing Affects Structure, Bioavailability and Immunogenicity of β-lactoglobulin. Foods, 9(7), 874. https://doi.org/10.3390/foods9070874



